Play all audios:
ABSTRACT BACKGROUND: A striking efficiency of interferon (IFN)-based anticancer therapy for advanced hepatocellular carcinoma (HCC) has been reported. Because its clinical efficiency greatly
depends on each patient's local response, prediction of local response is crucial. METHODS: Continuous exposure of IFN-_α_ to parental PLC/PRF/5 cells (PLC-P) and a limiting dilution
method resulted in the establishment of IFN-resistant cell clones (PLC-Rs). Microarray analyses of PLC-P and PLC-Rs identified insulin-like growth factor-binding protein 7 (IGFBP7) as one of
the most significantly downregulated genes in PLC-Rs. Changes in anticancer effects of IFN-_α_ were examined in HCC cells after genetic manipulation of IGFBP7 expression. The correlation
between immunohistochemically determined IGFBP7 expression and the response to IFN-_α_/5-fluorouracil (5-FU) therapy was investigated in surgically resected HCC specimens. RESULTS: PLC-R
cells showed a remarkable downregulation of IGFBP7 and resistance to IFN-_α_, compared with PLC-P. Parental PLC/PRF/5 cells transfected with short hairpin RNA against IGFBP7 showed a
significant resistance to IFN-_α_ relative to control cells (IC50 fold increase=14.38 times). Insulin-like growth factor-binding protein 7 transfection into PLC-R restored sensitivity to
IFN-_α_. In resected specimens, IGFBP7 expression significantly correlated with the response to IFN-_α_/5-FU therapy. CONCLUSION: IGFBP7 could be a useful predictor of the response to
IFN-based therapy in advanced HCC. SIMILAR CONTENT BEING VIEWED BY OTHERS EGFR INHIBITION REVERSES RESISTANCE TO LENVATINIB IN HEPATOCELLULAR CARCINOMA CELLS Article Open access 14 May 2022
PNMA1 IS A NOVEL IMMUNE MODULATOR AND THERAPEUTIC TARGET IN HEPATOCELLULAR CARCINOMA LINKED TO BILE ACID METABOLISM Article Open access 03 January 2025 DYNLL1 ACCELERATES CELL CYCLE VIA
ILF2/CDK4 AXIS TO PROMOTE HEPATOCELLULAR CARCINOMA DEVELOPMENT AND PALBOCICLIB SENSITIVITY Article 01 June 2024 MAIN The prognosis of patients with advanced hepatocellular carcinoma (HCC)
remains poor, particularly in patients with tumour thrombi in the major trunk of the portal vein, even after curative resection of the tumour (Tanaka et al, 1996; Yamakado et al, 1999;
Asahara, 1999 no. 47). In such a situation, conventional therapies have no clinical impact because of poor efficacy and possible complications (Furuse et al, 1997; Lee et al, 1997).
Therefore, a new strategy is required for patients with advanced HCC. Several studies have reported strong antitumour activity of interferon (IFN)-based combination chemotherapy to HCC,
irrespective of the lack of satisfactory results of IFN-_α_ monotherapy (Urabe et al, 1998; Leung et al, 1999; Chung et al, 2000; Patt et al, 2003; Obi et al, 2006). We have also reported
the clinical efficiency of IFN-_α_ and 5-fluorouracil (5-FU) (IFN-_α_/5-FU) therapy for advanced HCC and the underlying mechanisms of antitumour effects (Eguchi et al, 2000; Kondo et al,
2000, 2005; Sakon et al, 2002; Yamamoto et al, 2004; Ota et al, 2005; Nakamura et al, 2007; Wada et al, 2007, 2009; Damdinsuren et al, 2007a, 2007b; Nagano et al, 2007a, 2007b). These
previous studies showed that IFN-_α_ suppresses the proliferation of HCC cells that express type I IFN receptor type 2 (IFNAR2), and that the expression of IFNAR2 in HCC tissues was
significantly associated with a clinical response to IFN-_α_/5-FU therapy, suggesting that IFNAR2 expression might be useful in predicting the clinical response to such therapy (Ota et al,
2005; Nagano et al, 2007a). However, even a portion of patients expressing IFNAR2 showed resistance to the therapy, indicating the necessity of finding novel biological markers that can more
accurately predict the clinical response to IFN-_α_/5-FU therapy. Because the clinical outcome between responders and non-responders is markedly different, and to avoid the potentially
debilitating adverse effects of this therapy in non-responders, finding the predictive biomarker is crucial. In this study, IFN-resistant HCC cell clones were established and an
oligonucleotide microarray analysis was applied to these IFN-resistant cells and their parental cells. The microarray analysis identified that insulin-like growth factor (IGF)-binding
protein 7 (IGFBP7), which has been reported to have a tumour-suppressive activity through the induction of apoptosis in some cancers, was a key gene related to the response to this therapy
(Burger et al, 1998; Landberg et al, 2001; Mutaguchi et al, 2003; Sato et al, 2007; Lin et al, 2008; Wajapeyee et al, 2008). Furthermore, we confirmed that IGFBP7 significantly correlated
with the response to IFN-_α_/5-FU therapy in genetic manipulation experiments and to the clinical response in HCC tissue samples. These results indicate that IGFBP7 could be a suitable
