Play all audios:
ABSTRACT Inhibition of p38 kinase blocks the production of tumor-promoting factors in the multiple myeloma (MM) bone marrow microenvironment. Proteasome inhibitors MG132 and bortezomib have
been shown to have direct cytotoxic effects on MM cells. We show that a selective inhibitor of p38_α_, SCIO-469, enhances the ability of MG132 and bortezomib to induce the apoptosis of MM
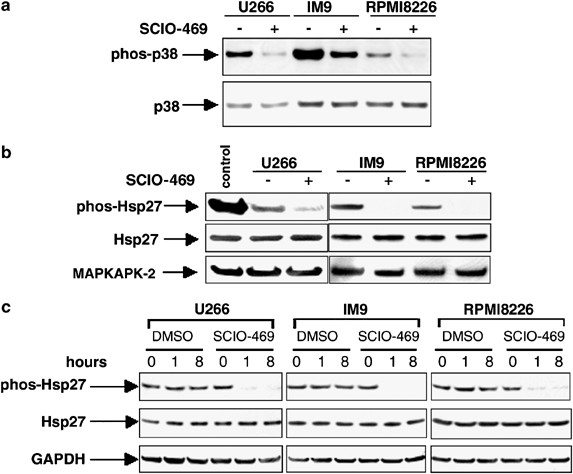
cells. Previously, we showed that p38 inhibition with SCIO-469 enhances MM cytotoxicity of bortezomib by inhibiting the transient expression and phosphorylation of Hsp27, a downstream target
of p38. Here we show that continued treatment of MM cells with bortezomib leads to a SCIO-469-enhanced downregulation of Hsp27 and to increased MM apoptosis. Furthermore, we show that p38
inhibition enhances the bortezomib-induced MM apoptosis by upregulation of p53 and downregulation of Bcl-XL and Mcl-1. In a mouse xenograft plasmacytoma model of MM, we found that inhibiting
p38 augments the effects of bortezomib in decreasing MM tumor growth _in vivo_. Thus, in addition to its role in suppressing an activated MM microenvironment, co-treatment with a p38
inhibitor, such as SCIO-469, may enhance the cytotoxicity of bortezomib by modulating pro-apoptotic and anti-apoptotic factors in MM cells, suggesting great potential for co-therapy. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal
Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices
may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS THE BCL-2 INHIBITOR ABT-199/VENETOCLAX SYNERGIZES WITH PROTEASOME INHIBITION VIA TRANSACTIVATION OF THE MCL-1 ANTAGONIST NOXA Article Open access 20
April 2022 HIGHLY SPECIFIC IMMUNOPROTEASOME INHIBITOR M3258 INDUCES PROTEOTOXIC STRESS AND APOPTOSIS IN KMT2A::AFF1 DRIVEN ACUTE LYMPHOBLASTIC LEUKEMIA Article Open access 19 May 2025 ROLE
OF TRIM24 IN THE REGULATION OF PROTEASOME-AUTOPHAGY CROSSTALK IN BORTEZOMIB-RESISTANT MANTLE CELL LYMPHOMA Article Open access 17 March 2025 REFERENCES * Hideshima T, Richardson P, Anderson
KC . Novel therapeutic approaches for multiple myeloma. _Immunol Rev_ 2003; 51: 164–166. Article Google Scholar * Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC . Adhesion of
human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. _Blood_ 1993; 82: 3712–3720. CAS PubMed Google Scholar * Podar K, Tai YT, Davies FE,
Lentzsch S, Sattler M, Hideshima T _et al_. Vascular endothelial growth factor triggers signaling cascades mediating multiple myeloma cell growth and migration. _Blood_ 2001; 98: 428–435.
Article CAS PubMed Google Scholar * Lee JC, Young PR . Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. _Leukocyte Biol_ 1996; 59: 152–157. Article
CAS Google Scholar * Hideshima T, Akiyama M, Hayashi T, Richardson P, Schlossman R, Chauhan D _et al_. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu.
