Play all audios:
ABSTRACT STUDY DESIGN: Hyper-reflexia, measured as a decrease of low frequency-dependent depression of the H-reflex, is known to occur in both humans and animals after spinal cord injury
(SCI). Previous studies have shown that passive exercise for 3 months could be used to restore low frequency-dependent depression of the H-reflex after SCI. OBJECTIVE: To determine the
effects of various periods of time on the ability of passive exercise to restore low frequency-dependent depression of the H-reflex. SETTING: Spinal Cord Injury Mobilization Program of the
Center for Translational Neuroscience, the research arm of the Jackson T Stephens Spine & Neuroscience Institute, Little Rock, AR, USA. METHODS: Adult rats underwent complete spinal cord
transection at the T10 level. The hindlimbs were passively exercised in different groups of rats for 1 h/day, 5 days/week for 15, 30, 45, 60, or 90 days, and low frequency-dependent
depression of the H-reflex was tested. RESULTS: Statistically significant low frequency-dependent depression of the H-reflex was evident by 30 days of exercise, although numerical reductions
were seen even at 15 days. There was a linear decrease in low frequency-dependent depression of the H-reflex with duration of passive exercise. CONCLUSIONS: Passive exercise can restore
frequency-dependent depression of spinal reflexes in a time-dependent manner if used following complete spinal transection. SPONSORSHIP: Supported by USPHS award RR020146 and the Arkansas
Biosciences Institute. SIMILAR CONTENT BEING VIEWED BY OTHERS SELF-MODULATION OF RECTUS FEMORIS REFLEX EXCITABILITY IN HUMANS Article Open access 19 May 2023 THE EFFECT OF INCORPORATING
WHOLE BODY VIBRATION INTO EXERCISE THERAPY ON THE CORTICOMOTOR EXCITABILITY OF THE QUADRICEPS IN ATHLETES FOLLOWING ANTERIOR CRUCIATE LIGAMENT RECONSTRUCTION Article Open access 23 April
2025 TRANSSPINAL STIMULATION AND STEP TRAINING ALTER FUNCTION OF SPINAL NETWORKS IN COMPLETE SPINAL CORD INJURY Article 03 July 2021 INTRODUCTION Spinal cord injury (SCI) results in numerous
deficits of the motor and sensory systems, including paralysis, anesthesia, and hyper-reflexia below the level of the lesion. Hyper-reflexia is evident in both humans and animals following
SCI. The physiological changes that have been postulated to contribute to hyper-reflexia include alpha motoneuron hyperexcitability,1, 2, 3 changes in the intrinsic properties of alpha
motoneurons,4, 5, 6, 7 reduced post-activation depression of transmission from Ia fibers,8, 9 synapse growth;10 alterations in morphology of alpha motoneurons,11 and decreased presynaptic
inhibition of Ia terminals.9, 12, 13, 14, 15 The time course of spinal changes after injury has been proposed to include an early postsynaptic mechanism, possibly involving an increase in
excitability and/or receptor upregulation, and a late change involving presynaptic mechanisms possibly involving synaptic growth in spared descending pathways and in reflex pathways.10 One
measure used by numerous investigators to quantify hyper-reflexia is the electrical analogue of the classic tendon jerk reflex, referred to as the Hoffman or H-reflex.2, 15, 16, 17, 18, 19
The H-reflex is a compound electromyographic (EMG) response elicited by the synaptic activation of motoneurons by muscle afferents following stimulation of muscle nerves. Thompson _et al_20
investigated four measures of H-reflex excitability in a contusion model of SCI in the rat. Results of their studies led these researchers to conclude that rate-sensitive depression of the
H-reflex was of particular importance in the assessment of hyper-reflexia following SCI. Other groups have reached similar conclusions regarding the importance of changes in H-reflex
rate-sensitive depression as a measure of the effects of SCI.21 In spinally intact individuals, the H-reflex demonstrates depressed amplitude, due to marked frequency-dependent depression,
once stimulus frequencies reach or exceed 1 Hz.22, 23 However, frequency-dependent depression of the H-reflex is less evident in patients or animals with chronic SCI.12, 13, 22, 24, 25
Previously, we reported the ability of long-term passive exercise therapy to restore frequency-dependent depression of the H-reflex in adult rats with complete spinal cord transections.24,
25 Specifically, we found that a period of 3 months of motorized bicycle exercise training (MBET), performed in episodes of 1 h/day, 5 days/week, was capable of restoring frequency-dependent
depression of the H-reflex to the level of intact animals.24, 25 The current studies were undertaken to determine the time course of this decrease in hyper-reflexia, and if shorter periods
of total exercise are capable of producing similar restoration of H-reflex frequency-dependent depression in adult rats following spinal cord transection. Preliminary results have been
reported.25 METHODS SURGERY Adult female Sprague–Dawley rats (Harlan, 200–250 g, _n_=40) underwent a lower thoracic laminectomy under ketamine (60 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.)