marker for predicting the clinical response to IFN-_α_/5-FU therapy. MATERIALS AND METHODS CELL LINES Human HCC cell lines, PLC/PRF/5 and HLE, were obtained from the Japan Cancer Research
Resources Bank (Tokyo, Japan), and Hep3B was obtained from the Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan). These cells were maintained in Dulbecco's
modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin and 100 mg ml−1 streptomycin at 37°C in a humidified incubator with 5% CO2 in air. ESTABLISHMENT
OF IFN-RESISTANT CELLS Parental PLC/PRF/5 cells (PLC-P) were exposed to IFN-_α_ at an initial concentration of 50 IU ml−1. At 2 weeks after exposure, surviving cells were continuously
exposed to sequentially increasing doses of 100 IU ml−1 (2 weeks), 200 IU ml−1 (2 weeks), 500 IU ml−1 (2 weeks), 1000 IU ml−1 (2 weeks), and 2000 IU ml−1. Through this process, we
successfully established IFN-resistant cells. By limiting the dilution of the established cells, 10 clones of PLC/PRF/5 cells resistant to IFN-_α_ were established. The clones were confirmed
as being resistant to IFN-_α_ stably over 20 passages. Among the 10 clones, three clones (PLC-Rs; PLC-R1, PLC-R2, and PLC-R3) were used in the experiments of this study. DRUGS AND REAGENTS
Purified human IFN-_α_ was kindly supplied by Otsuka Pharmaceutical Co. (Tokyo, Japan) and 5-FU and doxorubicin (DXR) by Kyowa Hakko Kirin Co. (Tokyo, Japan). Cisplatin (CDDP), insulin, and
IGF-1 were purchased from Nippon Kayaku Co. (Tokyo, Japan), Sigma-Aldrich Co. (St Louis, MO, USA), and Peprotech (Rocky Hill, NJ, USA), respectively. As for primary antibodies, polyclonal
goat anti-human IGFBP7 antibody and polyclonal rabbit anti-human IGFBP7 antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA) were used for immunohistochemistry and western blot
analysis, respectively. Antibodies to IFNAR2 and phosphotyrosine (p-Tyr) were purchased from Santa Cruz Biotechnology Inc; antibodies to signal transducer and activator of transcription
factor (STAT) 1, phosphorylated (Tyr 701) STAT (pSTAT) 1, Akt, and phosphorylated (Ser 473) Akt were from Cell Signaling Technology (Beverly, MA, USA); antibodies to STAT2, pSTAT2, and
insulin receptor substrate-1 (IRS-1) were from Millipore (Milford, MA, USA); and antibody to actin was from Sigma-Aldrich Co. PLASMID AND TRANSFECTION Plasmid coding for short hairpin RNA
(shRNA) against _IGFBP7_ and _IGFBP7_ expression plasmids was purchased from OriGene Technologies Inc. (Rockville, MD, USA). They were transfected into HCC cells using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) according to the instructions provided by the manufacturer. After transfection of the shRNA plasmid and _IGFBP7_ expression plasmid, stable transfectants were
selected and maintained by adding 1.0 _μ_g ml−1 of puromycin (Sigma-Aldrich Co.) and 600 _μ_g ml−1 of G418 (Gibco-BRL, Grand Island, NY, USA), respectively. The control vector plasmid
expressing non-effective shRNA was similarly introduced into cells to establish negative control cells for the shRNA plasmid. Empty vector plasmid was also similarly used to establish
negative control cells for the _IGFBP7_ expression plasmid. Successful transfection was confirmed by the coexpression of GFP. PLC-P transfected by shRNA plasmid against _IGFBP7_ and by the
negative control vector plasmid was named as PLC-P/shRNA (no. 1 and no. 2) and PLC-P/shRNA-NC, respectively. Short hairpin RNA no. 1 and no. 2 were different in sequence to shRNA. The PLC-Rs
transfected with the _IGFBP7_ expression plasmid and the negative control vector plasmid were named PLC-Rs/IGFBP7 and PLC-Rs/IGFBP7-NC, respectively. PATIENTS AND SPECIMENS The study
subjects were 30 patients with advanced HCC and recruited as described previously (Nagano et al, 2007a). All patients had multiple liver tumours in both lobes and tumour thrombi in the main
trunk of the portal vein, and each underwent palliative reduction surgery with tumour thrombectomy of the main trunk of the portal vein at the Osaka University Hospital between October 1999
and December 2004. The IFN-_α_/5-FU therapy for remnant multiple liver tumours was applied postoperatively, as described previously (Ota et al, 2005; Nagano et al, 2007a). Patients were
followed up after surgery, with a postoperative follow-up period of 18.2±19.7 months. Clinical response to therapy was evaluated according to the criteria of the Eastern Cooperative Oncology
Group (Oken et al, 1982). On the basis of the clinical response, responders were defined as patients with a complete response or partial response and non-responders were defined as patients
with a stable disease or progressive disease. The study protocol was approved by the Human Ethics Review Committee of Osaka University Hospital and a signed consent form was obtained from
