Play all audios:
The aim was to investigate the efficacy of neoadjuvant docetaxel–cisplatin and identify prognostic factors for outcome in locally advanced stage IIIA (pN2 by mediastinoscopy) non-small-cell
lung cancer (NSCLC) patients. In all, 75 patients (from 90 enrolled) underwent tumour resection after three 3-week cycles of docetaxel 85 mg m−2 (day 1) plus cisplatin 40 or 50 mg m−2 (days
1 and 2). Therapy was well tolerated (overall grade 3 toxicity occurred in 48% patients; no grade 4 nonhaematological toxicity was reported), with no observed late toxicities. Median overall
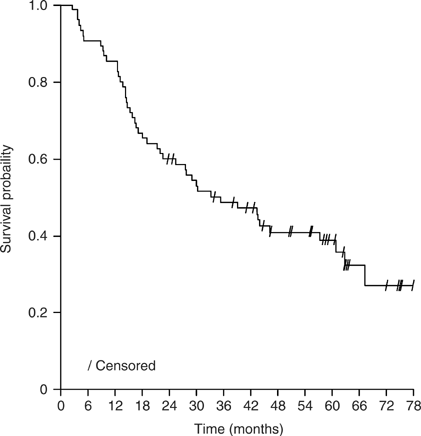
survival (OS) and event-free survival (EFS) times were 35 and 15 months, respectively, in the 75 patients who underwent surgery; corresponding figures for all 90 patients enrolled were 28
and 12 months. At 3 years after initiating trial therapy, 27 out of 75 patients (36%) were alive and tumour free. At 5-year follow-up, 60 and 65% of patients had local relapse and distant
metastases, respectively. The most common sites of distant metastases were the lung (24%) and brain (17%). Factors associated with OS, EFS and risk of local relapse and distant metastases
were complete tumour resection and chemotherapy activity (clinical response, pathologic response, mediastinal downstaging). Neoadjuvant docetaxel–cisplatin was effective and tolerable in
stage IIIA pN2 NSCLC, with chemotherapy contributing significantly to outcomes.
Surgery remains the best standard therapy for localised lung cancer. Results from randomised studies of early stage resectable non-small-cell lung cancer (NSCLC) have shown improved survival
from use of adjuvant chemotherapy (Non-small Cell Lung Cancer Collaborative Group, 1995; Arriagada et al, 2004; Strauss et al, 2004; Winton et al, 2004; Douillard et al, 2005). Improvements
in 5-year survival of 12–15% have been observed with adjuvant platinum-based doublet regimens in early stage disease (stage IB–IIIA) (Strauss et al, 2004; Winton et al, 2004; Douillard et
al, 2005), and adjuvant chemotherapy is now considered the standard of care after radical surgical resection. However, the associated toxicity and subjective poor tolerance of adjuvant
chemotherapy are a major limitation. Patient and physician compliance is low and even in trials only 35–85% of targeted dose intensity has been achieved. Moreover, a large randomised
intergroup trial failed to demonstrate any survival advantage when cisplatin and etoposide were added to postoperative radiation, compared with radiation alone for resected stage II–IIIA
disease (Keller et al, 2000).
Several small randomised studies (Pass et al, 1992; Rosell et al, 1994; Roth et al, 1994) have demonstrated a benefit from neoadjuvant treatment in locally advanced disease (stage IIIA). In
a large trial involving 355 patients with stage IB–IIIA NSCLC, a trend towards survival benefit was seen from neoadjuvant chemotherapy (median survival 37 months vs 26 months for surgery
alone; P=0.15) (Depierre et al, 2002). Encouragingly, preoperative chemotherapy appears to be better tolerated than adjuvant chemotherapy, with a high compliance of 90–95% (Depierre et al,
2002; Betticher et al, 2003). Similarly, preliminary results of a recent randomised trial of preoperative chemotherapy (paclitaxel and carboplatin) have shown a nonsignificant trend towards
improved progression-free survival and overall survival (OS) (Pisters et al, 2005).
The routine use of neoadjuvant chemotherapy in NSCLC remains controversial. Identification of predictive/prognostic factors to select patients who would derive the greatest benefit from
combined modality therapy will help clarify the most appropriate clinical use of neoadjuvant chemotherapy, particularly for patients with stage IIIA disease. We report here the final and
updated results of a large phase II trial in patients with pN2 (proven by mediastinoscopy) stage IIIA (International Union Against Cancer, UICC) disease. Analyses of potential factors
associated with the development of local relapse and distant metastases are also presented.
Initial results of this prospective, multicentre, phase II trial of docetaxel–cisplatin induction chemotherapy have been reported previously (Betticher et al, 2003). Briefly, all patients
were required to have mediastinoscopically proven, previously untreated, operable stage IIIA (T1–3 pN2 M0) NSCLC. Eligibility criteria included: age 18–75 years; World Health Organization
(WHO) performance status (PS) ⩽2; forced expiratory volume ⩾1.2 l s−1; normal cardiac and bone marrow (leucocyte >4.0 × 109 l−1, platelet >100 × 109 l−1) functions and adequate hepatic
(bilirubin within normal limits, aspartate aminotransferase and alanine aminotranferase ⩽1.5 × upper limit of normal (ULN), alkaline phosphatase ⩽2.5 × ULN) and kidney (creatinine clearance
>60 ml min−1) functions. The trial was approved by the local ethics committee at each participating centre, with informed, written consent obtained from all patients.
Patients were scheduled to receive cisplatin 80 mg m−2 as a 1-h infusion (divided over 2 days, on days 1 and 2) plus docetaxel 85 mg m−2 as a 1-h infusion on day 1, every 3 weeks for three
cycles providing haematological function was adequate (neutrophil >1.5 × 109 l−1, platelet >100 × 109 l−1). In view of the low toxicity observed in the first 36 patients, the protocol was
subsequently amended to increase the dose of cisplatin to 100 mg m−2 (50 mg m−2 on days 1 and 2) from patient 37 onwards. Resection was performed if there was no progressive disease (PD)
based on post-induction computed tomography (CT) scan and no contraindication emerging from pulmonary function testing. Postoperative mediastinal radiotherapy was to be administered to
patients with positive resection margin (R1 and R2) and/or involvement of the uppermost mediastinal lymph node. A daily dose of 2 Gy delivered in the central axis at the mid-plane was
administered 5 days/week to a cumulative dose of 60 Gy. The irradiated field included the bronchial stump, ipsilateral hilum and vascular shadows of the mediastinum bilaterally.
Postoperative chemotherapy was not administered.
One of the trial objectives was to identify possible prognostic factors of the combined modality treatment (chemotherapy, surgery and optional radiotherapy). We assessed the association of
OS, event-free survival (EFS; an event comprising PD, relapse or death), risk of local relapse and risk of distant metastases with each of 19 potential prognostic factors: baseline patient
characteristics (age, gender, PS, smoking habits, serum lactate dehydrogenase (LDH) and haemoglobin); baseline tumour characteristics (histology, tumour stage, differentiation, multilevel
involvement of mediastinal lymph nodes, and nodal enlargement on CT scan); type of surgery performed (right pneumonectomy, resection margin and complete resection) and activity of
chemotherapy (clinical response, pathological responses (⩾95% and