Play all audios:
ABSTRACT The efficacy and safety of oral valganciclovir was compared to ganciclovir i.v. in pre-emptive treatment of cytomegalovirus (CMV) in T-cell-depleted allogeneic stem cell transplant
(allo-SCT) recipients. A therapeutic guideline was developed to allow the safe application of valganciclovir in allo-SCT recipients requiring CMV therapy. In total, 107 consecutive
transplant recipients were evaluated. Cytomegalovirus DNA load in plasma was monitored longitudinally; details on antiviral therapy and treatment responses were analyzed retrospectively.
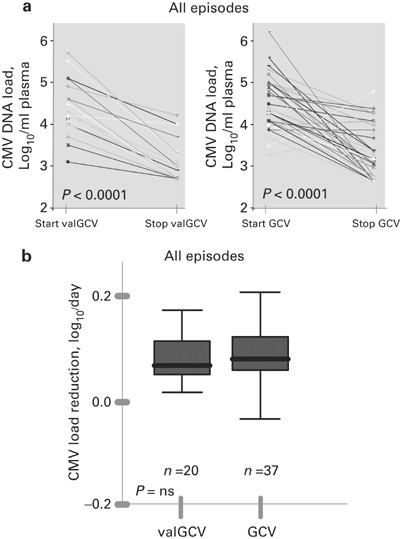
Fifty-seven CMV treatment episodes were recorded in 34 patients: 20 with valganciclovir (900 mg twice-daily) and 37 with ganciclovir (5 mg/kg twice-daily). Median CMV DNA load reduction was
0.079 and 0.069 log10 copies/ml/day in the ganciclovir and valganciclovir group, respectively. Good response on CMV DNA load (reduction below 3.0 log10 copies/ml) was observed in 75.7% of
ganciclovir and 80.0% of valganciclovir treatment episodes. Severe adverse effects were not observed and CMV-related disease did not occur. However, the percentage of patients receiving
erythrocyte transfusion was higher in the group of patients receiving ganciclovir as compared to valganciclovir (41 versus 20%, _P_=0.116). In conclusion, pre-emptive treatment with
valganciclovir and ganciclovir, led to similar reduction of CMV DNA load. Oral valganciclovir is an attractive and safe alternative for pre-emptive CMV treatment in T-cell-depleted allo-SCT
recipients. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe
to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF
Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact
customer support SIMILAR CONTENT BEING VIEWED BY OTHERS LETERMOVIR FOR CYTOMEGALOVIRUS INFECTION IN PEDIATRIC PATIENTS UNDERGOING ALLOGENIC HEMATOPOIETIC STEM CELL TRANSPLANTATION: A
REAL-LIFE STUDY BY THE INFECTIOUS DISEASES WORKING GROUP OF ITALIAN ASSOCIATION OF PEDIATRIC HEMATOLOGY-ONCOLOGY (AIEOP) Article 25 January 2024 NEW TRENDS IN THE MANAGEMENT OF
CYTOMEGALOVIRUS INFECTION AFTER ALLOGENEIC HEMATOPOIETIC CELL TRANSPLANTATION: A SURVEY OF THE INFECTIOUS DISEASES WORKING PARY OF EBMT Article 17 November 2022 EFFICACY OF PROPHYLACTIC
LETERMOVIR FOR CYTOMEGALOVIRUS REACTIVATION IN HEMATOPOIETIC CELL TRANSPLANTATION: A MULTICENTER REAL-WORLD DATA Article 02 November 2020 REFERENCES * Razonable RR . Epidemiology of
cytomegalovirus disease in solid organ and hematopoietic stem cell transplant recipients. _Am J Health Syst Pharm_ 2005; 62 (8 Suppl 1): S7–S13. PubMed Google Scholar * Boeckh M, Nichols
WG . The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. _Blood_ 2004;
103: 2003–2008. Article CAS Google Scholar * Gandhi MK, Khanna R . Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. _Lancet Infect Dis_ 2004; 4:
725–738. Article CAS Google Scholar * Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD . Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow
transplant. _Ann Intern Med_ 1993; 118: 173–178. Article CAS Google Scholar * Ljungman P . Beta-herpesvirus challenges in the transplant recipient. _J Infect Dis_ 2002; 186 (Suppl 1):
S99–S109. Article Google Scholar * Crumpacker CS . Ganciclovir. _N Engl J Med_ 1996; 335: 721–729. Article CAS Google Scholar * Emery VC . Prophylaxis for CMV should not now replace
pre-emptive therapy in solid organ transplantation. _Rev Med Virol_ 2001; 11: 83–86. Article CAS Google Scholar * Hart GD, Paya CV . Prophylaxis for CMV should now replace pre-emptive
therapy in solid organ transplantation. _Rev Med Virol_ 2001; 11: 73–81. Article CAS Google Scholar * Brown F, Banken L, Saywell K, Arum I . Pharmacokinetics of valganciclovir and
ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers. _Clin Pharmacokinet_ 1999; 37: 167–176. Article CAS Google Scholar * Pescovitz MD,
Rabkin J, Merion RM, Paya CV, Pirsch J, Freeman RB _et al_. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. _Antimicrob Agents Chemother_
2000; 44: 2811–2815. Article CAS Google Scholar * Jung D, Dorr A . Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. _J Clin Pharmacol_ 1999; 39:
800–804. Article CAS Google Scholar * Devyatko E, Zuckermann A, Ruzicka M, Bohdjalian A, Wieselthaler G, Rodler S _et al_. Pre-emptive treatment with oral valganciclovir in management of
CMV infection after cardiac transplantation. _J Heart Lung Transplant_ 2004; 23: 1277–1282. Article Google Scholar * Kalpoe JS, Schippers EF, Eling Y, Sijpkens YW, de Fijter JW, Kroes AC .
