Play all audios:
ABSTRACT Recreational abuse of toluene-containing volatile inhalants by adolescents is a significant public health problem. The mechanisms underlying the abuse potential of such substances
remain unclear, but could involve increased activity in mesoaccumbal dopamine (DA) afferents innervating the nucleus accumbens (ACB). Here, using _in vitro_ electrophysiology, we show that
application of behaviorally relevant concentrations of toluene directly stimulates DA neurons in the ventral tegmental area (VTA), but not surrounding midbrain regions. Toluene stimulation
of VTA neurons persists when synaptic transmission is reduced. Moreover, unlike _non_-DA neurons, the magnitude of VTA DA neuron firing does not decline during longer exposures designed to
emulate ‘huffing’. Using dual-probe _in vivo_ microdialysis, we show that perfusion of toluene _directly_ into the VTA increases DA concentrations in the VTA (somatodendritic release) and
its terminal projection site, the ACB. These results provide the first demonstration that even brief exposure to toluene increases action potential drive onto mesoaccumbal VTA DA neurons,
thereby enhancing DA release in the ACB. The finding that toluene stimulates mesoaccumbal neurotransmission by activating VTA DA neurons directly (independently of transynaptic inputs)
provide insights into the neural substrates that may contribute to the initiation and pathophysiology of toluene abuse. SIMILAR CONTENT BEING VIEWED BY OTHERS ETHANOL BLOCKS A NOVEL FORM OF
ILTD, BUT NOT ILTP OF INHIBITORY INPUTS TO VTA GABA NEURONS Article 10 March 2023 AGE-DEPENDENT EFFECTS OF VAPING ON THE PREFRONTAL CORTEX, VENTRAL TEGMENTAL AREA, AND NUCLEUS ACCUMBENS
Article Open access 21 November 2024 ACUTE AND PROTRACTED ABSTINENCE FROM METHAMPHETAMINE BIDIRECTIONALLY CHANGES INTRINSIC EXCITABILITY OF INDIRECT PATHWAY SPINY PROJECTION NEURONS IN THE
DORSOMEDIAL STRIATUM Article Open access 15 July 2022 INTRODUCTION Inhalant abuse represents a major health problem because of its association with future drug use (NSDUH, 2003) and severe
neurological/cognitive deficits (Filley, 2004). Many of these products (ie, spray paint, glue, etc.) including toluene and the abuse of pure toluene solvent, has been reported (Flanagan and
Ives, 1994). Although overall drug use by teenagers has declined since 1998, inhalant use has increased (NSDUH, 2003). Inhalant use begins in preadolescence and continues well into adulthood
(Rosenberg et al, 2002; NSDUH, 2003; Wu and Ringwalt, 2006; Cairney et al, 2005). Dopaminergic afferents arising from the ventral tegmental area (VTA) and projecting to the nucleus
accumbens (ACB) are crucial elements in the neural circuits that mediate arousal, motivation, and reinforcement (Wise, 2002). Increased dopamine (DA) neurotransmission within this
mesoaccumbal pathway mediates the reinforcing and psychomotor stimulant effects of drugs of abuse (Wise, 2002). In experimental animals, toluene inhalation produces reinforcing effects
(Bespalov et al, 2003; Blokhina et al, 2004; Funada et al, 2002; Weiss et al, 1979; Lee et al, 2006) and stimulates locomotor activity (Bowen, 2006; Riegel et al, 2003). Depletion of DA in
the ACB attenuates this latter effect (Riegel et al, 2003). Toluene also induces c-FOS activation in both the VTA and ACB (Lo and Chen, 2005), and increases the firing and bursting of VTA DA
neurons _in vivo_ (Riegel and French, 1999a). Although acute toluene exposure increases TTX-sensitive DA release in the dorsal striatum (Stengard et al, 1994), similar effects have not been
reported in the ACB (Kondo et al, 1995; Gerasimov et al, 2002b). Therefore, some investigators have concluded that a DA-independent (Spanagel and Weiss, 1999) or an, as yet, undetermined
(Hyman and Malenka, 2001) mechanism mediates the actions of toluene. Such conclusions, if incorrect, may confound future work seeking to identify the mechanisms underlying toluene abuse and
hamper the development of prevention strategies for toluene addiction (Beyer et al, 2001; Schiffer et al, 2006). DA neurons are spontaneously active (Grace and Bunney, 1983) and their
increased action potential firing stimulates DA release in the ACB (Wise, 2002). DA firing is influenced also by multiple mediators including various trans-synaptic inputs, local (GABAergic)
interneurons, and somatodendritic DA released from VTA DA neurons (Adell and Artigas, 2004; Marinelli et al, 2006; Beckstead et al, 2004). Therefore, toluene may influence one or more
neurotransmitter systems and the effects observed may vary depending upon the treatment regimen employed and the region or subregion examined. Indeed, the VTA is a heterogeneous structure
composed of DA and non-DA (ie, _γ_-GABA) neurons (Ikemoto and Wise, 2004; Van Bockstaele and Pickel, 1995). Furthermore, recent work has shown that infusion of ethanol and other drugs into
the posterior VTA elicits positive reinforcing effects, whereas anterior VTA infusion does not (Zangen et al, 2006; Rodd et al, 2004b; Ikemoto and Wise, 2004). Previous studies investigating
toluene did not determine where toluene acts to alter the activity of midbrain DA neurons, and if these changes are sufficient to increase DA release in the ACB (Riegel and French, 1999a).
The present studies determined whether _in vivo_ behaviorally relevant concentrations of toluene directly stimulate mesoaccumbal neurotransmission by a direct activation of VTA DA neurons.
_In vitro_ brain-slice experiments revealed that toluene displays anatomical specificity for VTA DA neurons that persists in treatments that reduce synaptic transmission and during longer,
escalating exposures designed to emulate ‘huffing’. Dual-probe microdialysis studies in awake animals revealed that perfusion of toluene into the VTA increases extracellular DA
concentrations in both the VTA and distal ACB. Parts of this data have been presented previously in preliminary form (Riegel and French, 2002). METHODS TOLUENE A stock solution of toluene in
polyoxyethylene-sorbitan mono-oleate (Tween-80, final concentration 0.02%) was prepared immediately before use with care taken to avoid any solvent loss. For electrophysiological
recordings, the stock solution was diluted in oxygenated aCSF (see below) and delivered (2 ml/min) to the recording chamber (35°C) by a gravity-feed (plasticizer-free) perfusion system.
Toluene was bath applied using two separate protocols. In _single applications_, each concentration of toluene was applied to the bath for 3 min to determine reversibility. Existing studies
indicate that inhalant abuse is characterized by a rapid intoxication (Meredith et al, 1989) and a related steep rise in brain toluene concentrations (Gerasimov et al, 2002a; Ameno et al,
1992; Kishi et al, 1988). Thus, as with other drugs (Balster and Schuster, 1973), the abuse liability of toluene may be affected by pharmacokinetic properties (Gerasimov et al, 2002a;
Lammers et al, 2005) and supplemental ‘huffs’ may be required to preserve the ‘high’ reported by users (NSDUH, 2003). Therefore, to better model these conditions, a _staircase paradigm_ was
employed. In this paradigm, escalating concentrations of toluene (seven concentrations at 3 min/concentration) were applied to the bath and the total duration of toluene exposure was
increased (21 min). Concentrations of toluene in perfusate samples taken from the recording chamber were determined by gas chromatograph (Riegel and French, 1999a) and were adjusted to match
_in vivo_ concentrations (4–79 μg/ml) observed in the blood of rodents (Riegel and French, 1999a) and humans exposed to toluene (Garriott et al, 1981). The drug reached the recording
chamber in ∼30 s, and the fluid in the chamber completely turned over in 3 min. For microdialysis, known aliquots of the toluene stock solution were diluted in aCSF and sealed immediately
just before reverse-dialysis. Data generated under conditions similar to those of the present study have shown that 10–30% of the drug concentration in the perfusion buffer reaches brain
tissue surrounding the dialysis probe (Yim and Gonzales, 2000; Gonzales et al, 1998). Thus, perfusate concentrations of toluene (1, 3, 10 mM) are expected to generate _in situ_
concentrations of 0.1, 0.3, and 1 mM, which are in accord with the _in vitro_ concentrations employed (23–822 μM) and previous _in vivo_ studies (for a discussion see Riegel et al, 2003).