anesthesia. A complete transection (Tx) of the spinal cord was made by aspiration and the transected ends of the cord retracted, producing a 2–3 mm cavity. Gelfoam was inserted into the
cavity to facilitate hemostasis and the dura was closed over the Tx site. Muscle and skin were sutured in separate layers, and animals were provided with dextrose–saline (5%, 1 ml/100 g body
weight, s.c.) to replace fluid lost during the surgical procedure. Penicillin (5000 U, i.m.) was administered immediately postoperatively, and animals were transferred to an incubator
maintained at 37.5°C until fully recovered from the anesthetic. The urinary bladder of each animal was expressed manually twice daily until reflexive voiding was established (10–14 days).
Animals were monitored for signs of urinary tract infection, and treatment with Baytril (enroflaxin 0.2 mg/day, i.m. for 10 days) was instituted as needed. All procedures were approved by
the Institutional Animal Use and Care Committee at UAMS. EXERCISE One group of rats (Tx only 90D, _n_=4) underwent no further treatment until reflex testing was carried out 90 days after Tx,
while a second group of transected animals (Tx only 30D, _n_=8) underwent no training but was tested for H-reflex frequency-dependent depression after 30 days, and a group of intact rats
served as nontransected controls (CONTROL, _n_=5). The remaining rats (_n_=23) were divided into five groups that received MBET daily. Exercise was provided for either 15 days (Tx+Ex 15D,
_n_=5), 30 days (Tx+Ex 30D, _n_=4), 45 days (Tx+Ex 45D, _n_=5), 60 days (Tx+Ex 60D, _n_=5), or 90 days (Tx+Ex 90D, _n_=4). The exercise regimen MBET involved suspending the rats on a sling
with the hindlimbs hanging down and the hind paws strapped to the pedals of a bicycle-type device, which was driven by a motor. The pedaling motion flexed one hindlimb and simultaneously
extended the contralateral one, while avoiding overstretching of either limb. Cycling speed was 30 rpm. Exercise sessions consisted of two 30-min episodes with 10 min of rest in between.
After the end of the training period, reflex testing was performed. The two Tx only groups also underwent reflex testing 30 days or 90 days after Tx, along with a group of intact rats (CTL),
for comparison with the MBET experimental groups. REFLEX TESTING Animals were anesthetized with ketamine (60 mg/kg, i.m.) and maintained with 10% doses as needed such that vibrissal and
pinna pinch reflexes were absent. Core body temperature was maintained at 36±1°C using a thermostatically controlled heat lamp. A bipolar cuff electrode was placed on the tibial nerve for
stimulation (0.1 ms pulses, cathode proximal on nerve). Exposed tissue was covered with mineral oil to prevent drying. A wire electrode was inserted subcutaneously in the digital
interosseous muscles between the fourth and fifth metatarsals for EMG recording as previously demonstrated,26, 27 and referenced to a clip applied to the skin on the digits. A ground
electrode was attached to the skin of the tail. Recordings were made using amplifier (Grass P511) filter settings of 3 Hz to 3 kHz with the 60 Hz notch filter in use. Responses to the
stimulus were digitized and averaged using a GW Instruments (Somerville, MA, USA) digitizer module and SuperScopeR software. H- AND M-WAVE RESPONSES IN THE RAT Stimulation of the tibial
nerve under the calcaneal tendon produced two responses, an early M-wave (∼2 ms latency), produced by direct activation of motoneuronal axons in the tibial nerve, and a later H-reflex (∼8 ms
latency), produced by activation of muscle afferents in the tibial nerve, which synapse monosynaptically on plantar motoneurons. The degree of stimulation that induced frequency-dependent
depression of the H-reflex was determined. The reflex was first tested at 0.2 Hz to determine threshold and maximal response levels. After discarding the first five responses in order to
obtain an average of the stabilized reflex, averages of 10 responses were obtained. Averages were compiled following stimulation at 0.2, 1, 5, and 10 Hz. The change in the response at