each patient. REAL-TIME QUANTITATIVE REVERSE TRANSCRIPTION-PCR For reverse transcriptase reaction, the extracted RNA, random hexamers, and Superscript II reverse transcriptase (Invitrogen)
were used according to the instructions supplied by the manufacturer. Real-time quantitative reverse transcription-PCR (qRT-PCR) was performed using designed oligonucleotide primers and
Light Cycler (Roche Diagnostics, Mannheim, Germany), and the amount of target gene expression was calculated. The expression of the target gene was normalised relative to the expression of
_porphobilinogen deaminase_ (_PBGD_), which was used as an internal control. The designed PCR primers were as follows: _IGFBP7_ forward primer 5′-CTGGGTGCTGGTATCTCCTC-3′, _IGFBP7_ reverse
primer 5′-TATAGCTCGGCACCTTCACC-3′; _PBGD_ forward primer 5′-TGTCTGGTAACGGCAATGCGGCTGCAAC-3′, _PBGD_ reverse primer 5′-TCAATGTTGCCACCACACTGTCCGTCT-3′. MICROARRAY EXPERIMENTS Microarray
experiments were conducted according to the method described previously (Noda et al, 2009). In brief, total RNA was purified by TRIzol reagent (Invitrogen) according to the instructions
provided by the manufacturer. The integrity of the purified RNA was assessed as being of high quality by Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) and RNA 6000 LabChip kits
(Yokokawa Analytical Systems, Tokyo, Japan). The purified RNAs obtained from PLC-P, PLC-R1, PLC-R2, and PLC-R3 were used as samples, and all samples were examined in duplicate. The samples
were mixed and hybridised on a microarray covering 30 336 human probes (AceGene Human 30 K; DNA Chip Research Inc and Hitachi Software Engineering Co, Kanagawa, Japan). The ratio of the
expression level of each gene was converted into a logarithmic scale (base 2) and the data matrix was normalised. In each sample, genes with missing values in more than two samples were
excluded from the analysis. A total of 28 761 genes out of 30 336 genes were finally available for the analysis. WESTERN BLOT ANALYSIS Cells grown to semiconfluence were lysed in RIPA buffer
(25 mM Tris (pH 7.5), 50 mM NaCl, 0.5% sodium deoxycholate, 2% Nonidet P-40, 0.2% sodium dodecyl sulphate, 1 mM phenylmethylsulphonyl fluoride, 1.6 _μ_g ml−1 aprotinin). Western blot
analysis was carried out as described previously (Kondo et al, 2005). GROWTH INHIBITORY ASSAY The growth inhibitory assay was assessed by the 3-(4-,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) (Sigma-Aldrich Co.) assay, as described previously (Eguchi et al, 2000). Briefly, cells were incubated for 72 h under several concentrations of IFN-_α_ and 5-FU.
After reincubation for 4 h with MTT solution, acid–isopropanol mixture was added to dissolve the resultant formazan crystals. The absorbance of the plate was measured in a microplate reader
at a wavelength of 570 nm with a 650 nm reference, and the results were expressed as a percentage of absorbance relative to that of untreated controls. ANNEXIN V ASSAY The binding of annexin
V was used as a sensitive method for measuring apoptosis, as described previously (Nakamura et al, 2007). At 24 h after treatment with IFN-_α_, PLC-P/shRNA and PLC-P/shRNA-NC cells were
stained by Annexin V-APC and propidium iodide (PI) (BD Biosciences, Franklin Lakes, NJ, USA), and analysed on a FACS Aria (BD Biosciences). Annexin V-positive and PI-negative cells,
considered as early apoptotic cells, were used for the assessment of apoptosis in this study (Lugli et al, 2005). MEASUREMENT OF CASPASE ACTIVITIES Caspase-3, caspase-8, and caspase-9
activities were measured using caspase-3, caspase-8, and caspase-9 colorimetric assay kits (Chemicon International Inc, Temecula, CA, USA). The measurement was performed in cell lysates
obtained from each cell 24 h after treatment with IFN-_α_, using the instructions provided by the manufacturer. IRS-1 IMMUNOPRECIPITATION After incubation for 12 h in serum-free medium,
cells were stimulated with 1 nM insulin or 10 nM IGF-1. The stimulated cells were lysed in lysis buffer (20 mM Tris (pH 7.4), 150 mM NaCl, 1.0% Triton-X-100, 1.0 mM EGTA, 1 mM
phenylmethylsulphonyl fluoride, 1.6 _μ_g ml−1 aprotinin, 10 _μ_g ml−1 leupeptin). Solubilised proteins were immunoprecipitated with anti-IRS-1 antibody, and tyrosine phosphorylation was
detected with anti-p-Tyr antibody. IMMUNOHISTOCHEMICAL STAINING Immunohistochemical staining for IGFBP7 in 30 HCC samples was performed by the method described previously (Kondo et al,
1999). Briefly, formalin-fixed, paraffin-embedded 4 _μ_m-thick sections were deparaffinised in xylene, then treated with an antigen retrieval procedure and incubated in methanol containing
0.3% hydrogen peroxide to block endogenous peroxidase. After incubation with normal protein block serum, the sections were incubated overnight at 4°C with an anti-IGFBP7 antibody as the