_IN VITRO_ EXTRACELLULAR ELECTROPHYSIOLOGY SLICE PREPARATION Brain slices were prepared from male Sprague–Dawley rats (PD 19-40) according to protocols approved by the University of Arizona
IACUC (Wang and French, 1993b). Control experiments in brain slices prepared from older male Sprague–Dawley rats (PD 60) showed analogous (see below) toluene-induced changes in firing
(_n_=9, data not shown). Coronal slices (400 μm) containing the VTA were incubated in oxygenated aCSF composed of (in mM) 124 NaCl, 2.5 KCl, 1.25 KH2PO4, 2.4 MgSO4, 2.5 CaCl2, 25.7 NaHCO3,
and 10 D-glucose. Where indicated, aCSF was altered by lowering Ca2+ and increasing Mg2+ to either 0.5 mM Ca2+/10 mM Mg2+ (Media-1) or 0.25 mM Ca2+/7.25 mM Mg2+ (Media-2). Flow rates were
kept at 2 ml/min to insure adequate infusion of the altered media, consistent with previous published studies (Bondy and Harrington, 1979; Washio and Inouye, 1978; Alderdice and Volle, 1978;
Scholfied, 1981). Incubation with such media for 5 min or greater has been shown to block synaptic transmission (Bondy and Harrington, 1979; Washio and Inouye, 1978; Alderdice and Volle,
1978; Scholfied, 1981; Hutter and Kostial, 1954). Extracellular electrophysiological recordings were made with glass microelectrodes filled with a solution of 2% pontamine sky-blue in 0.5 M
sodium acetate (Yin and French, 2000). Action potential signals were amplified, and filtered (1–10 kHz) (Wang and French, 1993a; Marinelli et al, 2006). RECORDINGS Recording sites were found
with a dissection microscope and confirmed histologically _post hoc_ (see below: ‘Localization of recording sites and microdialysis probes’). All locations were anatomically differentiated
by reference to the rat atlas (Paxinos and Watson, 1988). VTA neurons were identified according to conventional pharmacological and electrophysiological criteria (Grace and Bunney, 1983;
Grace and Bunney, 1984; Johnson and North, 1992; Cameron et al, 1997; Marinelli et al, 2006). DA neurons possess: (1) bi- or tri-phasic action potentials with a somatodendritic notch, (2)
action potential lasting >2 ms, (3) ∼0.5 Hz firing rates, (4) inhibition to DA (50–100 μM), and (5) insensitivity to 5-HT (60 μM). Non-DA (type-II) neurons possess: (1) biphasic
positive–negative action potentials lacking an S-D notch, (2) action potential lasting <2 ms, (3) 1–2 Hz firing rates, and (4) insensitivity to inhibition by DA (50–100 μM) and 5-HT (60
μM). _Non_-DA (type-III) neurons displayed: (1) positive–negative action potentials lacking a somatodendritic notch, (2) action potential lasting >2 ms, (3) ∼1.5–2 Hz firing rates, and
(4) inhibition to DA (50–100 μM) and 5-HT (60 μM). Consistent with previous electrophysiological studies, neurons in the interpeduncular nucleus (IPN) were classified by anatomical location
(Lena et al, 1993; Takagi, 1984) and DA neurons in the retrorubral field (RRF) were identified by electrophysiological/pharmacological criteria analogous to those used for VTA DA neurons
(Deutch et al, 1988). The identification of neurons in the rostral interstitial nucleus (RIN) was based upon _post hoc_ histological analysis (below). The _duration of inactivation_ was
defined as an episode of reduced action potential amplitude, below noise level, which sometimes occurred immediately after a period of pronounced neuronal excitation. Although the
reversibility of toluene's actions permitted multiple challenges, only one cell was recorded from each brain slice. _IN VIVO_ MICRODIALYSIS SURGERY Male Sprague–Dawley rats (PD 70-80)
were anesthetized with equithesin (1% pentobarbital, 2% magnesium sulfate, 4% chloral hydrate, 42% propyleneglycol, 11% ethanol, 3 ml/kg i.p.). Microdialysis guide cannulae (CMA/11, CMA
microdialysis, North Chelmsford, MA) were inserted stereotaxically (David Kopf instruments, Topanga, CA) and secured to the skull using stainless-steel screws and dental acrylic. Membrane
probe length (VTA: 1 mm; ACB: 2 mm) was adjusted for stereotaxic location (VTA: AP −5.6, _L_ −0.4, _V_ −7.8; ACB: AP +1.7, _L_ −1.4, _V_ −6.4), where AP, _L_, and, _V_ denote mm from bregma
(Paxinos and Watson, 1988). After surgery, the animals were housed individually and allowed to recover for 2–4 days before experiments were started. MICRODIALYSIS Microdialysis was performed
as described previously (Zapata and Shippenberg, 2005). The evening before the experiment, probes were inserted into the guide cannulae connected to a perfusion system consisting of 1 ml
gastight syringes mounted on microdialysis pumps (CMA/102) and fitted with FEP tubing (CMA microdialysis) connecting the probes through dual-channel, quartz-lined, low-resistance swivels.
Probes were perfused overnight (0.3 μl/min) and 1 h before experiments (1 μl/min) with aCSF (in mM: 145 NaCl, 2.8 KCl, 1.2 CaCl2, 1.2 MgCl2, 0.25 ascorbic acid, 5.4 D-glucose, pH 6.5–7.0
adjusted with NaOH). During experiments, the VTA superfusate alternated between aCSF, vehicle (Tween-80, final concentration in aCSF 0.02%), or toluene (1, 3, and 10 mM; 1 h/concentration).