various frequencies was calculated as the percent of the response at 0.2 Hz in order to determine depression of the H-reflex as a function of stimulation frequency. Following the frequency
series testing, the H-reflex amplitude was confirmed at 0.2 Hz for consistency. If the amplitude at recheck was less than 90% of the initial amplitude, the data was discarded. At the end of
the experiment, animals were euthanized with an overdose of barbiturate (Nembutal) and the Tx was confirmed either visually or histologically following transcardial perfusion with
paraformaldehyde (4%) and sucrose (20%). Sensory testing or assessment of spasticity was not carried out in this series of animals. MEASUREMENT AND STATISTICS The amplitude of the H-wave was
measured from the base line before the H-wave to the peak of the first (and largest) of its two components (Figure 1). Measurements from peak to peak of the two components gave similar
results so that only the baseline to peak measures are reported here. Results from animal groups were compared statistically using a two-way ANOVA test. Significant differences between
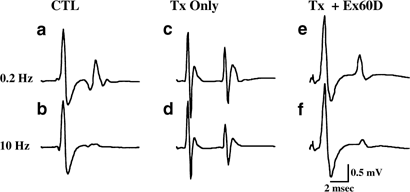
groups were tested using the Scheffe test, a conservative _post hoc_ comparison. Statistical significance was considered to be present at _P_<0.05. RESULTS Figure 1a shows representative
recordings from an intact CONTROL animal stimulated at 0.2 Hz (the 100% response), along with (Figure 1b) the response at 10 Hz (group mean±SE of the mean, ∼11±4% of the response at 0.2 Hz),
and the response from a Tx only 60D rat at 0.2 Hz (100%) (Figure 1c), and at 10 Hz for comparison (∼68±6%) (Figure 1d). Figure 1e and f show the responses from a Tx+Ex 60D rat at 0.2 Hz
(100%) and 10 Hz (∼22±7%), respectively. These recordings demonstrate the main effects of higher frequency stimulation leading to marked frequency-dependent depression in intact animals, of
Tx leading to decreased (increased percent) frequency-dependent depression, and of MBET leading to a restoration of frequency-dependent depression of the H-reflex. Figure 2 is a graph of the
habituation of the H-reflex following stimulation at 0.2, 1, 5, and 10 Hz in the following groups of animals: Tx only 90D, Tx+Ex 15D, Tx+Ex 30D, Tx+Ex 45D, Tx+Ex 60D, Tx+Ex 90D, and
CONTROL. The Tx only 30D group was not included because of its similarity with the other longer duration unexercised transected group (Tx only 90D). Statistically significant differences
between the Tx only 90D group and the other groups at each frequency are denoted by a single (*_P_<0.05) or a double (**_P_<0.01) asterisk. ANOVA of these groups showed statistically
significant differences across stimulation frequency (df=3, F=115.11, _P_<0.001), across experimental groups (df=7, F=6.77, _P_<0.001), and interaction between frequency and groups
(df=21, F=2.17, _P_<0.008). _Post hoc_ comparisons between all groups were undertaken, and those against the Tx only 90D group were considered the most relevant. When comparing this group
with the CONTROL animals, there were no significant differences at 1 Hz, but major differences at 5 Hz (_P_<0.01) and 10 Hz (_P_<0.01), indicating decreased frequency-dependent
depression (hyper-reflexia) 90 days after Tx. Such decreases in frequency-dependent depression were evident to a similar degree after only 30 days following Tx (Tx only 30D) compared to the
CONTROL group (_P_<0.01), suggesting that hyper-reflexia is manifested within 30 days and remains at similar levels in the chronic (90 day) condition. The effects of MBET for 15 days
(Tx+Ex 15D), while producing a numerical reduction of ∼15% at each frequency, were not statistically significant. However, after 30 days of MBET (Tx+Ex 30D), there were statistically
significant increases in frequency-dependent depression (decreased percent ∼30+%) at all frequencies tested, suggesting that passive exercise led to a restoration of H-reflex habituation.
