Play all audios:
ABSTRACT This study evaluated the disposition of the two atypical antipsychotics, amisulpride (AMS) and clozapine (CLZ), and its main metabolite _N_-desmethylclozapine (DCLZ), to their
target structures in the central nervous system by applying an _in vitro_ blood–brain barrier and blood–cerebrospinal fluid (CSF) barrier based on monolayers of porcine brain microvessel
endothelial cells (PMEC) or porcine choroid plexus epithelial cells (PCEC). Permeation studies through PMEC- and PCEC-monolayers were conducted for 60 min at drug concentrations of 1, 5, 10,
and 30 μM applied to the donor compartment. PMEC were almost impermeable for AMS (permeation coefficient, _P_<1 × 10−7 cm/s) in the resorptive direction, whereas transport in the
secretory direction was observed with a _P_ (±SD) of 5.2±3.6 × 10−6 cm/s. The resorptive _P_ of CLZ and DCLZ were 2.3±1.2 × 10−4 and 9.6±5.0 × 10−5 cm/s, respectively. For the permeation
across PCEC in the resorptive direction, a _P_ of 1.7±2.5 × 10−6 cm/s was found for AMS and a _P_ of 1.6±0.9 × 10−4 and 2.3±1.3 × 10−5 cm/s was calculated for CLZ and DCLZ, respectively.
Both, CLZ and DCLZ, could easily pass both barriers with about a five-fold higher permeation rate of CLZ at the PCEC. The permeation of AMS across the BBB was restricted partly due to an
efflux transport. It is thus suggested that AMS reaches its target structures via transport across the blood–CSF barrier. SIMILAR CONTENT BEING VIEWED BY OTHERS BRAIN-TO-BLOOD TRANSPORT OF
FLUORESCEIN IN VITRO Article Open access 26 October 2024 THE BLOOD–BRAIN BARRIER: STRUCTURE, REGULATION AND DRUG DELIVERY Article Open access 25 May 2023 STRATEGIES FOR DELIVERING
THERAPEUTICS ACROSS THE BLOOD–BRAIN BARRIER Article 01 March 2021 INTRODUCTION Amisulpride (AMS), a substituted benzamide, is regarded as an atypical antipsychotic due to the negligible risk
of extrapyramidal symptoms during therapy (Coukell et al, 1996). It exerts its antipsychotic action by selective blockade of postsynaptic dopaminergic D2 and D3 receptors (Martinot et al,
1996; Perrault et al, 1997; Castelli et al, 2001). At low concentrations, a preferential blockade of presynaptic D2/D3 receptors was postulated causing a more antidepressive-like action
(Perrault et al, 1997; Schoemaker et al, 1997). Furthermore, AMS preferentially antagonizes D2/D3 receptors in the limbic system in comparison to striatal areas (Scatton et al, 1997). The
predominant action of AMS in extrastriatal areas became especially obvious at low concentrations (Möller, 2001; Xiberas et al, 2001). Although it was speculated that at these low dosages
mainly D3 autoreceptors in cortical areas were antagonized (Scatton et al, 1997; Pani and Gessa, 2002), the reason for the limbic selectivity of AMS remains unclear. Like its predecessor,
sulpiride, it reveals some physicochemical peculiarities. AMS is, besides sulpiride, the most hydrophilic antipsychotic and elimination from the body is mainly accomplished by renal
clearance (Dufour and Desanti, 1988). Since AMS undergoes only negligible metabolism, differences in the distribution might mostly determine the availability of AMS at central dopaminergic
receptors. Only two studies are published regarding the disposition of AMS between the systemic circulation and peripheral compartments like the central nervous system (CNS) (Umbreit-Luik
and Dross, 1983; Dufour and Desanti, 1988). These studies were conducted in rats and reported a differential distribution between brain areas with or without blood–brain barrier (BBB) like
the epiphysis. While only less than 10% of the concentration in blood was found in areas protected by BBB, the concentration in, for example, the epiphysis was twice the blood concentration.
The relatively high AMS concentration in areas outside the BBB was also found in humans, resulting in a remarkable elevation of plasma prolactin by interfering with the dopaminergic control
of the hypophyseal hormones (Grunder et al, 1999). Different distributions in different brain areas have also been reported in a study assessing the local cerebral glucose use in rats after
AMS compared with haloperidol. For AMS, the glucose utilization was much higher in the temporal cortex compared with, for example, the nucleus accumbens (Cudennec et al, 1997). Striatal
dopamine D2 receptor occupancy in man was recently assessed by positron emission tomography (PET) (Martinot et al, 1996, Xiberas et al, 2001). It should be emphasized here that the striatum
might be not the best region to elucidate the therapeutic action of AMS, which is suggested to be more active in extrastriatal areas (Perrault et al, 1997; Schoemaker et al, 1997; Pani and
Gessa, 2002). This was corroborated by the study of Xiberas et al (2001), who revealed an accelerated occupancy of dopamine receptors in the extrastriatal compared with striatal areas at
relatively low serum concentrations of AMS. These studies in humans are in agreement with the findings in rat (see above). The reason for the regioselectivity is, however, still obscure.