Dialysis fractions were collected every 15 min, frozen (−80°C), and analyzed for DA content using HPLC-electrochemical detection within 48 h (Zapata and Shippenberg, 2005). Dialysate DA
concentrations were quantified using an external standard curve. The detection limit of DA in dialysate was 0.3 nM. LOCALIZATION OF RECORDING SITES AND MICRODIALYSIS PROBES The anterior
(−5.2 to −5.6 mm posterior to bregma) and posterior (−5.6 to −6.04 mm posterior to bregma) divisions of the VTA were defined as in other studies (Rodd et al, 2005; Zangen et al, 2006;
Ikemoto and Wise, 2004; Carlezon et al, 2000; Carlezon and Nestler, 2002; Rodd-Henricks et al, 2000). For electrophysiology, recording sites were confirmed by iontophoresis of pontamine
sky-blue dye at experiment completion (Wang and French, 1993a). Then, as described elsewhere (Johnson et al, 1996), brain slices were resectioned (5–10 μm), stained with cresyl violet, and
examined by light microscopy. Microdialysis probe placement was confirmed after brains were removed, frozen, and sectioned on a cryostat (30 μm) as previously described (Zapata and
Shippenberg, 2005). Only when probe location was confirmed in both the VTA and ACB (Paxinos and Watson, 1988), was data from that animal included for further analysis. DATA ANALYSIS For
electrophysiology, only one neuron per slice was tested. Changes in neuron firing rates are expressed as a change from baseline (Hz). Significant differences between treatments were
determined by a Student's paired _t_-test. All depicted action potential waveforms are averaged (⩾4 spikes/trace). For microdialysis, basal DA levels in dialysate were determined in
each animal and represent the average of four samples collected before vehicle perfusion and toluene exposure. For each concentration of toluene, four consecutive 15 min dialysate samples
were obtained. The averaged values at each toluene concentration were compared to the effect of vehicle. Statistical significance was determined using a repeated measure one-way ANOVA and
the Newman–Keuls test for _post hoc_ comparisons. DRUGS AND REAGENTS Toluene (analytical grade), benzene (reagent grade), and carbon disulfide (reagent grade) were purchased from Fischer
Scientific (Tustin, CA). DA and serotonin were purchased from Sigma Chemicals (St Louis, MO) and baclofen from RBI (Natick, MA). RESULTS _IN VITRO_ EXTRACELLULAR ELECTROPHYSIOLOGY Initial
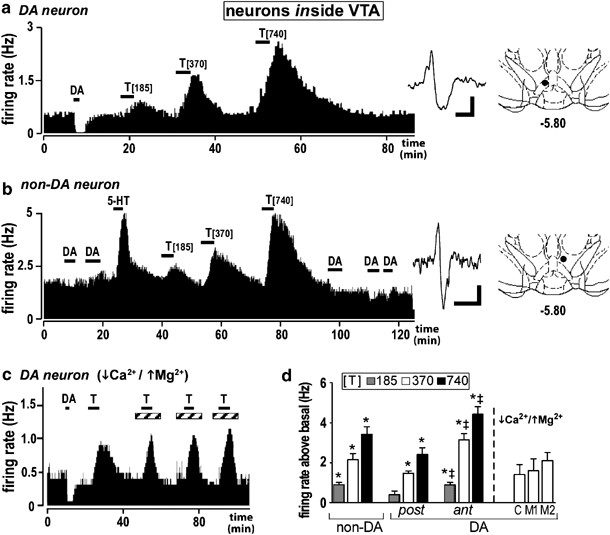
experiments using single applications determined whether toluene affects the excitability of VTA DA neurons in an isolated brain slice preparation. Short 3 min applications of toluene
stimulated VTA DA neurons. In all cases, the increase in firing was concentration-dependent and firing rates returned to baseline upon washout (Figure 1a) (maximum 3.4±0.3 Hz above baseline;
_n_=17; _p_<0.05, data not shown). The maximum increase in firing was greater in the anterior VTA (Figure 1d: 4.6±0.4 Hz above baseline; _n_=7), than the posterior VTA (Figure 1d:
2.8±0.3 Hz above baseline; _n_=10; _p_<0.01). These _in situ_ results resembled previous _in vivo_ studies, with respect to both the basal properties of DA neurons (Table 1) and the
reversible, toluene-induced increase in firing (Riegel and French, 1999a). In non-DA neurons, toluene stimulated the firing of type-II (Figure 1b; _n_=5) and type-III cells (_n_=7) (data not
shown, but a sample type-III cell is shown in Figure 3b). At both anterior and posterior VTA locations, toluene induced similar increases in non-DA neuron firing. So, the data were grouped
(Figure 1d: maximum 3.21±0.63 Hz above baseline; _n_=12). The stimulation of non-DA cells _in vitro_ differs from the inhibition of firing previously observed _in vivo_ (Riegel and French,
1999b), and may reflect the loss of monosynaptic glutamate inputs to neurons in a slice preparation (Tzschentke, 2001). To determine whether toluene increased VTA firing by altering
neurotransmitter release onto DA cells, we tested a low-Ca2+, high-Mg2+ aCSF media, previously shown to inhibit synaptic transmission (Brodie et al, 1990; Katz, 1970; Katz and Miledi, 1970).
The stimulatory properties of toluene (370 μM) were still apparent under these conditions (Figure 1c: 370 μM, single applications). As basal firing rates can vary under low Ca2+ conditions,
initial results with toluene in media-1 (0.5 mM Ca2+/10 mM Mg2+; Figure 1d: 1.73±0.86 Hz above baseline; _n_=5) were confirmed with experiments in media-2 (0.25 mM Ca2+/7.25 mM Mg2+; Figure
1d: 2.32±1.01 Hz above baseline; _n_=5). The magnitude of the toluene-induced increase in firing did not differ between media (Figure 1d; _p_>0.05, ANOVA), suggesting that toluene
directly activates VTA neurons. To determine if the stimulatory properties of toluene were unique to the VTA, three adjacent midbrain structures were tested using single applications. In the
IPN, the basal firing rate was 11.0±2.70 Hz (Table 1). Toluene inhibited the firing of neurons in the IPN (Figure 2a). The response was robust and concentration-dependent (Figure 2d:
maximum, −10.2±0.71 Hz below baseline; _n_=7). However, transient (<30 s) and concentration-independent increases were also noted in some neurons in the IPN (Figure 2a: <4 Hz, _n_=2).
In contrast, toluene did not significantly alter neuronal activity in the RRF (Figure 2b) or RIN (Figure 2c) (Figure 2e; maximum, 0.43±0.39 Hz above baseline, _n_=12; _p_>0.05). Basal
firing characteristics of neurons in the IPN and RRF are summarized in Table 1, and resemble those reported previously (Takagi, 1984; Deutch et al, 1988). The characteristics of neurons in
the RIN are shown (Table 1), although characterization of this region is lacking. Thus, these results demonstrate that behaviorally relevant concentrations of toluene (single applications)
selectively stimulate VTA neurons and that the increase in firing is similar in both DA and non-DA neurons. Behavioral responses to solvents are determined in part by the pattern of exposure
(Lammers et al, 2005). Therefore, prolonged _in vitro_ exposures were investigated. Using a staircase paradigm, we observed that cumulative applications of toluene stimulated VTA DA neurons
(Figure 3a; 22–823 μM) and non-DA neurons (Figure 3b; 22–633 μM). The increases in firing were concentration-dependent and reversed upon washout (Figure 3a and b). At DA neurons, the
efficacy of toluene did not change as a function of exposure paradigm. Thus, maximal activity under staircase conditions (Figure 3c: 4.07±0.44 Hz above baseline at 822 μM toluene, first
application, _n_=21) resembled firing under conditions using single applications (Figure 1d: 3.41±0.37 Hz above baseline at 740 μM toluene, _n_=17). In contrast, in non-DA neurons under
staircase conditions, the maximal efficacy of toluene declined ∼40% (Figure 3c: 2.19±0.32 Hz above baseline; at 633 μM, first application, _n_=9) relative to single applications (Figure 1d:
3.21±0.63 above baseline at 740 μM, _n_=12). The diminished efficacy is unlikely to be owing to tissue accumulation of toluene, because at comparable concentrations, the magnitude of the
increase in DA cell firing was similar during both short (single applications: 3 min) and long (staircase: 21 min) exposures. Furthermore, the attenuation of firing was observed only in
non-DA neurons during cumulative staircase conditions. More prolonged staircase exposure to toluene (>21 min) inactivated VTA DA (Figure 3a) and non-DA (Figure 3b) neurons and briefly