The effects of longer durations of MBET produced decreases in frequency-dependent depression that were variable at the lower frequencies (1 and 5 Hz), but linearly decrementing at 10 Hz. For
example, percent frequency-dependent depression decreased from ∼29% after 30 days (Tx+Ex 30D), to ∼28% after 45 days (Tx+Ex 45D), to ∼22% after 60 days (Tx+Ex 60D), to ∼7% after 90 days
(Tx+Ex 90D) of MBET. Figure 3 is a comparison of a delay of 30 days following Tx before testing the H-reflex in untrained rats (Tx only 30D) compared to those trained for 30 days after Tx
(Tx+Ex 30D) and to intact animals (CONTROL). Basically, ANOVA showed a significant difference across groups (df=2, F=12.22, _P_<0.001) and frequencies (df=3, F=50.46, _P_<0.001), as
well as interaction across these two factors (df=6, F=2.24, _P_<0.05). Tx only induced statistically significant decreases in frequency-dependent depression at 5 Hz (**_P_<0.01) and 10
Hz (**_P_<0.01) compared to CONTROL (++). MBET for 30 days induced an increase in H-reflex frequency-dependent depression (decreased percent) when comparing the effects of Tx
(*_P_<0.05) to Tx+Ex 30D (+) at both 5 and 10 Hz. These results show that the decrement in low frequency-dependent depression (1–10 Hz) of the H-reflex induced by Tx can be alleviated by
MBET in a period as short as 30 days. CONCLUSIONS The studies described provide confirmatory evidence that passive exercise in spinally transected animals can be used to restore low
frequency-dependent depression of the H-reflex.24, 25 The decrement in hyper-reflexia was evident in a MBET duration-dependent manner in Tx rats. This methodology has already been shown to
induce a similar effect in a spinal cord injured human subject.28 Before considering these ramifications, a number of issues and potential limitations should be considered. The H-reflex is a
reliable measure of spinal circuitry that is altered after an SCI. This reflex normally undergoes changes with a variety of rhythmic motions such as stepping,29 walking,30 running31 and
pedaling.32 In animals, the H-reflex has been a valuable tool to measure changes in spinal circuitry in both contusion20, 33 and transection models.23, 24 The H-reflex has also been used to
assess changes in spinal circuitry in the human, a recent effort concluding that (1) chronically paralyzed subjects showed suppression of H-reflexes to a lesser extent than able-bodied
normals or acutely paralyzed subjects, (2) those with acute paralysis showed similar H-reflex suppression as those within 40 weeks of their SCI, but had decreased frequency-dependent
depression after 44 weeks of paralysis (ie there was marked loss of frequency-dependent depression of the H-reflex in the ‘chronic’ condition), and (3) changes in muscle fatigue were
associated with a decrease in H-reflex suppression over time.13 The results described herein show that frequency-dependent depression of the H-reflex is decreased in the most ‘chronic’
condition tested, 90 days after Tx. Surprisingly, a similar level of hyper-reflexia was evident even at 30 days (Figure 3), suggesting that hyper-reflexia assumes a fairly ‘chronic’ level
within 30 days after Tx in the rat. These results are consistent with those of Thompson _et al_20 who reported significantly less frequency-dependent depression of the monosynaptic reflex at
28 and 60 days postinjury in spinal cord contused rats than in normal animals, but this effect was not evident 6 days after injury. Such results suggest that the onset of low
frequency-dependent depression of the H-reflex takes weeks to develop. Further studies are required to determine the time course of the decrement in frequency-dependent depression of the
H-reflex in the rat. Other forms of exercise in animal models also show promise in the ability to effect changes in lower limb function following SCI. For example, adult cats showed recovery
of full weight-bearing hindlimb stepping on a treadmill within a few weeks following complete spinal transection if treadmill training was implemented.34, 35 Completely spinalized cats that
were not treadmill-trained were 3 times less likely to step than those that were trained.36 If spinal cord hemisected rats are immobilized, the expected recovery is delayed and may not be
as great (compared to exercised animals) once use is restored.37 We showed that 90 days of MBET minimized loss of muscle mass in transected and exercised, compared to transected only,
rats.23, 24 Additionally, we showed that MBET restored frequency-dependent depression of the H-reflex to normal levels in exercised, transected rats.24, 25 The H-reflex of nonexercised Tx