_Blood_ 2003; 101: 703–705. Article CAS PubMed Google Scholar * Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M . Involvement of p38 mitogen-activated protein kinase signaling pathway
in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). _J Biol Chem_ 2000; 40: 31155–31161. Article Google Scholar * Hideshima T, Podar K, Chauhan D, Ishitsuka
K, Mitsiades C, Tai YT _et al_. p38 MAPK inhibition enhances bortezomib (bortezomib)-induced cytotoxicity against multiple myeloma cells. _Oncogene_ 2004; 23: 8766–8776. Article CAS
PubMed Google Scholar * Concannon CG, Gorman AM, Samali A . On the role of Hsp27 in regulating apoptosis. _Apoptosis_ 2003; 8: 61–70. Article CAS PubMed Google Scholar * Stokoe D,
Engel K, Campbell DG, Cohen P, Gaestel M . Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. _FEBS Lett_
1992; 313: 307–313. Article CAS PubMed Google Scholar * Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N _et al_. Hsp27 inhibits release of mitochondrial protein Smac in
multiple myeloma cells and confers dexamethasone resistance. _Blood_ 2003; 102: 3379–3386. Article CAS PubMed Google Scholar * Chauhan D, Li G, Podar K, Hideshima T, Shringarpure R,
Catley L _et al_. Blockade of Hsp27 overcomes Bortezomib/proteasome inhibitor bortezomib resistance in lymphoma cells. _Cancer Res_ 2003; 63: 6174–6177. CAS PubMed Google Scholar *
Mitsiades N, Mitsiades CS, Richardson PG, McMullan C, Poulaki V, Fanourakis G _et al_. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. _Proc Natl Acad Sci USA_
2002; 99: 14374–14379. Article CAS PubMed PubMed Central Google Scholar * Cory S, Huang DCS, Adams JM . The Bcl-2 family: roles in cell survival and oncogenesis. _Oncogene_ 2003; 22:
8590–8607. Article CAS PubMed Google Scholar * Spets H, Stromberg T, Georgii-Hemming P, Siljason J, Nilsson K, Jernberg-Wiklund H . Expression of the Bcl-2 family of pro- and
anti-apoptotic genes in multiple myeloma and normal plasma cells: regulation during interleukin 6 (IL-6)-induced growth and survival. _Eur J Haematol_ 2002; 69: 76–89. Article CAS PubMed
Google Scholar * Gauthier ER, Piche L, Lemieux G, Lemieux R . Role of bcl-X(L) in the control of apoptosis in murine myeloma cells. _Cancer Res_ 1996; 56: 1451–1456. CAS PubMed Google
Scholar * Wuilleme-Toumi S, Robillard N, Gomez P, Moreau P, Le Gouill S, Avet-Loiseau H _et al_. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival.
_Leukemia_ 2005; 19: 1248–1252. Article CAS PubMed Google Scholar * Derenne S, Monia B, Dean NM, Taylor JK, Rapp MJ, Harousseau JL _et al_. Antisense strategy shows that Mcl-1 rather
than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. _Blood_ 2002; 100: 194–199. Article CAS PubMed Google Scholar * Tu Y, Renner S, Xu F, Fleishman A, Taylor
J, Weisz J _et al_. BCL-X expression in multiple myeloma: possible indicator of chemoresistance. _Cancer Res_ 1998; 58: 256–262. CAS PubMed Google Scholar * Kimata H . GM1, a ganglioside
that specifically enhances immunoglobulin production and proliferation in human plasma cells. _Eur J Immunol_ 1994; 24: 2910–2913. Article CAS PubMed Google Scholar * Kisselev AF,
Goldberg AL . Proteasome inhibitors: from research tools to drug candidates. _Chem Biol_ 2001; 8: 739–758. Article CAS PubMed Google Scholar * Michels J, O'Neill JW, Dallman CL,
Mouzakiti A, Habens F, Brimmell M _et al_. Mcl-1 is required for Akata6 B-lymphoma cell survival and is converted to a cell death molecule by efficient caspase-mediated cleavage. _Oncogene_
2004; 23: 4818–4827. Article CAS PubMed Google Scholar * Richardson PG, Hideshima T, Anderson KC . Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of
multiple myeloma and other cancers. _Cancer Control_ 2003; 10: 361–369. Article PubMed Google Scholar * Elenitoba-Johnson KS, Jenson SD, Abbott RT, Palais RA, Bohling SD, Lin Z _et al_.
Involvement of multiple signaling pathways in follicular lymphoma transformation: p38-mitogen-activated protein kinase as a target for therapy. _Proc Natl Acad Sci USA_ 2003; 100: 7259–7264.
Article CAS PubMed PubMed Central Google Scholar * Karahashi H, Nagata K, Ishii K, Amano F . A selective inhibitor of p38 MAP kinase, SB202190, induced apoptotic cell death of a
lipopolysaccharide-treated macrophage-like cell line, J774.1. _Biochem Biophys Acta_ 2000; 1502: 207–223. CAS PubMed Google Scholar * Nguyn AN, Stebbins EG, Henson M, O'Young G, Choi
SJ, Quon D _et al_. Normalizing the bone marrow microenvironment with p38 inhibitor reduces multiple myeloma cell proliferation and adhesion and suppresses ostoclast formation. _Exp Cell
Res_ 2006, (in press). * Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P _et al_. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341.