Thus, the primary purpose of this study was to evaluate the overall disposition of AMS to its target structures in the CNS by applying an _in vitro_ BBB- and blood–cerebrospinal fluid (CSF)
barrier (BCB)-model based on monolayers of porcine brain microvessel endothelial cells (PMEC) or porcine choroid plexus epithelial cells (PCEC). Although this study mainly focused on the
permeation of AMS across BBB and BCB, it was also aimed at the comparison of the permeation of AMS with the permeation properties of clozapine (CLZ), another atypical antipsychotic. In
contrast to AMS, CLZ is highly lipophilic and was found to be readily and equally distributed to the CNS. In rats, a more than 15-fold higher concentrations in the brain compared with blood
was detected (Wilk and Stanley, 1978; Weigmann et al, 1999). In humans, a PET study by Nordstrom et al (1995) revealed rather high serotonin subtype 5-HT2C receptor occupancy even at low CLZ
serum concentrations. A second purpose of the study was therefore to evaluate possible different behavior of BBB and the BCB with respect to these different physicochemical substrates.
METHODS CHEMICALS Racemic AMS was kindly donated by Sanofi-Synthelabo (Quetigny, France). CLZ and its main metabolite _N_-desmethylclozapine (DCLZ) and verapamil were purchased from Sigma
(Taufkirchen, Germany). (−)-[Methoxy-3H]sulpiride (3H-SUL), specific activity 77 Ci/mmol, and [14C]mannitol (specific activity 50–63 mCi/mmol) were purchased from Perkin-Elmer (Boston, MA,
USA). Other chemicals for analytical purpose were of the highest quality commercially available. Chemicals for the preparation of cell culture- and assay-media are given in the text.
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC) ANALYSIS Quantitative analysis of racemic AMS, CLZ, and DCLZ was performed mainly according to a method recently established for the analysis of
sulpiride in human serum (Müller et al, 2001). In brief, 150 μl of the sample in assay medium had to be supplemented with 50 μl acetonitrile to obtain a UV-signal and a final injection
volume of 150 μl was injected by a Gilson 231 autoinjector (Abimed-Analysentechnik, Langenfeld, Germany) on a Lichrospher-CN analytical column (125 × 4.0 mm, 5 μm particle size;
MZ-Analysentechnik, Mainz, Germany) coupled to a UV-detector (LC-10, Shimadzu, Duisberg, Germany) set at 210 nm. The mobile phase consisted of 50% acetonitrile and 50% 0.008 M phosphate
buffer, pH 6.4 (vol/vol). Calibration samples were prepared in PBS buffer in a concentration range between 10 and 500 μg/l AMS (0.027–1.35 μM) and 20–5000 μg/l CLZ and DCLZ (0.061–15.2 and
0.064–16.0 μM, respectively). The limit of quantification was in the range of the lowest calibration sample and the calibration curves were always linear in the whole calibration range with
a correlation coefficient (_r_2) always exceeding 0.99. DETERMINATION OF THE PARTITION COEFFICIENT (LOG _P_) AND THE APPARENT PARTITION COEFFICIENT (LOG _D_(7.4)) Log _P_ values were
determined by potentiometric titration using a PCA 200 instrument (Sirius analytical instruments, Forrest Row, UK) according to the equation logP=log( 10 ( p k a − p k a (0) ) −1)−log(r),
where p_k_a(o) is the p_k_a in an octanol/water mixture and (_r_) is the volume ratio octanol/water (Takacs-Novak and Avdeef, 1996; Avdeef et al, 1999). Log _D_(7.4) was calculated from
_logD_(7.4) = log_P_ − log(1+ 10 p k a − pH ) and assessed by the shake-flask technique (Leo, 1987) by measuring the distribution of the ionized+nonionized form between 1-octanol and an
HBSS/HEPES buffer (pH 7.4). PERMEATION ACROSS CACO-2 CELLS P-gp-expressing Caco-2 cells (American Type Culture Collection, Rockville, MD) were cultivated (21–29 days) on polycarbonate filter
membranes using 1.13 cm2 Transwell™ cell culture inserts (0.4 μm pore size, polycarbonate membrane 12 mm filter, Corning Costar, Bodenheim, Germany). During this time, the development of
the monolayers was monitored by transepithelial electrical resistance (TEER) measurements using a Milicell™ ERS and chopstick electrodes (Milicel ERS, Milipore, Bedford, USA) and visual
control under a light microscope. As transport medium, Hank's Balanced Salt Solution (HBSS) supplemented with 10 mM MES adjusted to pH 7.4 was used throughout the experiments. Prior to