limited further firing. As noted in previous studies (Riegel and French, 1999a), this stimulation-induced inactivation was not owing to acute, irreversible cell death, because in all
instances, firing recovered to baseline when the perfusion media were replaced with toluene-free aCSF. With first applications, toluene inactivated non-DA neurons 2–3 times longer than DA
neurons (Figure 3a and b). To investigate if the toluene-induced inactivation of non-DA neurons was additive, the staircase paradigm was reapplied after firing resumed. The efficacy and
duration of inactivation during the two repetitive episodes were noted for comparison. In DA neurons (Figure 3a), maximal firing (Figure 3c; episode-1: 4.00±0.42 Hz above baseline, _n_=17;
episode-2: 4.09±0.49 Hz above baseline, _n_=9) and periods of inactivity (Figure 3d: episode-1: 2.50±1.11 min, _n_=17; episode-2: 2.82±0.96 min, _n_=9) remained the same. In non-DA neurons
(Figure 3b), however, maximal efficacy declined (Figure 3c episode-1: 2.2±0.2 Hz above baseline, _n_=9; episode-2: 1.3±0.3 Hz above baseline, _n_=3) and the duration of inactivation
increased (Figure 3d; episode-1: 11.3±1.6 min, _n_=9, _p_<0.01; episode-2: 28.2±2.3 min, _n_=3; _p_<0.001). Any assessment of toluene during a third episode was not possible, because
of the limitation of recording from a single neuron for longer than 2.5 h. Higher concentrations of toluene were not investigated because of the plateauing of neuronal stimulation. The
decline in efficacy and increase in the period of inactivation was only observed in non-DA neurons. Toluene (<1 mM) stimulated VTA DA neuron firing repeatedly without a significant
decline in efficacy. _IN VIVO_ MICRODIALYSIS Given these findings, we determined whether toluene can stimulate VTA DA neurons directly _in vivo_, and if so, whether this effect is sufficient
to increase mesoaccumbal DA release. Dual-probe microdialysis was used to measure concentrations of somatodendritic DA release in the VTA, an indirect marker of VTA DA neuron activity, and
DA released from DA terminals in the ACB. DA RELEASE IN THE VTA Toluene was infused directly into the VTA by reverse dialysis at concentrations of 1, 3, or 10 mM (see Methods). In the VTA,
infusion of vehicle resulted in a non significant shift (<15%) in basal dialysate DA concentrations (Figure 4a: 0.67±0.09 nM; _n_=9). In contrast, infusion of toluene increased
concentrations of somatodendritic VTA-DA (Figure 4a and b: F3,24=13.21, _p_<0.0001). A significant increase was observed in response to 3 and 10 mM toluene (Figure 4b; _p_<0.001 _vs_
vehicle). Significant increases in VTA DA were observed when toluene was infused into anterior or posterior VTA regions (Figure 4b; antVTA: F3,12=5.376, _p_=0.014; post VTA: F3,9=8.561,
_p_=0.0055 ANOVA). The anatomical location of all dialysis probes is shown in Figure 5. DA RELEASE IN THE ACB The basal concentration of ACB DA in dialysate was 1.82±0.43 nM. DA
concentrations in the ACB did not change following intra-VTA vehicle perfusion. However, reverse-dialysis of toluene into the VTA significantly increased DA levels in the ACB (Figure 4c and
d; _n_=11, F3,43=3.561, _p_=0.026, ANOVA). All toluene-induced increases in DA reversed within 60 min. The ACB-DA increases were significant, when toluene was infused into the posterior VTA
(Figure 4d; F3,18=4.772, _p_=0.013, ANOVA), but not the anterior VTA (Figure 4c and d; F3,9=1.613, _p_=0.250, ANOVA). This topographical selectivity (posterior VTA _vs_ anterior VTA)
parallels that previously observed in behavioral studies in which microinjection techniques were used to assess the effects of other drugs of abuse including both alcohol, and cannabinoids
(Zangen et al, 2006; Zangen et al, 2002; Ikemoto and Wise, 2004; Rodd-Henricks et al, 2000, 2002; Rodd et al, 2005). To confirm functional connectivity between the posterior VTA and ACB
probe placements, baclofen was infused into the VTA and DA levels after toluene washout and ACB DA levels were determined (Westerink et al, 1996). Baclofen produced a robust decline in ACB
DA concentrations (35±8% of vehicle; _n_=11; data not shown). Perfusion of toluene into regions anterior (_n_=5), dorsal (_n_=7), lateral (_n_=2), or ventral (_n_=2) to the VTA failed to
alter ACB-DA (Figure 4e and f) demonstrating the regional selectivity of this effect. Collectively, these data show that the effects of toluene on ACB-DA levels are specific to the posterior
VTA. DISCUSSION The present results indicate that toluene directly stimulates DA cell firing, thereby facilitating DA release in the ACB. Dopaminergic innervation of the ACB is a key
substrate for the reinforcing effects of other drugs of abuse (Wise, 2002). Nevertheless, despite intensive study, the neural substrates mediating the CNS actions of toluene have remained
elusive. Previous behavioral studies indicated that the locomotor activating effects of toluene require intact mesoaccumbal DA transmission (Riegel et al, 2003). Moreover, toluene increases
the activity of VTA DA neurons _in vivo_ (Riegel and French, 1999a). To date, however, it remained unclear whether toluene stimulated these neurons by direct actions on neurons _within the
VTA_ and if such activation was sufficient to increase DA release from distal DA terminals in the ACB. TOLUENE STIMULATES VTA NEURONS, LEADING TO DA RELEASE IN THE ACB Our data support the
premise that toluene-containing inhalants activate mesoaccumbal neurotransmission via direct actions on VTA DA neurons. First, toluene stimulated DA neurons in the VTA slice, a preparation
in which transynaptic inputs to DA cells are severed (Tzschentke, 2001). Similar effects were observed in slices incubated in aCSF containing low Ca2+/high Mg2+, conditions in which synaptic
transmission is inhibited (Katz, 1970; Katz and Miledi, 1970). Identical media were used previously to study the site of action for other drugs of abuse (Brodie et al, 1990; Sanghera et al,
1984; Trulson et al, 1987) including toluene (in other regions) (Magnusson et al, 1998). These findings suggest that toluene increases DA neuronal activity post-synaptically and
independently of afferent input. Second, _in vivo_ microdialysis showed that direct intra-VTA perfusion of toluene enhanced DA concentrations in the VTA and ACB. As VTA DA neuron cell bodies
are the only known source of DA in this region (Adell and Artigas, 2004), the former effect most likely reflects the well-described increase in somatodendritic DA release that occurs as a
consequence of increased firing of DA neurons (Adell and Artigas, 2004). Other drugs of abuse also increase somatodendritic DA release (Adell and Artigas, 2004). Work, however, from several
laboratories has shown that the VTA is functionally heterogeneous (Ikemoto and Wise, 2004; Ford et al, 2006). Experimental animals will work to obtain infusions of ethanol, opiates,
cannabinoids, or psychostimulants into the posterior VTA, whereas infusions into the anterior VTA are without effect (Rodd-Henricks et al, 2000, 2002; Rodd et al, 2005; Rodd et al, 2004b;
Zangen et al, 2006). Interestingly and consistent with this heterogeneity, microdialysis revealed that toluene was more effective in increasing ACB-DA concentrations when infused directly
into the posterior than in the anterior VTA. Third, the effects of toluene were anatomically specific. Unlike VTA DA cells, bath application of toluene did not alter DA neuronal firing in
the RRF or RIN. Furthermore, firing of GABAergic neurons in the IPN was inhibited. Similarly, reverse-dialysis of toluene into regions adjacent to the VTA did not alter ACB-DA levels.