_Blood_ 2003; 101: 1530–1534. Article CAS PubMed Google Scholar * Silvestris F, Tucci M, Cafforio P, Dammacco F . Fas-L upregulation by highly malignant myeloma plasma cells: role in the
pathogenesis of anemia and disease progression. _Blood_ 2001; 97: 1155–1164. Article CAS PubMed Google Scholar * Le Gouill S, Podar K, Harousseau JL, Anderson KC . Mcl-1 regulation and
its role in multiple myeloma. _Cell Cycle_ 2004; 3: 1259–1262. Article CAS PubMed Google Scholar * Bachelor MA, Bowden GT . Ultraviolet A-induced modulation of Bcl-XL by p38 MAPK in
human keratinocytes. _J Biol Chem_ 2004; 279: 42658–42668. Article CAS PubMed Google Scholar * Niwa M, Hotta K, Kanamori Y, Hatakeyama D, Hirade K, Katayama M _et al_. Involvement of p38
mitogen-activated protein kinase in heat shock protein 27 induction in human neutrophils. _Eur J Pharmacol_ 2003; 466: 245–253. Article CAS PubMed Google Scholar * Leuenroth SJ,
Grutkoski PS, Ayala A, Simms HH . Suppression of PMN apoptosis by hypoxia is dependent on Mcl-1 and MAPK activity. _Surgery_ 2000; 128: 171–177. Article CAS PubMed Google Scholar *
Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ _et al_. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. _Science_ 2003; 302:
1036–1038. Article CAS PubMed Google Scholar * Liu FT, Newland AC, Jia L . Bax conformational change is a crucial step for puma-mediated apoptosis in human leukemia. _Biochem Biophys
Res Commun_ 2003; 310: 956–962. Article CAS PubMed Google Scholar * Mackus WJ, Kater AP, Grummels A, Evers LM, Hooijbrink B, Kramer MH _et al_. Chronic lymphocytic leukemia cells display
p53-dependent drug-induced Puma upregulation. _Leukemia_ 2005; 19: 427–434. Article CAS PubMed Google Scholar * Adams JM . Ways of dying: multiple pathways to apoptosis. _Genes Dev_
2003; 17: 2481–2495. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Richard Brewer, former President of Scios Inc., for his heartfelt support and
inspiration for this project. All of the authors, except TH and KCA, are employed by Scios Inc., where SCIO-469, a potential product, was studied in the present work. This paper has not been
submitted elsewhere while under consideration for _Leukemia_. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Scios, Inc., Fremont, CA, USA T A Navas, A N Nguyen, M Reddy, J Y Ma, E
Haghnazari, M Henson, E G Stebbins, I Kerr, G O'Young, A M Kapoun, S Chakravarty, B Mavunkel, J Perumattam, G Luedtke, S Dugar, S Medicherla, A A Protter, G F Schreiner & L S
Higgins * Dana-Farber Cancer Institute, Boston, MA, USA T Hideshima & K C Anderson Authors * T A Navas View author publications You can also search for this author inPubMed Google
Scholar * A N Nguyen View author publications You can also search for this author inPubMed Google Scholar * T Hideshima View author publications You can also search for this author inPubMed
Google Scholar * M Reddy View author publications You can also search for this author inPubMed Google Scholar * J Y Ma View author publications You can also search for this author inPubMed
Google Scholar * E Haghnazari View author publications You can also search for this author inPubMed Google Scholar * M Henson View author publications You can also search for this author
inPubMed Google Scholar * E G Stebbins View author publications You can also search for this author inPubMed Google Scholar * I Kerr View author publications You can also search for this
author inPubMed Google Scholar * G O'Young View author publications You can also search for this author inPubMed Google Scholar * A M Kapoun View author publications You can also search
for this author inPubMed Google Scholar * S Chakravarty View author publications You can also search for this author inPubMed Google Scholar * B Mavunkel View author publications You can
also search for this author inPubMed Google Scholar * J Perumattam View author publications You can also search for this author inPubMed Google Scholar * G Luedtke View author publications
You can also search for this author inPubMed Google Scholar * S Dugar View author publications You can also search for this author inPubMed Google Scholar * S Medicherla View author
publications You can also search for this author inPubMed Google Scholar * A A Protter View author publications You can also search for this author inPubMed Google Scholar * G F Schreiner
View author publications You can also search for this author inPubMed Google Scholar * K C Anderson View author publications You can also search for this author inPubMed Google Scholar * L S
Higgins View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to L S Higgins. ADDITIONAL INFORMATION Supplementary
Information accompanies the paper on the Leukemia website (http://www.nature.com/leu) SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE S1 (JPG 47 KB) SUPPLEMENTARY FIGURE S2 (JPG 48 KB)
SUPPLEMENTARY FIGURE S3 (JPG 46 KB) SUPPLEMENTARY FIGURE LEGENDS (DOC 21 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Navas, T., Nguyen, A.,
Hideshima, T. _et al._ Inhibition of p38_α_ MAPK enhances proteasome inhibitor-induced apoptosis of myeloma cells by modulating Hsp27, Bcl-XL, Mcl-1 and p53 levels _in vitro_ and inhibits
tumor growth _in vivo_. _Leukemia_ 20, 1017–1027 (2006). https://doi.org/10.1038/sj.leu.2404200 Download citation * Received: 13 September 2005 * Revised: 22 December 2005 * Accepted: 17
January 2006 * Published: 13 April 2006 * Issue Date: 01 June 2006 * DOI: https://doi.org/10.1038/sj.leu.2404200 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative KEYWORDS * p38_α_ * myeloma * bortezomib * MG132 * Hsp27 * Mcl-1