the experiments, the cells were preincubated in transport buffer at 37°C at 5% CO2 and 90% relative humidity for 15 min. Each experiment was performed in triplicate at 37°C under continuous
stirring. Drug (AMS, CLZ, DCLZ) solutions (0.1 mM) were added to the donor side of the monolayers either in the absence or in the presence of verapamil (0.5 mM). Samples were taken from the
acceptor side at the beginning, after 30, 60, 90, and 120 min (end of the experiment). Samples were analyzed by HPLC and [14C]mannitol was applied to assess the cell monolayer integrity at
the end of the experiment. PMEC CELL CULTURE Primary brain endothelial cells were isolated from porcine brain homogenate by dispase digestion followed by a dextran density gradient
centrifugation. Endothelial cells were plated onto collagen-coated culture surfaces, subcultivated after 3 days and plated in a culture medium, consisting of Medium M199 (Biochrom, Berlin,
Germany), Earle's salts, 0.7 mM glutamine, antibiotics, and 10% ox serum (v/v), on rat tail collagen-coated Transwell™ filter inserts (Costar, Bodenheim, Germany). On the third day
after passage, the culture medium was replaced by serum-free assay medium (Hoheisel et al, 1998) consisting of medium DME/Ham's F12 (Biochrom, Berlin, Germany) with 0.7 mM glutamine
(Biochrom), antibiotics (Sigma), and 550 nM hydrocortisone (Sigma). The tightness of the monolayer was assessed directly prior to permeability studies by determination of the
transendothelial electrical resistance (TEER) using an ENDOHM-24™ chamber and the EVOHM™ voltmeter (World Precision Instruments, Berlin, Germany) and by evaluating the paracellular diffusion
of 14C-sucrose after the last permeability experiment was conducted. The preparation procedure is given in detail by Franke et al (2000). The mean (±SD) TEER was 1230±139 Ω cm2 (_N_=12) for
AMS, for CLZ and 1044±211 Ω cm2 (_N_=12) for DCLZ. The 14C-sucrose permeation was always in the range of 1 × 10−6 cm/s. PCEC CELL CULTURE PCEC from porcine choroid plexus were obtained by a
preparation basically described by Crook et al (1981), slightly modified by Gath et al (1997), and described in detail by Hakvoort et al (1998) and Haselbach et al (2001). In brief, porcine
choroid plexus tissue was digested by trypsin, released cells were centrifuged and thereafter resuspended in DME/HAM's F12 medium supplemented with 10% fetal bovine serum (Biochrom), 4
mM L-glutamin, 5 μg/ml insulin (Sigma), 20 μM cytosine arabinoside (Sigma), and 100 μg/ml antibiotics (Sigma). After 5 days, cells were seeded on laminin (Sigma)-coated permeable membranes
(Costar, Cambridge, MA, USA). After reaching confluence (7–9 days), cells were washed and incubated with a serum-free medium (DMA/HAM's F-12 medium (1 : 1) supplemented with 4 mM
L-glutamine and 5 μg/ml insulin). The mean (±SD) TEER was 1819±415 Ω cm2 (_N_=12) for AMS, 2022±403 Ω cm2 (_N_=12) for CLZ, and 1768±330 Ω cm2 (_N_=12) for DCLZ. PERMEABILITY STUDIES The
experimental setup for permeation studies across PMEC is described in detail by Franke et al (1999,2000). AMS, CLZ, or DCLZ were applied to the apical (luminal) side, representing the lumen
of a microvessel, and samples of 150 μl were taken from the basolateral acceptor compartment after 15, 30, 45, and 60 min. The sample volume was always replaced by an equal volume of fresh
medium. Each drug was tested at four different concentrations (1, 5, 10, and 30 μM) and three different filters/concentration were used. The concentrations were chosen to resemble utmost the
concentrations under therapeutic conditions in humans. Only AMS (30 μM) was tested in the opposite direction from basolateral to apical, representing the efflux from CNS into the blood. To
determine active transport, 3H-SUL, which is structurally related to AMS, was applied to both sides (apical and basolateral) in equimolar concentrations (1, 5, 10, and 30 μM). Radioactivity
at each side was measured after 0.5, 22.5, 29, 47, and 53 h in a beta-counter. For permeation studies across PCEC, drugs were applied to the basolateral side, which represents the blood
side, while samples were taken from the apical side, which represents the cerebrospinal fluid. The concentrations of drugs and sampling times were the same as described for PMEC permeation.