Anterior VTA perfusion of toluene failed to change ACB-DA, a finding consistent with anatomical studies showing that most DA neurons in this region do not innervate the ACB (Westerink et al,
1998; Tzschentke, 2001; Brog et al, 1993). However, there is an apparent trend to a delayed response, the magnitude of which may have been masked by a downward baseline drift in this
experimental group. This delayed response may reflect diffusion of toluene from the probe to more posterior VTA regions. The toluene-induced stimulation of anterior VTA DA neurons may be
related to the reported toluene-induced increase in DA release in the prefrontal cortex (Gerasimov et al, 2002b). Taken together, these findings indicate that by stimulating posterior VTA DA
cells, toluene increases extracellular DA concentrations in the distal ACB. CONCENTRATION DEPENDENCE AND NEUROBEHAVIORAL IMPLICATIONS Toluene is rapidly absorbed, distributed, and
eliminated from brain tissue (Gerasimov et al, 2002a; Kishi et al, 1988). Behavioral effects of toluene depend on both the brain concentrations and the pattern of exposure (Kishi et al,
1993; Lammers et al, 2005). Although inhalation exposure is relevant to inhalant abuse, the site (or sites) of action can be difficult to interpret. In both of our experimental approaches,
toluene was delivered directly to the VTA. The concentrations of toluene employed were those previously shown to be achieved in humans (Garriott et al, 1981) and experimental animals (see
discussion in Riegel et al, 2003). To simulate this exposure pattern _in vitro_, VTA slices were perfused with repeated, rapidly rising, escalating concentrations of toluene (staircase
paradigm). Extracellular recordings preserve action potential firing for long periods of time and are correlated with DA release in the ACB (Marinelli et al, 2006; Westerink et al, 1996). We
observed that VTA firing increased only over a narrow concentration range. At lower concentrations (<100 μM), toluene increased DA cell firing rates, but ACB DA concentrations were
unaltered. As noted elsewhere (Wise, 2002; Yeomans, 1989), the stimulation of unmyelinated fibers may have been insufficient to detect increased synaptic overflow of DA in distal terminals.
At intermediate concentrations, toluene increased both DA neuron firing and DA concentrations in the ACB. As discussed above (see Methods), retro-dialysis results in an estimated 10-fold
reduction in drug tissue concentration. Therefore, there is good concordance between the current results and previous _in vivo_ experiments, where blood toluene concentrations of 300 μM
stimulated VTA DA neurons (Riegel and French, 1999a). The firing of VTA DA neurons inactivated temporarily when toluene concentrations reached ∼1 mM. This inactivation persisted until
concentrations decreased, and is consistent with previous _in vivo_ inhalation studies showing inactivation of DA neurons when blood toluene concentrations exceeded ∼900 μM (Riegel and
French, 1999a). Thus, it is plausible that the lack of toluene-induced DA release in previous _in vivo_ studies results from an inactivation of VTA DA neurons induced by higher toluene
concentrations (Kondo et al, 1995; Gerasimov et al, 2002b). The lack of an accumbal DA response to 10 mM toluene may seem surprising given that this concentration increased extracellular DA
concentrations in the VTA (ie, somatodendritic release). The mechanisms mediating the differential effects of the higher toluene concentration on VTA and ACB DA levels are unclear.
Importantly, however, DA clearance in the VTA is less efficient than in terminal areas (Cragg and Greenfield, 1997). Thus, DA tissue concentrations may remain elevated even after suppression
of cell firing in the VTA. It is known that depolarization inhibits DA transporter function (Sonders et al, 1997). Sustained depolarization by toluene may further inhibit VTA DA uptake
resulting in impaired DA clearance in this region and an elevation of VTA DA levels. Interestingly, other organic solvents (eg, ethanol) inhibit DA uptake (Robinson et al, 2005). Therefore,
direct effects of toluene on transporter activity cannot be ruled out. In the staircase paradigm, VTA non-DA neurons were also stimulated. However, unlike DA cells, these neurons displayed
an inactivation threshold that became lower with repeated stimulation. Some VTA non-DA cells are GABA projection neurons (Cameron et al, 1997; Van Bockstaele and Pickel, 1995), which
innervate striatal cholinergic interneurons (Pickel and Chan, 1990) and cerebellar nuclei (Ikai et al, 1992). Although the functional role of these neurons is not completely understood (Van
Bockstaele and Pickel, 1995), their activation by low concentrations of toluene may contribute to TTX-sensitive increases in extracellular levels of GABA in these regions (Stengard and
O’Connor, 1994; Stengard et al, 1993) and to decreases in striatal ACh release (Stengard, 1994) during toluene inhalation. Other VTA non-DA cells are GABAergic interneurons (type-II) that
tonically inhibit VTA DA neurons _in vivo_ (Johnson and North, 1992). Although the mechanism underlying the shift in the inactivation point remains unclear, increases in the firing activity
of DA over GABA neurons would be expected to facilitate mesoaccumbal output. Finally, regarding the cellular targets, studies in cell expression systems indicate that toluene disrupts the
activity of numerous ion channels (Cruz et al, 1998; Bale et al, 2005; Beckstead et al, 2000), Ca2+ signaling (Westerink and Vijverberg, 2002; Westerink et al, 2002; Meulenberg and
Vijverberg, 2003), ATPases (Calderon-Guzman et al, 2005), and G-proteins (Tsuga et al, 1999; Tsuga and Honma, 2000; Tsuga et al, 2002). Additional studies will be needed to determine if one
or more of these mechanisms may be contributing to the toluene-induced excitation of VTA DA neurons. In conclusion, our results indicate that toluene selectively activates VTA DA neurons
projecting to the ACB. They identify a narrow concentration range within which toluene directly stimulates mesoaccumbal neurotransmission. Such findings suggest that previous conclusions,
that DA-independent mechanisms underlie the rewarding properties of inhalants, may be premature. Furthermore, given the alarming increase in inhalant abuse among youth (NSDUH, 2003),
additional studies examining the interaction of toluene and other inhalants with the mesoaccumbal DA reward pathway are warranted. REFERENCES * Adell A, Artigas F (2004). The somatodendritic
release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. _Neurosci Biobehavi Revi_ 28: 415–431. Article CAS Google Scholar * Alderdice MT,
Volle RL (1978). The increase in spontaneous transmitter release produced by beta-bungarotoxin and its modification by inorganic ions. _J Pharmacol Exp Ther_ 205: 58–68. CAS PubMed Google
Scholar * Ameno K, Kiriu T, Fuke C, Ameno S, Shinohara T, Ijiri I (1992). Regional brain distribution of toluene in rats and in a human autopsy. _Arch Toxicol_ 66: 153–156. Article CAS
PubMed Google Scholar * Bale AS, Tu Y, Carpenter-Hyland EP, Chandler LJ, Woodward JJ (2005). Alterations in glutamatergic and gabaergic ion channel activity in hippocampal neurons
following exposure to the abused inhalant toluene. _Neuroscience_ 130: 197–206. Article CAS PubMed Google Scholar * Balster RL, Schuster CR (1973). Fixed-interval schedule of cocaine
reinforcement: effect of dose and infusion duration. _J Exp Anal Behav_ 20: 119–129. Article CAS PubMed PubMed Central Google Scholar * Beckstead MJ, Grandy DK, Wickman K, Williams JT
(2004). Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. _Neuron_ 42: 939–946. Article CAS PubMed Google Scholar * Beckstead MJ, Weiner
JL, Eger EI, Gong DH, Mihic SJ (2000). Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. _Mol Pharmacol_ 57: 1199–1205. CAS PubMed Google
Scholar * Bespalov A, Sukhotina I, Medvedev I, Malyshkin A, Belozertseva I, Balster R _et al_ (2003). Facilitation of electrical brain self-stimulation behavior by abused solvents.