primary antibody. Thereafter, the sections were incubated with a biotin-conjugated secondary antibody (horse anti-goat antibody for IGFBP7) and with peroxidase-conjugated streptavidin. The
peroxidase reaction was then developed with 0.02% 3, 30-diaminobenzidine tetrachloride (Wako Pure Chemicals, Osaka, Japan) solution with 0.03% hydrogen peroxide. Finally, the sections were
counterstained with Meyer's haematoxylin. The IGFBP7 expression was defined as the presence of specific staining in the cytoplasm of cancer cells. Insulin-like growth factor-binding
protein 7 expression was evaluated as positive or negative. Two investigators (Y.T. and H.E.) independently assessed IGFBP7 expression without knowledge of the corresponding
clinicopathological data. The assessments were similar by the two investigators for all samples. STATISTICAL ANALYSIS Data are expressed as mean±s.d. Clinicopathological parameters were
compared using the _χ_2-test and continuous variables were compared using Student's _t_-test. Survival curves were computed using the Kaplan–Meier method, and differences between
survival curves were compared using the log-rank test. A _P_-value <0.05 denoted the presence of a statistically significant difference. Statistical analysis was performed using StatView
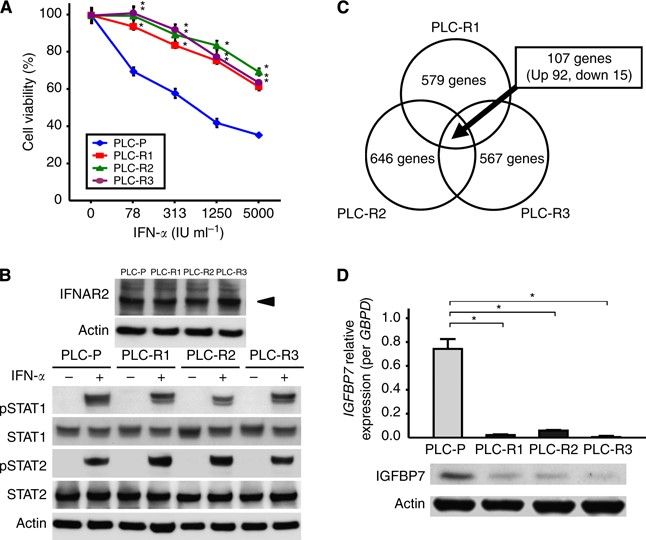
(version 5.0, SAS Institute Inc, Cary, NC, USA). RESULTS CHARACTERISTICS OF ESTABLISHED IFN-RESISTANT CELLS The morphology of PLC-Rs resembled that of PLC-P. Although PLC-Rs showed similar
growth curves compared with PLC-P in the absence of IFN-_α_ (data not shown), PLC-Rs were significantly resistant to IFN-_α_ compared with PLC-P, which was confirmed by MTT assays (Figure
1A). The expression levels of IFNAR2 were not different between PLC-P and PLC-Rs (Figure 1B). The protein level of STAT1 and STAT2, which directly bind to the intracellular domain of IFNAR2
and function as key molecules for signal transduction, was also not different between PLC-P and PLC-Rs treated with 1000 IU ml−1 of IFN-_α_ for 20 min (Figure 1B). Moreover, the
phosphorylation of STAT1 and STAT2 (pSTAT1 and pSTAT2), active forms of STATs, were also not different between these cells. _IGFBP7_ IS SIGNIFICANTLY DOWNREGULATED IN IFN-RESISTANT CELLS To
investigate the candidate genes involved in the response to IFN-_α_, microarray analysis was carried out with PLC-P and PLC-Rs. The analysis showed that, among the 28,761 genes, 579 (2.0%),
646 (2.2%), and 567 genes (2.0%) altered more than 1.5-fold in PLC-R1, PLC-R2, and PLC-R3, respectively. As shown in Figure 1C, 107 genes including 92 upregulated genes and 15 downregulated
genes (listed in Supplementary Table S1) were common among the above 579, 646, and 567 genes. Among these 107 genes, _IGFBP7_ was identified as one of the most downregulated genes with a
2.963-fold decrease. The downregulation of IGFBP7 in PLC-Rs compared with PLC-P was validated by real-time qRT-PCR and western blot analysis (Figure 1D). KNOCKDOWN OF _IGFBP7_ INDUCES
RESISTANCE TO IFN-_Α_ To evaluate the biological effect of _IGFBP7_, two kinds of plasmids coding for shRNA against IGFBP7 (no. 1 and no. 2) were transfected into PLC-P and named as
PLC-P/shRNA no. 1 and PLC-P/shRNA no. 2. The IGFBP7 expression was suppressed at both mRNA and protein levels in the established PLC-P/shRNAs, which was confirmed by qRT-PCR and western blot
analysis, respectively (Figure 2A). The MTT assay showed that PLC-P/shRNAs were significantly more resistant to IFN-_α_ than PLC-P/shRNA-NC (Figure 2B). On the basis of the measurement of
IC50, the fold increase of IC50 to IFN-_α_ was much larger than that to other drugs, including 5-FU, CDDP, and DXR, suggesting that chemoresistance acquired by IGFBP7 is specific to IFN-_α_
(Table 1). IFNAR2, STAT1, and STAT2 were similarly expressed in PLC-P/shRNA and PLC-P/shRNA-NC, and the IFN-_α_-induced pSTAT1 and pSTAT2 expressions were also not similar in the two cells
(Supplementary Figure S1A). As IGFBP7 has been shown to suppress tumour activity through induction of apoptosis (Landberg et al, 2001; Mutaguchi et al, 2003; Sato et al, 2007; Wajapeyee et
al, 2008), we evaluated the extent of apoptosis induced at 24 h after treatment of PLC-P/shRNA with 1000 IU ml−1 IFN-_α_. Annexin V assay using flow cytometry showed a significantly lower
percentage of early apoptotic cells in PLC-P/shRNA than in PLC-P/shRNA-NC (Figure 3C). Moreover, the activity of caspase-3, caspase-8, and caspase-9 induced by IFN-_α_ in PLC-P/shRNA was
significantly lower than that by PLC-P/shRNA-NC (Figure 3D). A plasmid coding for shRNA against IGFBP7 was transfected in other liver cancer cell lines (HLE and Hep3B). Both these cell lines
showed downregulated _IGFBP7_ expression (Supplementary Figure S2A). The transfected HLE and Hep3B cells were also resistant to IFN-_α_ treatment (500 IU ml−1) (Supplementary Figure S2B).