animals failed to show frequency-dependent depression at most stimulation frequencies. The present studies were designed to determine the time course of recovery induced by MBET on low
frequency-dependent depression of the H-reflex. MBET for only 15 days, while not inducing a statistically significant effect on H-reflex habituation, did have a numerical effect, increasing
frequency-dependent depression (decreasing percent inhibition) at 10 Hz from 69 to about 42%. MBET for 30, 45, and 60 days induced a similar, significant reduction in percent inhibition from
69 to around 22–29%. Not until 90 days of MBET was there a further reduction to around 7%. These findings suggest that MBET had a fairly rapid ameliorative effect on H-reflex
frequency-dependent depression, and could have added effects if continued long term. Numerous mechanisms have been proposed to account for the hyper-reflexia following SCI, including alpha
motoneuron hyperexcitability,1, 2, 3 alterations in motoneuronal morphology,11 synapse growth,12 changes in the intrinsic properties of alpha motoneurons,4, 5, 6, 7 and decreased presynaptic
inhibition of Ia terminals.9, 12, 13, 14, 15 Decreased presynaptic inhibition is frequently linked with decreased low-frequency depression of the H-reflex.14, 21, 38 It is thought that the
loss of inputs from descending pathways leads to a reorganization of spinal circuitry that promotes hyper-reflexia. MBET induces alternating contractions (via stretch reflexes) of
antagonists in the same limb and agonists in different limbs, as previously reported.39 We assume that the cycling motion elicited by MBET provides recurrent signaling that modifies this
hyper-reactive circuitry, thereby leading to a restitution of low frequency-dependent depression of the H-reflex. We have recently found preliminary evidence suggesting an additional
potential explanation. Complete Tx in adult rats was found to lead to an increase in neuronal gap junction gene expression.40, 41 Gap junctions allow synchronous activation by electrical
coupling of spinal motoneurons, but their expression ceases after 7–14 days postnatally.42 Tx thus may lead to an increase in neuronal (specifically Connexin 36) gap junction protein
expression. In addition, MBET for 30 days in Tx rats was found to normalize low frequency depression of the H-reflex and to lead to a decrease in Connexin 36 expression.41 We assume that Tx
effectively denervates motoneurons, which respond by increasing electrical coupling as in the neonatal state (motoneuronal coupling), a condition that fosters paroxysmal co-contraction of
agonists, such as is seen in hyper-reflexia and spasticity. MBET may act to decrease electrical coupling by passively forcing alternation leading to motoneuronal uncoupling. Additional
studies are required to substantiate and explore this interesting new avenue. We have also carried out studies on H-reflex frequency-dependent depression in human subjects.28 We tested a
total of 15 age- and sex-matched control subjects and six patients with SCI classified as ASIA C (no sensory or motor function below the level of the lesion, 4–8 years postinjury, ie all
chronic, >44 weeks postinjury). Frequency-dependent depression of the H-reflex in control human subjects differed somewhat from that in the intact rat, especially in that
frequency-dependent depression at even the highest frequency tested, 20 Hz, was about 10–15% in the human compared to 0% in the rat. The H-reflex in the control subjects decreased to 50–25%
of control at frequencies of 1, 3, and 5 Hz, then reached a plateau at this level for higher frequencies. In the SCI patients, frequency-dependent depression of the H-reflex was at 80–65% at
1, 3, 5, and 10 Hz, but decreased closer to normal levels at the higher frequencies tested.28 The use of MBET on one of these SCI subjects led to a normalization of H-reflex
frequency-dependent depression within 10 weeks.28 The H-reflex was at 65% at 10 Hz in this patient, but was reduced to 15% after 10 weeks of MBET. When MBET was discontinued, there was a
gradual return of hyper-reflexia over the next 3 weeks, ultimately returning to previous levels. This suggests that the effects of MBET, at least in the human, (a) require weeks to ensue,
and (b) are temporary but last beyond the duration of training, indicating that there is a plastic reorganization in spinal circuitry that can continue to be up or downregulated at the
spinal level after SCI. Moreover, there was anecdotal evidence from the subject that spasticity was reduced and bowel and bladder function improved. However, more detailed analyses of these
effects are required, along with measures of muscle mass to determine if MBET reduces muscle atrophy in the human as it does in the rat.23 The issue of spasticity requires some discussion.
Since spasticity includes both exaggerated reflexes and increased muscle tone, the H-reflex is not considered a selective measure of spasticity, which requires more specific assessment for
its quantification.14, 43, 45 In addition, the rat model is not a good one for spasticity, since rats show variable (to no) levels of spasticity, so that our results cannot address the level
of spasticity induced by Tx. Interestingly, the anti-spastic effects of L-dopa were proposed to be mediated by group II, but not group I, afferents,44 suggesting that spasticity involves
additional mechanisms to those tested by the H-reflex, which is thought to be mediated mainly by group I afferents. A complementary tool for measuring spastic hypertonia has been proposed
for use in humans,44 but such measures in the rat have not been established. Future studies require the quantitative assessment of both flaccid and spastic paralysis to determine if MBET can
modulate these sequelae. Partial weight bearing training (PWBT) has also been utilized in human patients with SCI to effect changes in locomotor function. Improvements in locomotion have
been produced in patients with incomplete, but not complete, SCI.46, 47 Exercise in the form of PWBT or overground training, combined with functional electrical stimulation (FES),48, 49 or
pharmacological intervention50 has also yielded promising improvements in locomotor function. However, methods required to assist locomotion as part of these training regimes are labor
intensive and physically demanding. More recently developed exercise methods that require fewer personnel to administer, such as the Dietz _et al_51 are quite expensive and thus fiscally
inaccessible for most patients. The use of MBET for restoring low frequency-dependent depression of reflexes as described here, and the potential for decreasing muscle atrophy after SCI
previously described,24, 25 suggest that MBET has promise in supplementing or even substituting for some of these techniques. Additionally, MBET can be delivered at low cost to the patient
with little physical assistance required. In summary, MBET appears to restore low frequency-dependent depression of the H-reflex in adult Tx rats with at least 30 days of training. A case
report of a human subject who underwent MBET supports the contention that such therapy may be beneficial in reducing hyper-reflexia in the human. While a number of parametric studies need to
be carried out, MBET appears to be promising for improving the quality of life of SCI victims. REFERENCES * Magladery JW, Teasdall RD, Park AM, Languth HW . Electrophysiological studies of
reflex activity in patients with lesions of the nervous system. I. A comparison of spinal motoneurone excitability following afferent nerve volleys in normal persons and patients with upper
motor neurone lesions. _Bull Johns Hopk Hosp_ 1952; 91: 219–244. CAS Google Scholar * Milanov I . Examination of the segmental pathophysiological mechanisms of spasticity. _Electromyogr
Clin Neurophysiol_ 1994; 34: 73–79. CAS PubMed Google Scholar * Laudau WM, Clare MH . Fusimotor function. VI. H-reflex, tendon jerk and reinforcement in hemiplegia. _Arch Neurol Psychiat_
1964; 10: 128–134. Article Google Scholar * Bennett DJ, Li Y, Harvey PJ, Gorassini M . Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. _J
Neurophysiol_ 2001; 86: 1972–1982. Article CAS PubMed Google Scholar * Eken T, Hultborn H, Kiehn G . Possible functions of transmitter-controlled plateau potentials in alpha
motoneurons. _Prog Brain Res_ 1989; 80: 257–267. Article CAS PubMed Google Scholar * Li Y, Bennett DJ . Persistent sodium and calcium currents cause plateau potentials in motoneruons of
chronic spinal rats. _J Neurophysiol_ 2003; 90: 857–869. Article CAS PubMed Google Scholar * Li Y, Li X, Harvey PJ, Bennett DJ . Effects of baclofen on spinal reflexes and persistent
inward currents in motoneurons of chronic spinal rats with spasticty. _J Neurophysiol_ 2004; 92: 2694–2703. Article CAS PubMed Google Scholar * Hultborn H . Changes in neuronal
properties and spinal reflexes during development of spasticity following spinal cord lesions and stroke: studies in animal models and patients. _J Rehab Med_ 2003; S41: 46–55. Article
Google Scholar * Nielsen J, Petersen N, Crone C . Changes in transmission across synapses of Ia afferents in spastic patients. _Brain_ 1995; 118: 995–1004. Article PubMed Google Scholar
* Little JW, Ditunno JF, Steins SA, Harris RM . Incomplete spinal cord injury: neuronal mechanisms of motor recovery and hyperreflexia. _Arch Phys Med Rehab_ 1999; 80: 587–599. Article CAS
Google Scholar * Kitzman P . Alteration in axial motoneuronal morphology in the spinal cord injured spastic rat. _Exper Neurol_ 2005; 192: 100–108. Article Google Scholar * Calancie B
et al. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. _Electroencephalogr and Clin Neurophysiol_
1993; 89: 177–186. Article CAS Google Scholar * Schindler-Ivens S, Shields RK . Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. _Exp Brain Res_
2000; 133: 233–241. Article CAS PubMed PubMed Central Google Scholar * Pierrot-Deseilligny P . Electrophysiological assessment of the spinal mechanisms underlying spasticity. _New
Trends Adv Tech Clin Neurophysiol_ 1990; 41: 264–273. Article CAS Google Scholar * Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E . A quantitative assessment of presynaptic inhibition
of Ia afferents in spastics: differences in hemiplegics and paraplegics. _Brain_ 1994; 117: 1449–1455. Article PubMed Google Scholar * Angel RW, Hofmann WW . The H-reflex in normal,
spastic, and rigid subjects. _Arch Neurol_ 1963; 8: 591–596. Article Google Scholar * Little JW, Halar EM . H-reflex changes following spinal cord injury. _Arch Phys Med Rehab_ 1985; 66:
19–22. CAS Google Scholar * Olsen PZ, Diamantopoulos E . Excitabiliity of spinal motor neurons in normal subjects and patients with spasticity, Parkinsonian rigidity, and cerebellar
hypotonia. _J Neurol Neurosurg Psychiatry_ 1967; 30: 325–331. Article CAS PubMed PubMed Central Google Scholar * Yablon SA, Stokic DS . Neurophysiologic evaluation of spastic
hypertonia. _Am J Phys Med Rehab_ 2004; 83: S10–S18. Article Google Scholar * Thompson FJ, Reier PJ, Lucas CC, Parme R . Altered patterns of reflex excitability subsequent to contusion
injury of the rat spinal cord. _J Neurophysiol_ 1992; 68: 1473–1486. Article CAS PubMed Google Scholar * Chen XY, Feng-Chen KC, Chen L, Stark DM, Wolpaw JR . Short-term and medium-term
effects of spinal cord tract transactions on soleus H-reflex in freely moving rats. _J Neurotrauma_ 2001; 18: 313–327. Article CAS PubMed Google Scholar * Ishikawa K, Ott K, Porter RW,
Stuart D . Low frequency depression of the H wave in normal and spinal man. _Exp Neurol_ 1966; 15: 140–156. Article CAS PubMed Google Scholar * Reese NB et al. Restoration of H-reflex
habituation by passive exercise in spinally transected rats. _American Phys Ther Assoc Combined Sections Meeting_. Nashville, TN 2004. Google Scholar * Skinner RD, Houle JD, Reese NB, Berry
CL, Garcia-Rill E . Effects of exercise and fetal spinal cord implants on the H-reflex in chronically spinalized adult rats. _Brain Res_ 1996; 729: 127–131. Article CAS PubMed Google
Scholar * Skinner RD, Houle JD, Reese NB, Dempster J, Garcia-Rill E . Amelioration of the H-reflex in chronically spinalized adult rats by exercise and fetal spinal cord implants. _Soc
Neurosci Abst_ 1998; 24: 2103. Google Scholar * Meinck HM . Occurrence of the H-reflex and the F wave in the rat. _Electroencephalogr Clin Neurophysiol_ 1976; 41: 530–533. Article CAS
PubMed Google Scholar * Cliffer KD et al. Consistent repeated M- and H-wave recording in the hind limb of rats. _Muscle Nerve_ 1998; 21: 1405–1413. Article CAS PubMed Google Scholar *
Kiser TS, Reese NB, Maresh T, Skinner RD, Garcia-Rill E . Exercise-induced normalization of frequency-dependent inhibition of the H-reflex in a spinal cord injured human subject. _J Spinal
Cord Med_ in press. * Crenna P, Frigo C . Excitability of the soleus H reflex arc during walking and stepping in man. _Exp Brain Res_ 1978; 66: 49–60. Google Scholar * Brooke JD, Collins
DF, Boucher S, McIlroy W . Modulation of human short latency reflexes between standing and walking. _Brain Res_ 1991; 548: 172–178. Article CAS PubMed Google Scholar * Capaday C, Stein
RB . Difference in the amplitude of the human soleus H-reflex in walking and running. _J Physiol_ 1987; 392: 513–522. Article CAS PubMed PubMed Central Google Scholar * Brooke JD,
McIlroy WE, Collins DF . Movement and H reflex modulation. I. Pedalling versus matched controls. _Brain Res_ 1992; 582: 78–84. Article CAS PubMed Google Scholar * Thompson FJ, Parmer R,
Reier PJ . Alteration in rate modulation of reflexes to lumbar motoneurons after mid thoracic spinal cord injury in the rat. I. Contusion Injury. _J Neurotrauma_ 1998; 15: 495–508. Article
CAS PubMed Google Scholar * Barbeau H, Rossignol S . Recovery of locomotion after chronic spinalization in the adult cat. _Brain Res_ 1987; 412: 84–95. Article CAS PubMed Google
Scholar * Lovely RG, Gregor RG, Roy RR, Edgerton VR . Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. _Exp Neurol_ 1986; 92: 421–435. Article
CAS PubMed Google Scholar * de Leon RD, Hodgson JA, Roy RR, Edgerton VR . Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats.
