Play all audios:
Dear Editor One of the several mechanisms accounting for HIV-1-induced lymphodepletion resides in the capacity of the envelope glycoprotein complex (Env) expressed on HIV-1 infected cells to
interact with CD4 and a suitable coreceptor (CXCR4 or CCR5) expressed on non-infected cells, thereby triggering cell fusion.1 The vast majority of syncytium-inducing HIV-1 variants employs
CXCR4 as a coreceptor, and a strong correlation between CD4+ T cell decline and infection by syncytium-induced HIV-1 variants has been established. Formation of syncytia _in vitro_ is
generally followed by cell death, either by apoptosis or necrosis, depending on the cell types engaged in the process.2,3 _In vivo_, in lymphoid tissues from AIDS patients, syncytium
formation is accompanied by overexpression of tissue transglutaminase, a marker of apoptosis.4 In a model culture system of syncytium-dependent cell death, HeLa cells stably transfected with
a lymphotropic HIV-1 _Env_ gene (HeLa Env) were fused by co-culture with CD4/CXCR4-expressing HeLa cells (Hela CD4).5,6 As an internal control, HeLa cell expressing a monotropic HIV-1
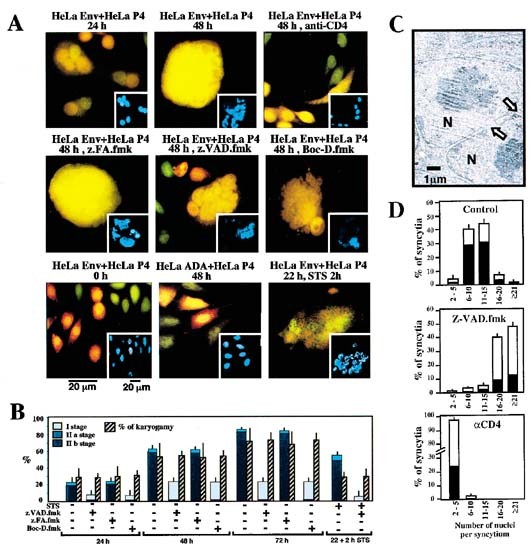
envelope (ADA, that does not interact with the CXCR4 co-receptor expressed on HeLa CD4) failed to form syncytia with HeLa CD4 cells and did not undergo apoptosis7 (Figure 1A). Fusion events
were monitored by means of two stable, non-toxic CellTracker fluorescent dies with which HeLa Env (CellTracker Green) or HeLa CD4 cells (CellTracker Orange) were pre-incubated. After 24 h of
co-culture, juxtaposed nuclei from both cell types could be clearly distinguished within a common cytoplasm, the center of syncytia (Figure 1A) whose overall organization roughly
recapitulates that of normal cells.1 After 48 h, however, nucleoplasm fusion (karyogamy) occurred in ∼50% of syncytia, as detectable by the blending of the two CellTracker dies within the
nucleus. Concomitantly, an increasing percentage of syncytia spontaneously exhibited apoptotic chromatin condensation, as detected with the Hoechst 33342 dye (Figure 1A,B). Karyogamy induced
by the ENV-CD4/CXCR4 interaction involved circumscript fusions of the nuclear envelopes and occurred in a fraction of cells which are lacking signs of nuclear chromatin condensation (Figure
1C). It thus may be uncoupled from apoptotic chromatin condensation, as also suggested by kinetic analyses (Figure 1B). What is then the functional relationship between syncytium formation,
karyogamy, and apoptosis? Freshly formed syncytia (24 h) driven into apoptosis by a short-term incubation (2 h) with staurosporin (STS) exhibited a similar degree of chromatin condensation
as syncytia undergoing apoptosis spontaneously (48 h). However, neither STS (Figure 1) nor other pro-apoptotic agents such as etoposide (not shown) did increase the frequency of syncytia
exhibiting karyogamy, indicating that apoptosis induced by exogenous stimuli obeys other principles than spontaneous syncytial apoptosis. The two pan-caspase inhibitors Boc-D.fmk and
Z-VAD.fmk (but not the chemically related cathepsin inhibitor Z-FA.fmk) suppressed nuclear chromatin condensation (Figure 1A,B) as well as the detachment of HeLa Env/HeLa CD4 syncytia from
the culture substrate (not shown). Moreover, inhibition of apoptosis by the caspase inhibitor Z-VAD.fmk allowed for the formation of much larger syncytia as compared to control HeLa Env/HeLa
CD4 cocultures (Figure 1D), suggesting that caspase activation with the consequent irreversible loss of cellular functions limits the recruitment of cells into growing syncytia. In strict
contrast to their effects on chromatin condensation (Figure 1A,B) and syncytial size (Figure 1D), Boc-D.fmk or Z-VAD.fmk failed to prevent nuclear fusion (Figure 1A,B), suggesting that,