As IGFBP7 has been reported to bind insulin and IGF (Oh, 1998; Subramanian et al, 2007), it could be conceivable that IGFBP7 induces resistance by interfering with insulin and/or IGF
signalling. To verify this possibility, we examined the effect of IGFBP7 on the phosphorylation of IRS-1 and Akt, major transducers of insulin and IGF signalling. As shown in Supplementary
Figure S1B, there were no significant differences in the phosphorylation of IRS-1 or Akt between PLC-P/shRNA and PLC-P/shRNA-NC. This result suggests that IGFBP7-related resistance occurs in
an insulin- and IGF-independent manner. TRANSFECTION OF IGFBP7 RESTORES SENSITIVITY TO IFN-_Α_ Next, _IGFBP7_ expression plasmid was transfected into PLC-R1 (PLC-R1/IGFBP7). Upregulation of
IGFBP7 in PLC-R1/IGFBP7 compared with PLC-R1/IFGBP7-NC was confirmed by qRT-PCR and western blot analysis (Figure 3A). By the MTT assay, PLC-R1/IGFBP7 partially but significantly restores
sensitivity to IFN-_α_ compared with PLC-R1/IGFBP7-NC (Figure 3B). IGFBP7 IS A USEFUL PREDICTOR OF CLINICAL RESPONSE TO IFN-_Α_/5-FU THERAPY To confirm whether IGFBP7 expression is
associated with the clinical response to IFN-_α_/5-FU therapy, HCC samples of 30 patients who underwent IFN-_α_/5-FU therapy postoperatively were immunohistochemically stained for IGFBP7
expression. Whereas the expression levels of IGFBP7 in cancerous lesions varied among the patients, a homogenous staining for IGFBP7 was observed in the cytoplasm of cells in non-cancerous
sections (Figure 4). Among the 30 patients examined, 12 (40.0%) showed positive staining, whereas 18 (60.0%) patients were negative for IGFBP7. Of the IGFBP7-positive patients, 66.7% (8 of
12) were histologically evaluated as responders to the therapy, whereas only 11.1% (2 of 18) of IGFBP7-negative patients were responders, suggesting that IGFBP7 expression was significantly
associated with response to therapy (_P_<0.05) (Table 2). The sensitivity, specificity, and accuracy for the prediction to IFN-_α_/5-FU therapy by IGFBP7 were 80.0% (8 of 10), 80.0% (16
of 20), and 80.0% (24 of 30). None of the other clinicopathological factors tested, apart from IFNAR2, were associated with response to the therapy (Supplementary Table S2). Finally, we
examined the correlations between postoperative prognosis and various clinicopathological factors including IGFBP7 status. The postoperative overall survival in IGFBP7-positive patients was
significantly better than that in IGFBP7-negative patients (_P_<0.05, Figure 5). Furthermore, multivariate analysis of overall survival using two significant factors identified in the
univariate analyses showed that, in addition to IFNAR2, IGFBP7 status was an independent and significant determinant of overall survival (Table 3), indicating that IGFBP7 is a potentially
useful marker for the prediction of clinical response to IFN-_α_/5-FU therapy. DISCUSSION In this study, gene expression profiling identified significant suppression of IGFBP7 in PLC-Rs
compared with PLC-P. Insulin-like growth factor-binding protein 7, also known as IGFBP-rP1 and MAC25, can inhibit the proliferation of cancer cells, and its expression is downregulated in
certain cancers (Burger et al, 1998; Landberg et al, 2001; Mutaguchi et al, 2003; Sato et al, 2007; Lin et al, 2008; Wajapeyee et al, 2008). It is also reported that IGFBP7 suppression is
associated with rapid tumour growth and tumour invasiveness (Burger et al, 1998; Sato et al, 2007; Lin et al, 2008). However, there are no reports of the association between IGFBP7
expression and sensitivity to chemotherapeutic drugs. In this study, IGFBP7 was suppressed by shRNA transfection in HCC cells and the transfected cells acquired resistance to IFN-_α_. The
association between IGFBP7 expression and response to IFN-_α_ was also confirmed in experiments using IGFBP7-overexpressing cells. Considering that IGFBP7-suppressed cells showed a smaller
percentage of apoptosis than control cells, the acquired resistance was thought to result from the impediment of apoptosis. The suppression of apoptosis by downregulation of IGFBP7 was
consistent with that found in previous studies (Burger et al, 1998; Landberg et al, 2001; Mutaguchi et al, 2003; Sato et al, 2007; Lin et al, 2008; Wajapeyee et al, 2008). In addition to
resistance to IFN-_α_, IGFBP7-suppressed cells showed modest but significant resistance to other drugs. Taking into consideration the fact that IGFBP7 promotes apoptosis even in the absence
of any drugs, the acquisition of resistance to both IFN-_α_ and other drugs may be quite natural. However, the fold increase in acquired resistance to IFN-_α_ was much larger than that to
other drugs as confirmed by measurements of IC50, suggesting that IGFBP7 is specifically related to the resistance to IFN-_α_. Moreover, from the experiments of insulin- and IGF signalling,
this effect of IGFBP7 was suggestive to occur in an insulin- and IGF-independent manner. Furthermore, to clarify the mechanism of IGFBP7-specific IFN resistance, we examined IFNAR2
expression and IFN signalling and compared them between PLC-P and PLC-Rs and between PLC-P/shRNA and PLC-P/shRNA-NC. The IFN signalling was evaluated by the expression of STAT1 and STAT2,
and by IFN-_α_-induced expression of pSTAT1 and pSTAT2. The results showed no significant differences in the expression of IFNAR2 and IFN signalling between PLC-P and PLC-Rs or between
PLC-P/shRNA and PLC-P/shRNA-NC. On the other hand, Wajapeyee et al (2008) reported that IGFBP7 induces apoptosis through increased SMARCB1 upregulation by the recruitment of STAT1 to the
binding site of the SMARCB1 promoter. Another study reported that STAT1 is recruited to the SMARCB1 promoter by IFN, suggesting that IFN-induced STAT1 recruitment to the SMARCB1 promoter is
possibly one of the mechanisms of IFN-induced apoptosis (Hartman et al, 2005). It might therefore be possible that STAT1 recruitment could be prevented antagonistically when IGFBP7 is
suppressed, leading to a higher resistance to IFN-_α_ than to other drugs. In this study, however, pSTAT1 expression was not different between PLC-P and PLC-Rs or between PLC-P/shRNA and
PLC-P/shRNA-NC, and there were no significant differences in the SMARCB1 expression evaluated by the result of microarray between PLC-P and PLC-Rs. These results indicate that IGFBP7-related
IFN resistance is based not on SMARCB1 but on a novel mechanism, which should be clarified in the future. The present study revealed that, in addition to the significant association between
IGFBP7 status and the clinical response to IFN-_α_/5-FU therapy, the IGFBP7 status as well as IFNAR2, was an independent prognostic factor in HCC patients undergoing IFN-_α_/5-FU therapy.