All transport experiments were conducted at 37°C. To correct for possible additional contribution of the coated filters to the total permeation, diffusion of drugs (only AMS and CLZ) through
coated filters without cell monolayers was tested (1, 5, 10, and 30 μM; samples taken after 5, 10, 15, and 20 min). CALCULATION Permeability coefficients (_P_) were calculated according to
Pardridge et al (1990) using the equation _P_(_cm_/_s_) = _dQ_/_dt_ × _V_)/(_A_ × _C__0_) where d_Q_/d_t_ is the translocation rate, _V_ is the volume of the acceptor compartment, _A_ is the
filter surface (1.13 cm2), and _C_0 is the initial concentration. To correct for the filter contribution, the total endothelial permeability coefficient (_P_) was divided by the blank
filter diffusion (_P_f) and given as percentage of unrestricted diffusion through blank filter. If no concentration dependency was detectable, _P_ was calculated as mean±SD from all
concentrations after 60 min of incubation. Kinetic analyses were performed by means of nonlinear least square curve fitting using the GraFit program (version 4.03, Erithacus Software Ltd,
Staines, UK). RESULTS LOG _P_ AND LOG _D_(7.4) VALUES The potentiometric method and the shake-flask method gave comparable results for all drugs tested. The exact log _P_ and log _D_(7.4)
values are given in Table 1. While the difference between log _P_ and log _D_(7.4) was only negligible in the case of CLZ, the difference was almost 10-fold for DCLZ and about 50-fold for
AMS. PERMEATION THROUGH COATED BLANK FILTER Diffusion through coated blank filter was about 10-fold lower for AMS compared with CLZ (2.18 × 10−5 _vs_ 3.75 × 10−4 cm/s and 3.6 × 10−5 _vs_ 4.7
× 10−4 cm/s for PMEC and PCEC, respectively). PERMEATION ACROSS CACO-2 CELLS The permeation of AMS showed a 2.68 higher secretory flux (basolateral to apical, b–a) compared to the
resorptive flux (apical to basolateral, a–b), as shown in Table 2. Furthermore, addition of 0.5 mM verapamil increased the flux in the resorptive direction from 0.22±0.02 to 0.46±0.03 × 10−5
cm/s (Table 2). While the flux in the secretory direction was slightly higher than in the resorptive direction (2.1±0.5 × 10−5 _vs_ 3.3±0.2 × 10−5 cm/s) for DCLZ, there was no difference
between the resorptive and the secretory flux for CLZ with 3.11±0.1 × 10−5 and 3.42±0.6 × 10−5, respectively. PERMEATION ACROSS PMEC AMS was not able to permeate PMEC in considerable
concentrations in the resorptive direction. Only with one of three preparations, measurable amounts were found in the acceptor compartment at the highest concentration of 30 μM and after
60-min incubation time. The approximated _P_ (<10−7 cm/s) was even lower than that of sucrose (about 10−6 cm/s). On the other hand, a considerable permeation in the secretory direction
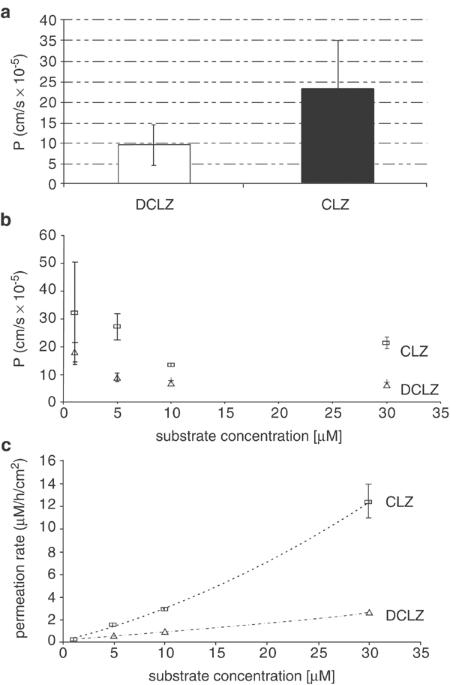
(basolateral to apical) was found with a _P_ (mean±SD) of 5.2±3.6 × 10−6 cm/s. The _P_ of CLZ and DCLZ in the resorptive direction was more than a magnitude higher with _P_=2.3±1.2 × 10−4
and 0.96±0.5 × 10−4 cm/s, respectively (Figure 1). Concentration dependence became only visible for DCLZ (Figure 1) pointing to a saturable process at concentrations higher than 1 μM. At
maximum, 50% of the applied CLZ dose was found at the basolateral side, while only 12% were measurable for DCLZ. Compared with the permeation through the blank filter, the permeation through
PMEC made up 62±5.4% for CLZ but <1% for AMS. Permeation kinetics of CLZ and DCLZ over time were best described by a quadratic function (best fit of the data), with _y_=0
0.0061_x_2+0.227_x_+0.1032 (_R_2=0.9995) and _y_=0.001_x_2+0.055_x_+0.1427 (_R_2=0.9999) for CLZ and DCLZ, respectively, assuming slightly nonlinear kinetics with accelerated permeation
rates at higher concentrations (Figure 1). No active transport was found for 3H-SUL (Figure 2). PERMEATION ACROSS PCEC All drugs tested were able to permeate plexus epithelial cells from the
basolateral (blood) to the apical (plexus) side. The _P_ values were, however, rather different with 1.7±2.5 × 10−6 cm/s for AMI, 2.3±1.2 × 10−5 cm/s for DCLZ, and 1.6±0.95 × 10−4 cm/s for
CLZ (Figure 3). Permeation across PCEC compared with blank filter diffusion was 9.3±6.6 and 62±5.4% for AMS and CLZ, respectively (Figure 3). The permeation kinetics was mostly linear over
the entire concentration range for all substances (Figure 3). DISCUSSION This study was the first that investigated the permeation of two atypical antipsychotics through biomembranes that
restrict free access to the CNS. The study revealed tremendous differences between the two atypical antipsychotics, CLZ and AMS. As these are structurally unrelated compounds—the former is a
highly lipophilic dibenzazepine with a p_k_a of about 7.4, AMS is a more hydrophilic substituted benzamide with a p_k_a of about 9.08—differences had been expected (Doan et al, 2002).