karyogamy precedes in a caspase-independent fashion. To further investigate the impact of ongoing fusion events between individual cells/nuclei and existing syncytia (or fusion between
syncytia), the monoclonal antibody Leu 3a (which blocks the CDR2 region of the CD4 D1 domain) was added to cocultures. Blockade of CD4 by adding Leu3a from the beginning of cocultures
completely abolished cell fusion (Figure 1A). When added after 24 h of culture, Leu 3a led to a net reduction of the median number of nuclei per syncytium, strongly inhibited karyogamy
(Figure 1A), and, concomitantly, reduced the frequency of apoptotic nuclear events observed 72 h (Figure 1D) after initiation of coculture. Altogether, these data suggest that the size of
syncytia determines the probability of karyogamy and spontaneous apoptosis and that, inversely, apoptotic caspase activation limits ongoing syncytium formation. More importantly, these data
suggest also that both karyogamy and apoptosis may be mechanistically coupled to non-physiologial syncytium formation. To our knowledge, this is the first description of nuclear fusion in
somatic mammalian cells. The molecular mechanisms of nuclear fusion or karyogamy has previously only been explored in the context of sexual reproduction of unicellular fungi such as
_Saccharomyces cerevisiae_.8 It will be interesting to learn whether similar mechanisms also participate in cell fusion-induced karyogamy of somatic mammalian cells. Supported by a special
grant by the Ligue Nationale contre le Cancer, as well as by grants from ANRS, FRM, the European Commission (to G Kroemer). KF Ferri receives a fellowship from the French Ministry of
Science. E Jacotot receives an ANRS fellowship. M Geuskens is a senior research associate of the Belgian National Fund for Scientific Research. REFERENCES * Sylwester A _et al_. 1997 _J.
Immunol._ 158: 3996–4007 * Laurent Crawford AG _et al_. 1993 _AIDS Res. Hum. Retroviruses_ 9: 761–773 * Plymale DR _et al_. 1999 _AIDS_ 13: 1827–1839 Article CAS Google Scholar * Amendola
A _et al_. 1996 _Proc. Natl. Acad. Sci. USA_ 93: 11057–11062 * Ferri KF _et al_. 2000 _J. Exp. Med._ in press * Ferri FK _et al_. 2000 _Ann. NY Acad. Sci._ in press * Dragic T _et al_. 1992
_J. Virol._ 66: 4794–4802 * Rose MD . 1996 _Annu. Rev. Cell. Dev. Biol._ 12: 663–695 Article CAS Google Scholar * Schwartz O _et al_. 1994 _Virology_ 198: 360–365 Article CAS Google
Scholar * Pleskoff O _et al_. 1997 _Science_ 276: 1874–1878 Article CAS Google Scholar * Daugas E _et al_. 2000 _FASEB. J._ 14: 729–739 Download references AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Centre National de la Recherche Scientifique, UMR1599, Institut Gustave Roussy, 39 rue Camille-Desmoulins, Villejuif, F-94805, France K F Ferri, E Jacotot & G Kroemer *
Laboratory of Molecular Parasitology, Université, Libre de Bruxelles, Gosselies, B-6041, Belgium M Geuskens Authors * K F Ferri View author publications You can also search for this author
inPubMed Google Scholar * E Jacotot View author publications You can also search for this author inPubMed Google Scholar * M Geuskens View author publications You can also search for this
author inPubMed Google Scholar * G Kroemer View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to G Kroemer. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ferri, K., Jacotot, E., Geuskens, M. _et al._ Apoptosis and karyogamy in syncytia induced by the HIV-1-envelope
glycoprotein complex. _Cell Death Differ_ 7, 1137–1139 (2000). https://doi.org/10.1038/sj.cdd.4400748 Download citation * Published: 09 November 2000 * Issue Date: 01 November 2000 * DOI:
https://doi.org/10.1038/sj.cdd.4400748 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative