Play all audios:
ABSTRACT The t(11; 22)(p13; q12) translocation associated with desmosplastic small round cell tumor results in a chimeric molecule fusing the amino terminal domain (NTD) of the EWS1 gene to
three of the four carboxy-terminal zinc fingers of the WT1 tumor suppressor gene. Since the DNA binding domains of WT1 and EWS/WT1 are structurally different, we have assessed the functional
consequences of the EWS/WT1 fusion. We find that the EWS/WT1 protein has a higher binding affinity for a given recognition target than the WT1 product. This is unlike other fusion products
involving translocation of the NTD of EWS to DNA binding domains in which DNA binding specificity and affinity is not changed. We demonstrate that EWS/WT1 is a nuclear protein and that the
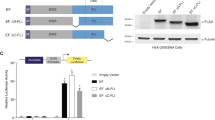
NTD of EWS contains (a) nuclear localization signal(s). We also find that the integrity of a domain within the WT1 zinc fingers, responsible for mediating interaction between WT1 and the
transcriptional repressor par-4, is disrupted in the EWS/WT1 fusion product. Deletion analysis of the NTD of EWS indicated that integrity of the entire domain was necessary to achieve full
transactivation potential. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 50 print issues and online access $259.00 per year only $5.18 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS THE FLI PORTION OF EWS/FLI CONTRIBUTES A TRANSCRIPTIONAL REGULATORY FUNCTION THAT IS DISTINCT AND SEPARABLE FROM ITS
DNA-BINDING FUNCTION IN EWING SARCOMA Article Open access 18 June 2021 FUNCTIONAL CHARACTERIZATION OF NPM1–TYK2 FUSION ONCOGENE Article Open access 18 January 2022 A MXI1-NUTM1 FUSION
PROTEIN WITH MYC-LIKE ACTIVITY SUGGESTS A NOVEL ONCOGENIC MECHANISM IN A SUBSET OF _NUTM1_-REARRANGED TUMORS Article 01 September 2020 AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Biochemistry, McGill University, 3655 Drummond Street, Montreal, H3G 1Y6, Quebec, Canada Jungho Kim & Jerry Pelletier * Department of Biology, Hong Kong University of
Science and Technology, Clear Water Bay, Kowloon, Hong Kong Kevin Lee * Department of Oncology, McGill University, 3655 Drummond Street, Montreal, H3G 1Y6, Quebec, Canada Jerry Pelletier
Authors * Jungho Kim View author publications You can also search for this author inPubMed Google Scholar * Kevin Lee View author publications You can also search for this author inPubMed
Google Scholar * Jerry Pelletier View author publications You can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE
THIS ARTICLE Kim, J., Lee, K. & Pelletier, J. The DNA binding domains of the WT1 tumor suppressor gene product and chimeric EWS/WT1 oncoprotein are functionally distinct. _Oncogene_ 16,
1021–1030 (1998). https://doi.org/10.1038/sj.onc.1201616 Download citation * Received: 20 June 1997 * Accepted: 03 October 1997 * Published: 05 March 1998 * Issue Date: 26 February 1998 *
DOI: https://doi.org/10.1038/sj.onc.1201616 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * EWS/WT1 * DSRCT * oncogene * cancer genetics *
transcription factor