Interestingly, while the partition between octanol and buffer expressed as log _P_ was 175-fold higher for CLZ, the apparent partition (log _D_(7.4)) at a more physiological pH of 7.4 was
almost 4000-fold higher for CLZ. As the permeation experiments were conducted at physiological pH, log _D_(7.4) seemed to correlate much better with the permeation through microvessel
endothelial cells than log _P_. Both drugs have to pass the BBB to exert their pharmacological effects. It was thus unexpected that AMS obviously did not penetrate the BBB model. It should
be mentioned that the permeability of AMS through the cell-free filter was about 10-fold lower compared with CLZ. The difference in the permeation of AMS through PMEC could, however, not be
solely explained by this methodological issue since the difference was more than 1000-fold comparing CLZ and AMS in the resorptive direction. The poor permeation in the resorptive direction
was even more restricted by a remarkable transport in the secretory direction. This prominent flux in the secretory direction also became visible when the Caco-2 model was applied. A ratio
>2 of the apical to basolateral permeability (resorptive direction) _vs_ apical to basolateral permeability (secretory direction) across Caco-2 monolayers was reported to be indicative of
P-glycoprotein (Pgp) substrates (Polli et al, 2001). We would, therefore, regard AMS as a Pgp substrate, while CLZ and DCLZ did not fulfill the criteria of a Pgp-sensitive drug. DCLZ had a
lower permeability rate than CLZ, which was in good agreement with our findings in rats after oral administration of CLZ (Weigmann et al, 1999). In contrast to PMEC, permeation of AMS across
plexus epithelial cells was higher and more comparable to the permeation of CLZ and DCLZ. The difference between CLZ and DCLZ was even more pronounced at the PCEC-monolayer with about a
10-fold higher _P_ of CLZ. The generally higher permeation rates of CLZ might be also caused by an active transport through these barriers as it had been shown to play a role for the
synthetic opioid fentanyl (Henthorn et al, 1999). The lack of transport of AMS across this BBB model was a rather surprising result. It seems to be caused by the additive effects of
disadvantageous physicochemical properties and an efflux transport. The latter might be catalyzed by Pgp, which has been detected at the luminal side of porcine microvessel endothelial cells
(Miller et al, 2000). In a recent study, the structurally related benzamide SUL was suggested to be a substrate of Pgp (Baluom et al, 2001). Our own experiments, however, did not provide
any evidence for an active transport of SUL at the PMEC and at a concentration of 10 μM, SUL was able to permeate PMEC with a _P_ of 2.2 × 10−6 cm/s. This pointed to different properties of
these chemically related drugs with respect to the CNS disposition. Pgp could also be the reason for the accelerated transport across PCEC, since it was found to be expressed at the luminal
side of plexus epithelial cells (Rao et al, 1999). Besides Pgp, multidrug resistance associated protein (MRP2) was detected at the luminal side of PMEC (Miller et al, 2000) and at the
basolateral (blood-) side of PCEC (MRP1) (Gao and Meier, 2001). Quite recently, the so-called brain multidrug resistance protein (BMDP), a new member of the ABCG subfamily, was found to be
expressed in high rates in PMEC (Eisenblätter and Galla, 2002; Eisenblätter et al, 2003). It might participate in drug efflux at the BBB and needs to be considered. While AMS is likely to be
a substrate of Pgp, it is still unknown if one of the drugs tested might be a substrate of other transporters like MRP, BMDP, or the organic anion transporter protein (OATP), which is
expressed especially at the BCB (Angeletti et al, 1997). Furthermore, this is only an _in vitro_ model of the BBB and BCB and conclusions from our results on the _in vivo_ situation in
humans should be drawn rather cautiously. Limitations of the applied BBB model includes the fact that porcine cells were used that do not necessarily express the same transporter proteins at
the same rate as in human tissue. Secondly, it is an artificial system and thus even though the expressed proteins are highly conserved, the expression rate might differ substantially from
the _in vivo_ situation (Miller et al, 2000; Gutmann et al, 1999). The most important structural difference, however, is related to the fact that we have no brain tissue behind the acceptor
side. Brain tissue might act as a lipophilic sponge by increasing the flux rates, especially of lipophilic drugs that are not a substrate of an efflux transporter. Despite these limitations,
it can be concluded that AMS is evidently not able to permeate the BBB to a considerable extent. It is thus worth hypothesizing about the route of AMS to its targets in the brain. CSF