_Pharmacol Biochemi Behav_ 75: 199–208. Article CAS Google Scholar * Beyer CE, Stafford D, LeSage MG, Glowa JR, Steketee JD (2001). Repeated exposure to inhaled toluene induces behavioral
and neurochemical cross-sensitization to cocaine in rats. _Psychopharmacology (Berl)_ 154: 198–204. Article CAS Google Scholar * Blokhina EA, Dravolina OA, Bespalov AY, Balster RL,
Zvartau EE (2004). Intravenous self-administration of abused solvents and anesthetics in mice. _Eur J Pharmacol_ 485: 211–218. Article CAS PubMed Google Scholar * Bondy SC, Harrington ME
(1979). Calcium-dependent release of putative neurotransmitters in the chick visual system. _Neuroscience_ 4: 1521–1527. Article CAS PubMed Google Scholar * Bowen SE (2006). Increases
in amphetamine-like discriminative stimulus effects of the abused inhalant toluene in mice. _Psychopharmacology (Berl)_ 186: 517–524. Article CAS Google Scholar * Brodie MS, Shefner SA,
Dunwiddie TV (1990). Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area _in vitro_. _Brain Res_ 508: 65–69. Article CAS PubMed Google Scholar * Brog
JS, Salyapongse A, Deutch AY, Zahm DS (1993). The patterns of afferent innervation of the core and shell in the ‘accumbens’ part of the rat ventral striatum: immunohistochemical detection of
retrogradely transported fluorogold. _J Comp Neurol_ 338: 255–278. Article CAS PubMed Google Scholar * Cairney S, Maruff P, Burns CB, Currie J, Currie BJ (2005). Neurological and
cognitive recovery following abstinence from petrol sniffing. _Neuropsychopharmacol_ 30: 1019–1027. Article Google Scholar * Calderon-Guzman D, Hernandez-Islas JL, Espitia Vazquez IR,
Barragan-Mejia G, Hernandez-Garcia E, del Angel DS _et al_ (2005). Effect of toluene and cresols on Na+,K+-ATPase, and serotonin in rat brain. _Regul Toxicol Pharmacol_ 41: 1–5. Article CAS
PubMed Google Scholar * Cameron DL, Wessendorf MW, Williams JT (1997). A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids.
_Neuroscience_ 77: 155–166. Article CAS PubMed Google Scholar * Carlezon Jr WA, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL _et al_ (2000). Distinct sites of opiate reward and
aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. _J Neurosci_ 20: 62RC. Article Google Scholar * Carlezon Jr WA, Nestler EJ (2002). Elevated
levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? _Trends Neurosci_ 25: 610–615. Article CAS PubMed Google Scholar * Cragg SJ, Greenfield SA (1997).
Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. _J Neurosci_ 17: 5738–5746. Article CAS
PubMed PubMed Central Google Scholar * Cruz SL, Mirshahi T, Thomas B, Balster RL, Woodward JJ (1998). Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and
non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. _J Pharmacol Exp Ther_ 286: 334–340. CAS PubMed Google Scholar * Deutch AY, Goldstein M, Baldino Jr F, Roth RH (1988).
Telencephalic projections of the A8 dopamine cell group. _Ann NY Acad Sci_ 537: 27–50. Article CAS PubMed Google Scholar * Filley CM (2004). The effects of toluene on the central nervous
system. _J Neuropathol Exp Neurol_ 63: 1–12. Article CAS PubMed Google Scholar * Flanagan RJ, Ives RJ (1994). Volatile substance abuse. _Bull Narc_ 46: 49–78. CAS PubMed Google
Scholar * Ford CP, Mark GP, Williams JT (2006). Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. _J Neurosci_ 26: 2788–2797. Article CAS
PubMed PubMed Central Google Scholar * Funada M, Sato M, Makino Y, Wada K (2002). Evaluation of rewarding effect of toluene by the conditioned place preference procedure in mice. _Brain
Res Brain Res Protoc_ 10: 47–54. Article CAS PubMed Google Scholar * Garriott JC, Foerster E, Juarez L, de la GF, Mendiola I, Curoe J (1981). Measurement of toluene in blood and breath
in cases of solvent abuse. _Clin Toxicol_ 18: 471–479. Article CAS PubMed Google Scholar * Gerasimov MR, Ferrieri RA, Schiffer WK, Logan J, Gatley SJ, Gifford AN _et al_ (2002a). Study
of brain uptake and biodistribution of [11C]toluene in non-human primates and mice. _Life Sci_ 70: 2811–2828. Article CAS PubMed Google Scholar * Gerasimov MR, Schiffer WK, Marstellar D,
Ferrieri R, Alexoff D, Dewey SL (2002b). Toluene inhalation produces regionally specific changes in extracellular dopamine. _Drug Alcohol Dependence_ 65: 243–251. Article CAS PubMed
Google Scholar * Gonzales RA, McNabb J, Yim HJ, Ripley T, Bungay PM (1998). Quantitative microdialysis of ethanol in rat striatum. _Alcohol Clin Exp Res_ 22: 858–867. Article CAS PubMed
Google Scholar * Grace AA, Bunney BS (1983). Intracellular and extracellular electrophysiology of nigral dopaminergic neurons-1. Identification and characterization. _Neuroscience_ 10:
301–315. Article CAS PubMed Google Scholar * Grace AA, Bunney BS (1984). The control of firing pattern in nigral dopamine neurons: burst firing. _J Neurosci_ 4: 2877–2890. Article CAS
PubMed PubMed Central Google Scholar * Hutter OF, Kostial J (1954). Effect of magnesium and calcium ions on the release of acetylcholine. _J Physiol (Lond)_ 124: 234–241. Article CAS
Google Scholar * Hyman SE, Malenka RC (2001). Addiction and the brain: the neurobiology of compulsion and its persistence. _Nat Rev Neurosci_ 2: 695–703. Article CAS PubMed Google
Scholar * Ikai Y, Takada M, Shinonaga Y, Mizuno N (1992). Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex
and deep cerebellar nuclei. _Neuroscience_ 51: 719–728. Article CAS PubMed Google Scholar * Ikemoto S, Wise RA (2004). Mapping of chemical trigger zones for reward. _Neuropharmacology_
47: 190–201. Article CAS PubMed Google Scholar * Johnson SM, Trussell DC, McRitchie DA, Halliday GM, Hardman CD (1996). Anatomical and immunohistochemical identification of
catecholaminergic neurones in brain slice preparations used in electrophysiology. _J Neurosci Methods_ 64: 83–93. Article CAS PubMed Google Scholar * Johnson SW, North RA (1992). Two
types of neurone in the rat ventral tegmental area and their synaptic inputs. _J Physiol_ 450: 455–468. Article CAS PubMed PubMed Central Google Scholar * Katz B (1970). On the quantal
mechanism of neural transmitter release, Nobel Lecture, December 12, 1970. * Katz B, Miledi R (1970). Further study of the role of calcium in synaptic transmission. _J Physiol_ 207: 789–801.