Similar reduction of cytomegalovirus DNA load by oral valganciclovir and intravenous ganciclovir on pre-emptive therapy after renal and renal-pancreas transplantation. _Antivir Ther_ 2005;
10: 119–123. CAS PubMed Google Scholar * Singh N, Wannstedt C, Keyes L, Gayowski T, Wagener MM, Cacciarelli TV . Efficacy of valganciclovir administered as preemptive therapy for
cytomegalovirus disease in liver transplant recipients: impact on viral load and late-onset cytomegalovirus disease. _Transplantation_ 2005; 79: 85–90. Article CAS Google Scholar * Barge
RM, Osanto S, Marijt WA, Starrenburg CW, Fibbe WE, Nortier JW _et al_. Minimal GVHD following _in-vitro_ T cell-depleted allogeneic stem cell transplantation with reduced-intensity
conditioning allowing subsequent infusions of donor lymphocytes in patients with hematological malignancies and solid tumors. _Exp Hematol_ 2003; 31: 865–872. Article CAS Google Scholar *
Barge RM, Brouwer RE, Beersma MF, Starrenburg CW, Zwinderman AH, Hale G _et al_. Comparison of allogeneic T cell-depleted peripheral blood stem cell and bone marrow transplantation: effect
of stem cell source on short- and long-term outcome. _Bone Marrow Transplant_ 2001; 27: 1053–1058. Article CAS Google Scholar * Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE,
Clift RA _et al_. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. _Transplantation_ 1974; 18: 295–304. Article CAS
Google Scholar * Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE _et al_. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle
patients. _Am J Med_ 1980; 69: 204–217. Article CAS Google Scholar * Kalpoe JS, Kroes AC, de Jong MD, Schinkel J, de Brouwer CS, Beersma MF _et al_. Validation of clinical application of
cytomegalovirus plasma DNA load measurement and definition of treatment criteria by analysis of correlation to antigen detection. _J Clin Microbiol_ 2004; 42: 1498–1504. Article CAS Google
Scholar * Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B _et al_. Efficacy and safety of valganciclovir vs oral ganciclovir for prevention of cytomegalovirus disease in
solid organ transplant recipients. _Am J Transplant_ 2004; 4: 611–620. Article CAS Google Scholar * Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease
in transplant recipients. _Clin Infect Dis_ 2002; 34: 1094–1097. Article Google Scholar * Mattes FM, Hainsworth EG, Geretti AM, Nebbia G, Prentice G, Potter M _et al_. A randomized,
controlled trial comparing ganciclovir to ganciclovir plus foscarnet (each at half dose) for preemptive therapy of cytomegalovirus infection in transplant recipients. _J Infect Dis_ 2004;
189: 1355–1361. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS There was no financial support and no conflicts of interest are reported. AUTHOR INFORMATION Author notes *
P L J van der Heiden and J S Kalpoe: Both authors contributed equally to this paper. AUTHORS AND AFFILIATIONS * Department of Hematology, Leiden University Medical Center, Leiden, The
Netherlands P L J van der Heiden, R M Barge & R Willemze * Department of Medical Microbiology, Leiden University Medical Center, Leiden, The Netherlands J S Kalpoe & A C M Kroes *
Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands E F Schippers Authors * P L J van der Heiden View author publications You can also search for
this author inPubMed Google Scholar * J S Kalpoe View author publications You can also search for this author inPubMed Google Scholar * R M Barge View author publications You can also search
for this author inPubMed Google Scholar * R Willemze View author publications You can also search for this author inPubMed Google Scholar * A C M Kroes View author publications You can also
search for this author inPubMed Google Scholar * E F Schippers View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to E
F Schippers. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE van der Heiden, P., Kalpoe, J., Barge, R. _et al._ Oral valganciclovir as pre-emptive
therapy has similar efficacy on cytomegalovirus DNA load reduction as intravenous ganciclovir in allogeneic stem cell transplantation recipients. _Bone Marrow Transplant_ 37, 693–698 (2006).
https://doi.org/10.1038/sj.bmt.1705311 Download citation * Received: 15 November 2005 * Revised: 16 January 2006 * Accepted: 16 January 2006 * Published: 27 February 2006 * Issue Date: 01
April 2006 * DOI: https://doi.org/10.1038/sj.bmt.1705311 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * cytomegalovirus * ganciclovir *
valganciclovir * pre-emptive treatment * allogeneic stem cell transplantation * CMV DNA load