functions probably not only include mechanical protection of the brain and a sink action but also nutrient supply and even drug delivery to the brain and from the brain to the periphery
(Ghersi-Egea and Strazielle, 2001; King et al, 2001). Results of our study suggest that AMS might be at least partially distributed to its targets via the ventricular CSF. This would explain
the higher concentrations measured in brain areas more close to the ventricles like the hypothalamus compared with structures like the thalamus and might be one reason for the proposed
preferential extrastriatal action of AMS. It would be an interesting new aspect in the development of psychotropic drugs if certain drugs can actually enter the CNS via the CSF. However, the
hypothesis needs to be proven in future experiments. If we focus on the chemically related benzamide antipsychotics sulpiride and AMS, the latter had about a 10-fold higher affinity to
dopamine D2 and D3 receptors than sulpiride (_in vitro_ dissociation constants, _K_i, at the D2 receptor 1.3 _vs_ 10 nmol/l for AMS and sulpiride, respectively; for reference, see Coukell et
al, 1996). Moreover, AMS was so far suggested to permeate better into brain tissue due to its slightly higher lipophilicity. However, from _in vivo_ radioligand binding studies using
[3H]raclopride, sulpiride and AMS displaced [3H]raclopride binding with a comparable ED50 for limbic structures (14.6 _vs_ 17.3 mg/kg i.p.) and for the striatum (45.5 _vs_ 43.6 mg/kg i.p.)
(Schoemaker et al, 1997). This discrepancy between the _in vitro_ and _in vivo_ properties can be easily explained by the restricted permeation of AMS across the BBB as shown in our
experiments. The higher _in vitro_ affinity of AMS to its target receptor is thus obviously overshadowed by the poor CNS permeability. This is another example of the importance of evaluating
the CNS permeability of psychotropic drugs to assess their actual therapeutic efficacy. REFERENCES * Angeletti RH, Novikoff PM, Juvvadi SR, Fritschy J-M, Meier PJ, Wolkoff AW (1997). The
choroid plexus epithelium is the site of the organic anion transport protein in the brain. _Proc Natl Acad Sci USA_ 94: 283–286. Article CAS Google Scholar * Avdeef A, Box KJ, Comer JEA,
Gilges M, Hadley M, Hibbert C _et al_ (1999). PH-metric LogP11. pKa determination of water-insoluble drugs in organic solvent-water mixtures. _J Pharm Biomed Anal_ 20: 631–641. Article CAS
Google Scholar * Baluom M, Friedmann M, Rubinstein A (2001). Improved intestinal absorption of sulpiride in rats with synchronized oral delivery systems. _J Control Release_ 70: 139–147.
Article CAS Google Scholar * Castelli MP, Mocci I, Sanna AM, Gessa GL, Pani L (2001). (-) S amisulpride binds with high affinity to cloned dopamine D3 and D2 receptors. _Eur J Pharmacol_
432: 143–147. Article CAS Google Scholar * Coukell AJ, Spencer CM, Benfield P (1996). Amisulpride. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy
in the management of schizophrenia. _CNS Drugs_ 6: 237–256. Article CAS Google Scholar * Crook RB, Kasagami H, Prusiner SB (1981). Culture and characterization of epithelial cells from
bovine choroid plexus. _J Neurochem_ 37: 845–854. Article CAS Google Scholar * Cudennec A, Fage D, Bénavidès J, Scatton B (1997). Effects of amisulpride, an atypical antipsychotic which
blocks preferentially presynaptic dopamine autoreceptors, on integrated functional cerebral activity in the rat. _Brain Res_ 768: 257–265. Article CAS Google Scholar * Doan KMM, Humphreys
JE, Webster LO, Wring SA, Shampine LJ, Serabjit-Singh CJ _et al_ (2002). Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS
marketed drugs. _J Pharmacol Exp Ther_ 303: 1029–1037. Article CAS Google Scholar * Dufour A, Desanti C (1988). Pharmacocinétique et métabolisme de l’amisulpride. _Ann Psychiatry_ 3:
298–305. Google Scholar * Eisenblätter T, Galla H-J (2002). A new multidrug resistance protein at the blood–brain barrier. _Biochem Biophys Res Commun_ 293: 1273–1278. Article Google
Scholar * Eisenblätter T, Hüwel S, Galla H-J (2003). Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood–brain barrier. _Brain Res_ 971:
221–231. Article Google Scholar * Franke H, Galla H-J, Beuckmann CT (1999). An improved low-permeability _in vitro_-model of the blood–brain barrier: transport studies on retinoids,
sucrose, haloperidol, caffeine and mannitol. _Brain Res_ 818: 65–71. Article CAS Google Scholar * Franke H, Galla H-J, Beuckmann CT (2000). Primary cultures of brain microvessel
endothelial cells: a valid and flexible model to study drug transport through the blood–brain barrier _in vitro_. _Brain Res Protoc_ 5: 248–256. Article CAS Google Scholar * Gao B, Meier
PJ (2001). Organic anion transport across the choroid plexus. _Microsc Res Techniq_ 52: 60–64. Article CAS Google Scholar * Gath U, Hakvoort A, Wegener J, Decker S, Galla H-J (1997).