Article CAS PubMed PubMed Central Google Scholar * Kishi R, Harabuchi I, Ikeda T, Katakura Y, Miyake H (1993). Acute effects of trichloroethylene on blood concentrations and
performance decrements in rats and their relevance to humans. _Br J Ind Med_ 50: 470–480. CAS PubMed PubMed Central Google Scholar * Kishi R, Harabuchi I, Ikeda T, Yokota H, Miyake H
(1988). Neurobehavioural effects and pharmacokinetics of toluene in rats and their relevance to man. _Br J Ind Med_ 45: 396–408. CAS PubMed PubMed Central Google Scholar * Kondo H, Huang
J, Ichihara G, Kamijima M, Saito I, Shibata E _et al_ (1995). Toluene induces behavioral activation without affecting striatal dopamine metabolism in the rat: behavioral and microdialysis
studies. _Pharmacol Biochem Behav_ 51: 97–101. Article CAS PubMed Google Scholar * Lammers JHCM, van Asperen J, de Groot D, Rijcken WRP (2005). Behavioural effects and kinetics in brain
in response to inhalation of constant or fluctuating toluene concentrations in the rat. _Environ Toxicol Pharmacol_ 19: 625–634. Article CAS PubMed Google Scholar * Lee DE, Gerasimov MR,
Schiffer WK, Gifford AN (2006). Concentration-dependent conditioned place preference to inhaled toluene vapors in rats. _Drug Alcohol Depend_ 85: 87–90. Article CAS PubMed Google Scholar
* Lena C, Changeux JP, Mulle C (1993). Evidence for ‘preterminal’ nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. _J Neurosci_ 13: 2680–2688. Article CAS
PubMed PubMed Central Google Scholar * Lo PS, Chen HH (2005). Immunohistochemical localization of toluene-induced c-Fos protein expression in the rat brain. _Toxicol Lett_ 157: 151–160.
Article CAS PubMed Google Scholar * Magnusson AK, Sulaiman MR, Dutia MB, Tham R (1998). Effects of toluene on tonic firing and membrane properties of rat medial vestibular nucleus
neurones _in vitro_. _Brain Res_ 779: 334–337. Article CAS PubMed Google Scholar * Marinelli M, Rudick CN, Hu XT, White FJ (2006). Excitability of dopamine neurons: modulation and
physiological consequences. _CNS Neurol Disord Drug Targets_ 5: 79–97. Article CAS PubMed Google Scholar * Meredith TJ, Ruprah M, Liddle A, Flanagan RJ (1989). Diagnosis and treatment of
acute poisoning with volatile substances. _Hum Toxicol_ 8: 277–286. Article CAS PubMed Google Scholar * Meulenberg CJW, Vijverberg HPM (2003). Selective inhibition of
[gamma]-aminobutyric acid type A receptors in human IMR-32 cells by low concentrations of toluene. _Toxicology_ 190: 243–248. Article CAS PubMed Google Scholar * NSDUH (2003). The NSDUH
report [electronic resource: http://oas.samhsa.gov/nhsda/2k3nsduh/2k3ResultsW.pdf]/_National Survey on Drug Use and Health. 2003_. Office of Applied Studies, US Dept of Health & Human
Services, SAMHSA, 2003; National Survey on Drug Use and Health (US) United States. Substance Abuse and Mental Health Services Administration Office of Applied Studies: Rockville, MD. *
Paxinos G, Watson C (1988). _The Rat Brain Atlas_. Academic Press: San Diego. Google Scholar * Pickel VM, Chan J (1990). Spiny neurons lacking choline acetyltransferase immunoreactivity are
major targets of cholinergic and catecholaminergic terminals in rat striatum. _J Neurosci Res_ 25: 263–280. Article CAS PubMed Google Scholar * Riegel AC, Ali SF, French ED (2003).
Toluene-induced locomotor activity is blocked by 6-hydroxydopamine lesions of the nucleus accumbens and the mGluR2/3 agonist LY379268. _Neuropsychopharmacology_ 28: 1440–1447. Article CAS
PubMed Google Scholar * Riegel AC, French ED (1999a). An electrophysiological analysis of rat ventral tegmental dopamine neuronal activity during acute toluene exposure. _Pharmacol
Toxicol_ 85: 37–43. Article CAS PubMed Google Scholar * Riegel AC, French ED (1999b). The susceptibility of rat non-dopamine ventral tegmental neurons to inhibition during toluene
exposure. _Pharmacol Toxicol_ 85: 44–46. Article CAS PubMed Google Scholar * Riegel AC, French ED (2002). Abused inhalants and central reward pathways: electrophysiological and
behavioral studies in the rat. _Ann NY Acad Sci_ 965: 281–291. Article CAS PubMed Google Scholar * Robinson DL, Volz TJ, Schenk JO, Wightman RM (2005). Acute ethanol decreases dopamine
transporter velocity in rat striatum: _in vivo_ and _in vitro_ electrochemical measurements. _Alcohol Clin Exp Res_ 29: 746–755. Article CAS PubMed Google Scholar * Rodd ZA, Bell RL,
Zhang Y, Murphy JM, Goldstein A, Zaffaroni A _et al_ (2005). Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of
alcohol-preferring (P) rats: involvement of dopamine and serotonin. _Neuropsychopharmacology_ 30: 330–338. Article CAS PubMed Google Scholar * Rodd ZA, Melendez RI, Bell RL, Kuc KA,
Zhang Y, Murphy JM _et al_ (2004b). Intracranial self-administration of ethanol within the ventral tegmental area of male wistar rats: evidence for involvement of dopamine neurons. _J
Neurosci_ 24: 1050–1057. Article CAS PubMed PubMed Central Google Scholar * Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ (2000). Regional heterogeneity for the
intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. _Psychopharmacology (Berl)_ 149: 217–224. Article CAS Google Scholar * Rodd-Henricks
ZA, Melendez RI, Zaffaroni A, Goldstein A, McBride WJ, Li TK (2002). The reinforcing effects of acetaldehyde in the posterior ventral tegmental area of alcohol-preferring rats. _Pharmacol
Biochem Behav_ 72: 55–64. Article CAS PubMed Google Scholar * Rosenberg NL, Grigsby J, Dreisbach J, Busenbark D, Grigsby P (2002). Neuropsychologic impairment and MRI abnormalities
associated with chronic solvent abuse. _J Toxicol Clin Toxicol_ 40: 21–34. Article PubMed Google Scholar * Sanghera MK, Trulson ME, German DC (1984). Electrophysiological properties of
mouse dopamine neurons: _in vivo_ and _in vitro_ studies. _Neuroscience_ 12: 793–801. Article CAS PubMed Google Scholar * Schiffer WK, Lee DE, Alexoff DL, Ferrieri R, Brodie JD, Dewey SL
(2006). Metabolic correlates of toluene abuse: decline and recovery of function in adolescent animals. _Psychopharmacology (Berl)_ 186: 159–167. Article CAS Google Scholar * Scholfied CN
(1981). Electrophysiology of isolated mammalian CNS preparations. In: Kerkut GA, Wheal HV (eds). _Electrophysiology of Isolated Mammalian CNS Preparations_. Academic Press: New York. pp
133–152. Google Scholar * Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG (1997). Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and
psychostimulants. _J Neurosci_ 17: 960–974. Article CAS PubMed PubMed Central Google Scholar * Spanagel R, Weiss F (1999). The dopamine hypothesis of reward: past and current status.
_Trends Neurosci_ 22: 521–527. Article CAS PubMed Google Scholar * Stengard K (1994). Effect of toluene inhalation on extracellular striatal acetylcholine release studied with
microdialysis. _Pharmacol Toxicol_ 75: 115–118. Article CAS PubMed Google Scholar * Stengard K, Hoglund G, Ungerstedt U (1994). Extracellular dopamine levels within the striatum increase
during inhalation exposure to toluene: a microdialysis study in awake, freely moving rats. _Toxicol Lett_ 71: 245–255. Article CAS PubMed Google Scholar * Stengard K, O’Connor WT
(1994). Acute toluene exposure decreases extracellular gamma-aminobutyric acid in the globus pallidus but not in striatum: a microdialysis study in awake, freely moving rats. _Eur J
Pharmacol_ 292: 43–46. CAS PubMed Google Scholar * Stengard K, Tham R, O’Connor WT, Hoglund G, Ungerstedt U (1993). Acute toluene exposure increases extracellular GABA in the cerebellum
of rat: a microdialysis study. _Pharmacol Toxicol_ 73: 315–318. Article CAS PubMed Google Scholar * Takagi M (1984). Actions of cholinergic drugs on cells in the interpeduncular nucleus.