Because our 30 patients in this study are those with far advanced HCC, it is quite reasonable that the clinical response to the therapy correlates well with the prognosis after the therapy.
These results indicate that prediction of response and prognosis by evaluating IGFBP7 and IFNAR2 is useful in this clinical setting. In summary, IGFBP7 was selected on the basis of the
results of the microarray analysis using established IFN-resistant HCC cell lines. The expression of IGFBP7 in tumour tissue correlated significantly with the response to IFN-_α_/5-FU
therapy. This correlation was also confirmed in genetic manipulation experiments. Our findings suggest that IGFBP7 could be a novel marker for the prediction of the clinical response to
IFN-_α_/5-FU therapy. CHANGE HISTORY * _ 16 NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _
REFERENCES * Asahara T, Itamoto T, Katayama K, Nakahara H, Hino H, Yano M, Ono E, Dohi K, Nakanishi T, Kitamoto M, Azuma K, Itoh K, Shimamoto F (1999) Hepatic resection with tumor
thrombectomy for hepatocellular carcinoma with tumor thrombi in the major vasculatures. _Hepatogastroenterology_ 46: 1862–1869 CAS PubMed Google Scholar * Burger AM, Zhang X, Li H,
Ostrowski JL, Beatty B, Venanzoni M, Papas T, Seth A (1998) Down-regulation of T1A12/mac25, a novel insulin-like growth factor binding protein related gene, is associated with disease
progression in breast carcinomas. _Oncogene_ 16: 2459–2467 Article CAS Google Scholar * Chung YH, Song IH, Song BC, Lee GC, Koh MS, Yoon HK, Lee YS, Sung KB, Suh DJ (2000) Combined
therapy consisting of intraarterial cisplatin infusion and systemic interferon-alpha for hepatocellular carcinoma patients with major portal vein thrombosis or distant metastasis. _Cancer_
88: 1986–1991 Article CAS Google Scholar * Damdinsuren B, Nagano H, Monden M (2007a) Combined intra-arterial 5-fluorouracil and subcutaneous interferon-α therapy for highly advanced
hepatocellular carcinoma. _Hepatol Res_ 37 (Suppl 2): S238–S250 Article CAS Google Scholar * Damdinsuren B, Nagano H, Wada H, Noda T, Natsag J, Marubashi S, Miyamoto A, Takeda Y, Umeshita
K, Doki Y, Dono K, Monden M (2007b) Interferon α receptors are important for antiproliferative effect of interferon-α against human hepatocellular carcinoma cells. _Hepatol Res_ 37: 77–83
Article CAS Google Scholar * Eguchi H, Nagano H, Yamamoto H, Miyamoto A, Kondo M, Dono K, Nakamori S, Umeshita K, Sakon M, Monden M (2000) Augmentation of antitumor activity of
5-fluorouracil by interferon α is associated with up-regulation of p27Kip1 in human hepatocellular carcinoma cells. _Clin Cancer Res_ 6: 2881–2890 CAS PubMed Google Scholar * Furuse J,
Iwasaki M, Yoshino M, Konishi M, Kawano N, Kinoshita T, Ryu M, Satake M, Moriyama N (1997) Hepatocellular carcinoma with portal vein tumor thrombus: embolization of arterioportal shunts.