Porcine choroid plexus cells in culture: maintenance of barrier properties and apical secretion of CSF components. _Eur J Cell Biol_ 74: 68–78. CAS PubMed Google Scholar * Ghersi-Egea
J-F, Strazielle N (2001). Brain drug delivery, drug metabolism, and multidrug resistance at the choroid plexus. _Microsc Res Techniq_ 52: 83–88. Article CAS Google Scholar * Grunder G,
Wetzel H, Schlosser R, Anghelescu I, Hillert A, Lange K _et al_ (1999). Neuroendocrine response to antipsychotics: effects of drug type and gender. _Biol Psychiatry_ 45: 89–97. Article CAS
Google Scholar * Gutmann H, Toeroek M, Fricker G, Huwyler J, Beglinger C, Drewe J (1999). Modulation of multidrug resistance protein expression in porcine brain capillary endothelial
cells _in vitro_. _Drug Metab Dispos_ 27: 937–941. CAS PubMed Google Scholar * Hakvoort A, Haselbach M, Galla H-J (1998). Active transport properties of porcine choroid plexus cells in
culture. _Brain Res_ 795: 247–256. Article CAS Google Scholar * Haselbach M, Wegener J, Decker S, Engelbertz C, Galla H-J (2001). Porcine choroid plexus epithelial cells in culture:
regulation of barrier properties and transport processes. _Microsc Res Techniq_ 52: 137–152. Article CAS Google Scholar * Henthorn TK, Liu Y, Mahapatro M, Ng K (1999). Active transport of
fentanyl by the blood–brain barrier. _J Pharmacol Exp Ther_ 289: 1084–1089. CAS PubMed Google Scholar * Hoheisel D, Nitz T, Franke H, Wegener J, Hakwoort A, Tilling T _et al_ (1998).
Hydrocortisone reinforces the blood–brain barrier properties in a serum free cell culture system. _Biochem Biophys Res Commun_ 244: 312–316. Article CAS Google Scholar * King M, Su W,
Chang A, Zuckerman A, Pasternak GW (2001). Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. _Nat Neurosci_ 4: 268–274. Article
CAS Google Scholar * Leo AJ (1987). Some advantages of calculating octanol-water partition coefficients. _J Pharm Sci_ 76: 166–168. Article CAS Google Scholar * Martinot JL,
Pailére-Martinot M-L, Poirier ME, Dao-Castellana MH, Loc’h C, Maziére B (1996). _In vivo_ characteristics of dopamine D2 receptor occupancy by amisulpride in schizophrenia.
_Psychopharmacology_ 124: 154–158. Article CAS Google Scholar * Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G (2000). Xenobiotic transport across isolated brain
microvessels studied by comfocal microscopy. _Mol Pharmacol_ 58: 1357–1367. Article CAS Google Scholar * Möller H-J (2001). Amisulpride: efficacy in the management of chronic patients
with predominant negative symptoms of schizophrenia. _Eur Arch Psychiatry Clin Neurosci_ 251: 217–224. Article Google Scholar * Müller MJ, Härtter S, Köhler D, Hiemke C (2001). Serum
levels of sulpiride enantiomers after oral treatment with racemic sulpiride in psychiatric patients: a pilot study. _Pharmacopsychiatry_ 34: 27–32. Article Google Scholar * Nordstrom AL,
Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G (1995). D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. _Am J
Psychiatry_ 152: 1444–1449. Article CAS Google Scholar * Pani L, Gessa GL (2002). The substituted benzamide and their clinical potential on dysthymia and on the negative symptoms of
schizophrenia. _Mol Psychiatry_ 7: 247–253. Article CAS Google Scholar * Pardridge WM, Triguero D, Yang J, Cancilla PA (1990). Comparison of _in vitro_ and _in vivo_ models of drug
transcytosis through the blood–brain barrier. _J Pharmacol Exp Ther_ 253: 884–891. CAS PubMed Google Scholar * Perrault GH, Depoortere R, Morel E, Sanger DJ, Scatton B (1997).