_J Neurophysiol_ 1998; 79: 1329–1340. Article CAS PubMed Google Scholar * Little JW, Harris RM, Lerner SJ . Immobilization impairs recovery after spinal cord injury. _Arch Phys Med
Rehab_ 1991; 72: 408–412. CAS Google Scholar * Trimble MH, Kukulka CG, Behrman AL . The effect of treadmill gait training on low-frequency depressioin of the soleus H-reflex: comparison of
a spinal cord injured man to normal subjects. _Neurosci Lett_ 1998; 246: 186–188. Article CAS PubMed Google Scholar * Houle JD, Morris K, Skinner RD, Garcia-Rill E, Peterson C . Effects
of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. _Muscle Nerve_ 1999; 22: 846–856. Article CAS PubMed Google Scholar * Yates C, Zhou
Y, Mitchell D, Reese NB, Skinner RD, Garcia-Rill E . Motoneuronal coupling after spinal cord injury (SCI) leading to hyperreflexia: increased expression of Connexin 36 (Cx36) in the spinal
cord after transection (Tx). _Neurosci Abstr_ in press. * Skinner RD, Yates C, Zhou Y, Mitchell D, Reese NB, Garcia-Rill E . Motoneuronal uncoupling by passive exercise in spinal cats:
decreased expression of Connexin 36 (Cx36). _Neurosci Abstr_ in press. * Walton KD, Navarrete R . Postnatal changes in motoneurone electrotonic coupling studied in the _in vitro_ rat lumbar
spinal cord. _J Physiol_ 1991; 433: 283–305. Article CAS PubMed PubMed Central Google Scholar * Katz R, Rymer W . Spastic hypertonia: mechanisms and measurement. _Arch Phys Med Rehabil_
1989; 70: 144–155. CAS PubMed Google Scholar * Eriksson J, Olausson B, Jankowska E . Antispastic effects of L-dopa. _Exp Brain Res_ 1996; 111: 296–304. Article CAS PubMed Google
Scholar * Benz EN, Hornby TG, Bode RK, Scheidt RA, Schmit BD . A physiologically based clinical measure for spastic reflexes in spinal cord injury. _Arch Phys Med Rehab_ 2005; 86: 52–59.
Article Google Scholar * Wernig A, Nanassy A, Muller S . Laufband (LB) therapy in spinal cord lesioned persons. _Prog Brain Res_ 2000; 128: 89–97. Article CAS PubMed Google Scholar *
Wirz M, Colombo G, Dietz V . Long term effects of locomotor training in spinal humans. _J Neurol Neurosurg Psychiatry_ 2001; 71: 93–96. Article CAS PubMed PubMed Central Google Scholar
* Sadowsky CL . Electrical stimulation in spinal cord injury. _Neurorehabilitation_ 2001; 16: 165–169. CAS PubMed Google Scholar * Wieler M et al. Multicenter evaluation of electrical
stimulation systems for walking. _Arch Phys Med Rehab_ 1999; 80: 495–500. Article CAS Google Scholar * Barbeau H, Norman KE . The effect of noradrenergic damage on the recovery of walking
after spinal cord injury. _Spinal Cord_ 2003; 41: 137–143. Article CAS PubMed Google Scholar * Dietz V, Miller R, Colombo G . Locomotor activity in spinal man: significance of afferent
input from joint and load receptors. _Brain_ 2002; 125: 2626–2634. Article PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Physical
Therapy, University of Central Arkansas, Conway, AR, USA N B Reese * Department of Neurobiology and Developmental Sciences, Center for Translational Neuroscience, College of Medicine,
University of Arkansas for Medical Sciences (UAMS), Little Rock, AR, USA R D Skinner, D Mitchell, C Yates, C N Barnes, T S Kiser & E Garcia-Rill Authors * N B Reese View author
publications You can also search for this author inPubMed Google Scholar * R D Skinner View author publications You can also search for this author inPubMed Google Scholar * D Mitchell View
author publications You can also search for this author inPubMed Google Scholar * C Yates View author publications You can also search for this author inPubMed Google Scholar * C N Barnes
View author publications You can also search for this author inPubMed Google Scholar * T S Kiser View author publications You can also search for this author inPubMed Google Scholar * E
Garcia-Rill View author publications You can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Reese,
N., Skinner, R., Mitchell, D. _et al._ Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. _Spinal Cord_ 44, 28–34 (2006).
https://doi.org/10.1038/sj.sc.3101810 Download citation * Published: 26 July 2005 * Issue Date: 01 January 2006 * DOI: https://doi.org/10.1038/sj.sc.3101810 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative KEYWORDS * hyper-reflexia * rehabilitation * spasticity * spinal cord injury (SCI)