_Radiology_ 204: 787–790 Article CAS Google Scholar * Hartman SE, Bertone P, Nath AK, Royce TE, Gerstein M, Weissman S, Snyder M (2005) Global changes in STAT target selection and
transcription regulation upon interferon treatments. _Genes Dev_ 19: 2953–2968 Article CAS Google Scholar * Kondo M, Nagano H, Sakon M, Yamamoto H, Morimoto O, Arai I, Miyamoto A, Eguchi
H, Dono K, Nakamori S, Umeshita K, Wakasa K, Ohmoto Y, Monden M (2000) Expression of interferon α/β receptor in human hepatocellular carcinoma. _Int J Oncol_ 17: 83–88 CAS PubMed Google
Scholar * Kondo M, Nagano H, Wada H, Damdinsuren B, Yamamoto H, Hiraoka N, Eguchi H, Miyamoto A, Yamamoto T, Ota H, Nakamura M, Marubashi S, Dono K, Umeshita K, Nakamori S, Sakon M, Monden
M (2005) Combination of IFN-α and 5-fluorouracil induces apoptosis through IFN-α/β receptor in human hepatocellular carcinoma cells. _Clin Cancer Res_ 11: 1277–1286 Article CAS Google
Scholar * Kondo M, Yamamoto H, Nagano H, Okami J, Ito Y, Shimizu J, Eguchi H, Miyamoto A, Dono K, Umeshita K, Matsuura N, Wakasa K, Nakamori S, Sakon M, Monden M (1999) Increased expression
of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. _Clin Cancer Res_ 5: 4005–4012 CAS PubMed Google Scholar *
Landberg G, Ostlund H, Nielsen NH, Roos G, Emdin S, Burger AM, Seth A (2001) Downregulation of the potential suppressor gene IGFBP-rP1 in human breast cancer is associated with inactivation
of the retinoblastoma protein, cyclin E overexpression and increased proliferation in estrogen receptor negative tumors. _Oncogene_ 20: 3497–3505 Article CAS Google Scholar * Lee HS, Kim
JS, Choi IJ, Chung JW, Park JH, Kim CY (1997) The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal
vein obstruction. A prospective controlled study. _Cancer_ 79: 2087–2094 Article CAS Google Scholar * Leung TW, Patt YZ, Lau WY, Ho SK, Yu SC, Chan AT, Mok TS, Yeo W, Liew CT, Leung NW,
Tang AM, Johnson PJ (1999) Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. _Clin Cancer Res_ 5: 1676–1681 CAS
PubMed Google Scholar * Lin J, Lai M, Huang Q, Ruan W, Ma Y, Cui J (2008) Reactivation of IGFBP7 by DNA demethylation inhibits human colon cancer cell growth _in vitro_. _Cancer Biol Ther_
7: 1896–1900 Article CAS Google Scholar * Lugli E, Troiano L, Ferraresi R, Roat E, Prada N, Nasi M, Pinti M, Cooper EL, Cossarizza A (2005) Characterization of cells with different
mitochondrial membrane potential during apoptosis. _Cytometry A_ 68: 28–35 Article Google Scholar * Mutaguchi K, Yasumoto H, Mita K, Matsubara A, Shiina H, Igawa M, Dahiya R, Usui T (2003)
Restoration of insulin-like growth factor binding protein-related protein 1 has a tumor-suppressive activity through induction of apoptosis in human prostate cancer. _Cancer Res_ 63:
7717–7723 CAS PubMed Google Scholar * Nagano H, Miyamoto A, Wada H, Ota H, Marubashi S, Takeda Y, Dono K, Umeshita K, Sakon M, Monden M (2007a) Interferon-α and 5-fluorouracil combination
therapy after palliative hepatic resection in patients with advanced hepatocellular carcinoma, portal venous tumor thrombus in the major trunk, and multiple nodules. _Cancer_ 110: 2493–2501
Article CAS Google Scholar * Nagano H, Sakon M, Eguchi H, Kondo M, Yamamoto T, Ota H, Nakamura M, Wada H, Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Dono K, Umeshit K, Nakamori S,
Monden M (2007b) Hepatic resection followed by IFN-α and 5-FU for advanced hepatocellular carcinoma with tumor thrombus in the major portal branch. _Hepatogastroenterology_ 54: 172–179 CAS
PubMed Google Scholar * Nakamura M, Nagano H, Sakon M, Yamamoto T, Ota H, Wada H, Damdinsuren B, Noda T, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Nakamori S, Dono K, Monden M
(2007) Role of the Fas/FasL pathway in combination therapy with interferon-α and fluorouracil against hepatocellular carcinoma _in vitro_. _J Hepatol_ 46: 77–88 Article CAS Google Scholar
* Noda T, Nagano H, Takemasa I, Yoshioka S, Murakami M, Wada H, Kobayashi S, Marubashi S, Takeda Y, Dono K, Umeshita K, Matsuura N, Matsubara K, Doki Y, Mori M, Monden M (2009) Activation
of Wnt/β-catenin signalling pathway induces chemoresistance to interferon-α/5-fluorouracil combination therapy for hepatocellular carcinoma. _Br J Cancer_ 100: 1647–1658 Article CAS Google
Scholar * Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M (2006) Combination therapy of intraarterial 5-fluorouracil and systemic
interferon-α for advanced hepatocellular carcinoma with portal venous invasion. _Cancer_ 106: 1990–1997 Article CAS Google Scholar * Oh Y (1998) IGF-independent regulation of breast
cancer growth by IGF binding proteins. _Breast Cancer Res Treat_ 47: 283–293 Article CAS Google Scholar * Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982)
Toxicity and response criteria of the Eastern Cooperative Oncology Group. _Am J Clin Oncol_ 5: 649–655 Article CAS Google Scholar * Ota H, Nagano H, Sakon M, Eguchi H, Kondo M, Yamamoto
T, Nakamura M, Damdinsuren B, Wada H, Marubashi S, Miyamoto A, Dono K, Umeshita K, Nakamori S, Wakasa K, Monden M (2005) Treatment of hepatocellular carcinoma with major portal vein
thrombosis by combined therapy with subcutaneous interferon-α and intra-arterial 5-fluorouracil; role of type 1 interferon receptor expression. _Br J Cancer_ 93: 557–564 Article CAS Google
Scholar * Patt YZ, Hassan MM, Lozano RD, Brown TD, Vauthey JN, Curley SA, Ellis LM (2003) Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b