Psychopharmacological profile of amisulpride: an antipsychotic with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. _J Pharmacol Exp Ther_ 280: 73–82. CAS
Google Scholar * Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO _et al_ (2001). Rational use of _in vitro_ P-glycoprotein assays in drug discovery. _J Pharmacol Exp Ther_
299: 620–628. CAS PubMed Google Scholar * Rao VV, Dahlheimer JL, Bardgeti ME, Snyder AZ, Finch RA, Sartorellei AC _et al_ (1999). Choroid plexus epithelial expression of _MDR1_ P
glycoprotein and multidrug resistance-associated protein contribute to the blood–cerebrospinal-fluid drug-permeability barrier. _Proc Natl Acad Sci USA_ 96: 3900–3905. Article CAS Google
Scholar * Scatton B, Claustre Y, Cudennec A, Oblin A, Perrault GH, Sanger DJ _et al_ (1997). Amisulpride: from animal pharmacology to therapeutic action. _Int Clin Psychopharmacol_ 12(Suppl
2): S29–S36. Article Google Scholar * Schoemaker H, Claustre Y, Fage D, Rouquier L, Chergui K, Curet O _et al_ (1997). Neurochemical characteristics of amisulpride, and atypical dopamine
D2/D3 receptor antagonist with both presynaptic and limbic selectivity. _J Pharmacol Exp Ther_ 280: 83–97. CAS Google Scholar * Takacs-Novak K, Avdeef A (1996). Interlaboratory study of
LogP determination by shake-flask and potentiometric methods. _J Pharm Biomed Anal_ 14: 1405–1413. Article CAS Google Scholar * Umbreit-Luik M, Dross K (1983). Regional distribution of
three related benzamides (sulpiride, sultopride and DAN 2163) in the brain of the rat. _Arch Pharmacol_ 324(Suppl): R68. Google Scholar * Weigmann H, Härtter S, Fischer V, Dahmen N, Hiemke
C (1999). Distribution of clozapine and desmethylclozapine between blood and brain in rats. _Eur Neuropsychopharmacol_ 9: 253–256. Article CAS Google Scholar * Wilk S, Stanley M (1978).
Clozapine concentrations in brain regions: relationship to dopamine metabolite increase. _Eur J Pharmacol_ 15: 101–107. Article Google Scholar * Xiberas X, Martinot JL, Mallet L, Artiges
E, Canal M, Loc’h C _et al_ (2001). _In vivo_ extrastriatal and striatal D2 dopamine receptor blockade by amisulpride in schizophrenia. _J Clin Psychopharmacol_ 21: 207–214. Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by a grant from the Deutsche Forschungsgemeinschaft (Hi 399/5-1). The generous financial aid provided by Dr Eich
and Sanofi-Synthelabo (Berlin, Germany) is gratefully acknowledged. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Psychiatry, University of Mainz, Mainz, Germany Sebastian
Härtter & Christoph Hiemke * Department of Biochemistry, University of Muenster, Muenster, Germany Sabine Hüwel, Tina Lohmann & Hans-Joachim Galla * Department of Pharmacy,
University of Mainz, Mainz, Germany Amal Abou el ela & Peter Langguth Authors * Sebastian Härtter View author publications You can also search for this author inPubMed Google Scholar *
Sabine Hüwel View author publications You can also search for this author inPubMed Google Scholar * Tina Lohmann View author publications You can also search for this author inPubMed Google
Scholar * Amal Abou el ela View author publications You can also search for this author inPubMed Google Scholar * Peter Langguth View author publications You can also search for this author
inPubMed Google Scholar * Christoph Hiemke View author publications You can also search for this author inPubMed Google Scholar * Hans-Joachim Galla View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Sebastian Härtter. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Härtter, S., Hüwel, S., Lohmann, T. _et al._ How Does the Benzamide Antipsychotic Amisulpride get into the Brain?—An _In Vitro_ Approach Comparing Amisulpride with Clozapine.
_Neuropsychopharmacol_ 28, 1916–1922 (2003). https://doi.org/10.1038/sj.npp.1300244 Download citation * Received: 31 January 2003 * Revised: 25 April 2003 * Accepted: 30 April 2003 *
Published: 22 May 2003 * Issue Date: 01 November 2003 * DOI: https://doi.org/10.1038/sj.npp.1300244 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
KEYWORDS * amisulpride * clozapine * _in vitro_ * blood–brain barrier * blood–CSF barrier * antipsychotics