_Exp Neurol_ 84: 358–363. Article CAS PubMed Google Scholar * Trulson ME, Trulson TJ, Arasteh K (1987). Recording of mouse ventral tegmental area dopamine-containing neurons. _Exp
Neurol_ 96: 68–81. Article CAS PubMed Google Scholar * Tsuga H, Haga T, Honma T (2002). Effects of toluene exposure on signal transduction: toluene reduced the signaling via stimulation
of human muscarinic acetylcholine receptor m2 subtypes in CHO cells. _Jpn J Pharmacol_ 89: 282–289. Article CAS PubMed Google Scholar * Tsuga H, Honma T (2000). Effects of short-term
toluene exposure on ligand binding to muscarinic acetylcholine receptors in the rat frontal cortex and hippocampus. _Neurotoxicol Teratol_ 22: 603–606. Article CAS PubMed Google Scholar
* Tsuga H, Wang RS, Honma T (1999). Effects of toluene on regulation of adenylyl cyclase by stimulation of G-protein-coupled receptors expressed in CHO cells. _Jpn J Pharmacol_ 81: 305–308.
Article CAS PubMed Google Scholar * Tzschentke TM (2001). Pharmacology and behavioral pharmacology of the mesocortical dopamine system. _Prog Neurobiol_ 63: 241–320. Article CAS PubMed
Google Scholar * Van Bockstaele EJ, Pickel VM (1995). GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. _Brain Res_ 682: 215–221.
Article CAS PubMed Google Scholar * Wang T, French ED (1993a). Effects of phencyclidine on spontaneous and excitatory amino acid-induced activity of ventral tegmental dopamine neurons:
an extracellular _in vitro_ study. _Life Sci_ 53: 49–56. Article CAS PubMed Google Scholar * Wang T, French ED (1993b). Electrophysiological evidence for the existence of NMDA and
non-NMDA receptors on rat ventral tegmental dopamine neurons. _Synapse_ 13: 270–277. Article CAS PubMed Google Scholar * Washio HM, Inouye ST (1978). The effect of calcium and magnesium
on the spontaneous release of transmitter at insect motor nerve terminals. _J Exp Biol_ 75: 101–112. CAS PubMed Google Scholar * Weiss B, Wood RW, Macys DA (1979). Behavioral toxicology
of carbon disulfide and toluene. _Environ Health Perspect_ 30: 39–45. Article CAS PubMed PubMed Central Google Scholar * Westerink BH, Kwint HF, deVries JB (1996). The pharmacology of
mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. _J Neurosci_ 16: 2605–2611. Article CAS PubMed PubMed
Central Google Scholar * Westerink BHC, Enrico P, Feimann J, De Vries JB (1998). The pharmacology of mesocortical dopamine neurons: a dual-probe microdialysis study in the ventral
tegmental area and prefrontal cortex of the rat brain. _J Pharmacol Exp Ther_ 285: 143–154. CAS PubMed Google Scholar * Westerink RHS, Klompmakers AA, Westenberg HGM, Vijverberg HPM
(2002). Signaling pathways involved in Ca2+- and Pb2+-induced vesicular catecholamine release from rat PC12 cells. _Brain Res_ 957: 25–36. Article CAS PubMed Google Scholar * Westerink
RHS, Vijverberg HPM (2002). Toluene-induced, Ca2+-dependent vesicular catecholamine release in rat PC12 cells. _Neurosci Lett_ 326: 81–84. Article CAS PubMed Google Scholar * Wise RA
(2002). Brain reward circuitry: insights from unsensed incentives. _Neuron_ 36: 229–240. Article CAS PubMed Google Scholar * Wu LT, Ringwalt CL (2006). Inhalant use and disorders among
adults in the United States. _Drug Alcohol Depend_ 85: 1–11. Article CAS PubMed PubMed Central Google Scholar * Yeomans JS (1989). Two substrates for medial forebrain bundle
self-stimulation: myelinated axons and dopamine axons. _Neurosci Biobehav Rev_ 13: 91–98. Article CAS PubMed Google Scholar * Yim HJ, Gonzales RA (2000). Ethanol-induced increases in
dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. _Alcohol_ 22: 107–115. Article CAS PubMed Google Scholar *
Yin R, French ED (2000). A comparison of the effects of nicotine on dopamine and non-dopamine neurons in the rat ventral tegmental area: an _in vitro_ electrophysiological study. _Brain Res
Bull_ 51: 507–514. Article CAS PubMed Google Scholar * Zangen A, Ikemoto S, Zadina JE, Wise RA (2002). Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior
differences within the ventral tegmental area and lack of effect in nucleus accumbens. _J Neurosci_ 22: 7225–7233. Article CAS PubMed PubMed Central Google Scholar * Zangen A, Solinas
M, Ikemoto S, Goldberg SR, Wise RA (2006). Two brain sites for cannabinoid reward. _J Neurosci_ 26: 4901–4907. Article CAS PubMed PubMed Central Google Scholar * Zapata A, Shippenberg
TS (2005). Lack of functional D2 receptors prevents the effects of the D3-preferring agonist (+)-PD 128907 on dialysate dopamine levels. _Neuropharmacology_ 48: 43–50. Article CAS PubMed
Google Scholar Download references ACKNOWLEDGEMENTS We thank Drs Karten, Krause, Beckstead, Ford, and Williams for critically reading the manuscript. Support for A Riegel was provided by
NIDA T-334180. A Zapata and T Shippenberg were supported by the National Institute on Drug Abuse, Intramural Research Program. AUTHOR INFORMATION Author notes * Arthur C Riegel and Agustin
Zapata: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ, USA Arthur C Riegel
& Edward D French * Vollum Institute, Oregon Health & Science University, Portland, OR, USA Arthur C Riegel * US Department of Health and Human Services, Intramural Research Program,
Integrative Neuroscience Section, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD, USA Agustin Zapata & Toni S Shippenberg Authors * Arthur C Riegel View
author publications You can also search for this author inPubMed Google Scholar * Agustin Zapata View author publications You can also search for this author inPubMed Google Scholar * Toni S
Shippenberg View author publications You can also search for this author inPubMed Google Scholar * Edward D French View author publications You can also search for this author inPubMed
Google Scholar CORRESPONDING AUTHOR Correspondence to Arthur C Riegel. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Riegel, A., Zapata, A.,
Shippenberg, T. _et al._ The Abused Inhalant Toluene Increases Dopamine Release in the Nucleus Accumbens by Directly Stimulating Ventral Tegmental Area Neurons. _Neuropsychopharmacol_ 32,
1558–1569 (2007). https://doi.org/10.1038/sj.npp.1301273 Download citation * Received: 24 May 2006 * Accepted: 06 October 2006 * Published: 10 January 2007 * Issue Date: 01 July 2007 * DOI:
https://doi.org/10.1038/sj.npp.1301273 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * inhalant abuse * ventral tegmental area (VTA) * nucleus
accumbens (ACB) * electrophysiology * microdialysis * dopamine (DA)