for treatment of hepatocellular carcinoma. _J Clin Oncol_ 21: 421–427 Article CAS Google Scholar * Sakon M, Nagano H, Dono K, Nakamori S, Umeshita K, Yamada A, Kawata S, Imai Y, Iijima
S, Monden M (2002) Combined intraarterial 5-fluorouracil and subcutaneous interferon-α therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. _Cancer_
94: 435–442 Article CAS Google Scholar * Sato Y, Chen Z, Miyazaki K (2007) Strong suppression of tumor growth by insulin-like growth factor-binding protein-related protein
1/tumor-derived cell adhesion factor/mac25. _Cancer Sci_ 98: 1055–1063 Article CAS Google Scholar * Subramanian A, Sharma AK, Banerjee D, Jiang WG, Mokbel K (2007) Evidence for a tumour
suppressive function of IGF1-binding proteins in human breast cancer. _Anticancer Res_ 27: 3513–3518 CAS PubMed Google Scholar * Tanaka A, Morimoto T, Yamaoka Y (1996) Implications of
surgical treatment for advanced hepatocellular carcinoma with tumor thrombi in the portal vein. _Hepatogastroenterology_ 43: 637–643 CAS PubMed Google Scholar * Urabe T, Kaneko S,
Matsushita E, Unoura M, Kobayashi K (1998) Clinical pilot study of intrahepatic arterial chemotherapy with methotrexate, 5-fluorouracil, cisplatin and subcutaneous interferon-α-2b for
patients with locally advanced hepatocellular carcinoma. _Oncology_ 55: 39–47 Article CAS Google Scholar * Wada H, Nagano H, Yamamoto H, Arai I, Ota H, Nakamura M, Damdinsuren B, Noda T,
Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Doki Y, Dono K, Nakamori S, Sakon M, Monden M (2007) Combination therapy of interferon-α and 5-fluorouracil inhibits tumor angiogenesis in
human hepatocellular carcinoma cells by regulating vascular endothelial growth factor and angiopoietins. _Oncol Rep_ 18: 801–809 CAS PubMed Google Scholar * Wada H, Nagano H, Yamamoto H,
Noda T, Murakami M, Kobayashi S, Marubashi S, Eguchi H, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M (2009) Combination of interferon-alpha and 5-fluorouracil inhibits endothelial cell
growth directly and by regulation of angiogenic factors released by tumor cells. _BMC Cancer_ 9: 361 Article Google Scholar * Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR (2008)
Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. _Cell_ 132: 363–374 Article CAS Google Scholar * Yamakado K, Tanaka N, Nakatsuka
A, Matsumura K, Takase K, Takeda K (1999) Clinical efficacy of portal vein stent placement in patients with hepatocellular carcinoma invading the main portal vein. _J Hepatol_ 30: 660–668
Article CAS Google Scholar * Yamamoto T, Nagano H, Sakon M, Wada H, Eguchi H, Kondo M, Damdinsuren B, Ota H, Nakamura M, Marubashi S, Miyamoto A, Dono K, Umeshita K, Nakamori S, Yagita H,
Monden M (2004) Partial contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway to antitumor effects of interferon-α/5-fluorouracil against
hepatocellular carcinoma. _Clin Cancer Res_ 10: 7884–7895 Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Surgery, Graduate
School of Medicine, Osaka University, 2-2 Yamadaoka E-2, Suita, Osaka, 565-0871, Japan Y Tomimaru, H Eguchi, H Wada, T Noda, M Murakami, S Kobayashi, S Marubashi, Y Takeda, M Tanemura, Y
Doki, M Mori & H Nagano * Division of Health Sciences, Graduate School of Medicine, Osaka University, 2-2 Yamadaoka E-2, Suita, Osaka, 565-0871, Japan K Umeshita Authors * Y Tomimaru
View author publications You can also search for this author inPubMed Google Scholar * H Eguchi View author publications You can also search for this author inPubMed Google Scholar * H Wada
View author publications You can also search for this author inPubMed Google Scholar * T Noda View author publications You can also search for this author inPubMed Google Scholar * M
Murakami View author publications You can also search for this author inPubMed Google Scholar * S Kobayashi View author publications You can also search for this author inPubMed Google
Scholar * S Marubashi View author publications You can also search for this author inPubMed Google Scholar * Y Takeda View author publications You can also search for this author inPubMed
Google Scholar * M Tanemura View author publications You can also search for this author inPubMed Google Scholar * K Umeshita View author publications You can also search for this author
inPubMed Google Scholar * Y Doki View author publications You can also search for this author inPubMed Google Scholar * M Mori View author publications You can also search for this author
inPubMed Google Scholar * H Nagano View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to H Nagano. ADDITIONAL
INFORMATION Supplementary Information accompanies the paper on British Journal of Cancer website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES S1 AND S2 (PPT 256 KB) SUPPLEMENTARY
INFORMATION (DOC 152 KB) RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0
Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tomimaru, Y., Eguchi,
H., Wada, H. _et al._ Insulin-like growth factor-binding protein 7 alters the sensitivity to interferon-based anticancer therapy in hepatocellular carcinoma cells. _Br J Cancer_ 102,
1483–1490 (2010). https://doi.org/10.1038/sj.bjc.6605669 Download citation * Revised: 18 March 2010 * Accepted: 29 March 2010 * Published: 20 April 2010 * Issue Date: 11 May 2010 * DOI:
https://doi.org/10.1038/sj.bjc.6605669 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * hepatocellular carcinoma * interferon-_α_ *
5-fluorouracil * insulin-like growth factor-binding protein 7 (IGFBP7)