Play all audios:
Non-steroidal anti-inflammatory drugs (NSAIDs) and hormone therapy (HT) independently decrease the risk of colorectal cancer. However, their role in altering survival after a colorectal
cancer diagnosis is not well established.
We examined the association between the use of these common medications before diagnosis and colorectal cancer survival among women in western Washington State diagnosed with incident
colorectal cancer from 1997 to 2002. Cases were ascertained using the Surveillance, Epidemiology and End Results cancer registry; mortality follow-up was completed through linkages to the
National Death Index. Cox proportional hazards regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs).
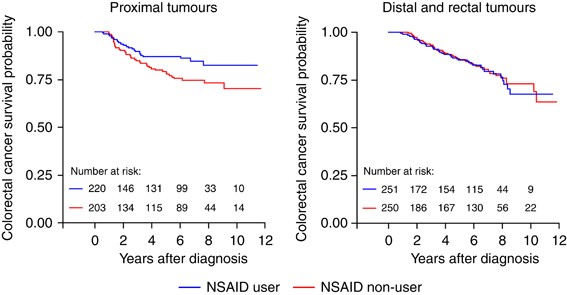
We observed no overall association between colorectal cancer survival and pre-diagnostic NSAID use. However, when stratified by tumour sub-site, NSAID use was associated with a reduced risk
of colorectal cancer mortality for women diagnosed with proximal (HR=0.55; 95% CI: 0.32–0.92), but not distal or rectal (HR=1.32; 95% CI: 0.83–2.10) tumours. The usage of HT was not
associated with colorectal cancer survival overall or by tumour sub-site.
Usage of NSAIDs before diagnosis may be associated with improved colorectal cancer survival among women diagnosed with proximal tumours. The usage of HT does not appear to have a function in
altering colorectal cancer mortality.
Non-steroidal anti-inflammatory drug (NSAID) use and hormone therapy (HT) use have each been consistently shown to be associated with a significantly reduced risk of developing colorectal
cancer (Giovannucci et al, 1994; Newcomb and Storer, 1995; Rossouw et al, 2002; Gambacciani et al, 2003; Chlebowski et al, 2004; Chan et al, 2005; Bardia et al, 2007; Newcomb et al, 2007b;
Baron, 2009; Cole et al, 2009). However, few studies have investigated the role of these common medications in subsequent mortality after a diagnosis of colorectal cancer.
Only three observational studies in the literature to date have addressed the relationship between NSAID use and colorectal cancer survival (Fuchs and Heseltine et al, 2005; Chan et al,
2009; Zell et al, 2009). A study of stage III colorectal cancer patients (n=846 men and women combined) enrolled in a randomized chemotherapy trial observed that consistent aspirin use was
associated with 52% lower risk of either cancer recurrence or mortality (Fuchs and Heseltine et al, 2005). A report from the Nurses’ Health Study (NHS) and Health Professionals Follow-up
Study, (n=840 women; 439 men) found that regular aspirin use after a diagnosis of colorectal cancer was associated with a 29% reduced risk of colorectal cancer-specific mortality (Chan et
al, 2009). Finally, a recent investigation in the California Teachers Study (CTS) cohort (n=621 women) observed that regular NSAID use before diagnosis among women was associated with a 42%
reduced rate of colorectal cancer mortality (Zell et al, 2009).
Three previous studies have observed inverse associations between regular HT use and the risk of death from colorectal cancer (Calle et al, 1995; Slattery et al, 1999; Mandelson et al,
2003), although the design of one study precluded it from distinguishing between the effects of HT on reduced cancer incidence from those of HT on survival after diagnosis (Calle et al,
1995). In contrast, results from the Women's Health Initiative and a recent large, population-based study of women with large bowel cancer both observed no association between HT use and
colorectal cancer survival (Ritenbaugh et al, 2008; Newcomb et al, 2009).
We investigated the association between both NSAID use and HT use before cancer diagnosis and subsequent death among female colorectal cases identified from the population-based
Surveillance, Epidemiology and End Results (SEER) registry in 13 counties of western Washington State.
Details of case ascertainment have been published elsewhere (Newcomb et al, 2007a, 2007b). Briefly, eligible case subjects included women, aged between 50 and 74 years, residing in 13
counties in western Washington State, who were diagnosed between 1997 and 2002 with incident, invasive colorectal cancer. Women aged between 20 and 49 years diagnosed during the same time
period in the Puget Sound counties (3 of the 13) were also eligible for inclusion. Cases were reported to the Cancer Surveillance System, a population-based registry that is part of the
National Cancer Institute's SEER program. Eligibility was limited to English-speaking subjects with available telephone numbers. With physician approval, the study subjects received an
introductory letter in the mail and were followed-up with a telephone call. A total of 1614 eligible women were identified. Of these cases, 100 were deceased, 151 were lost to follow-up
before interview and 181 refused to participate or did not complete the baseline interview, resulting in a final sample size of 1173 cases. Cases were interviewed and enrolled an average of
8.1 months (s.d.=3.2) after colorectal cancer diagnosis. Analyses were restricted to Caucasian women (n=1051) because of sparse data among women of other races. The study was approved by the
Institutional Review Board of the Fred Hutchinson Cancer Research Center in accordance with assurances filed with and approved by the US Department of Health and Human Services.
A structured 60-min telephone interview was used to obtain information from all cases on established and potential risk factors for colorectal cancer. Interviewer-collected information
included data on history of NSAID and exogenous hormone use, menstrual and reproductive history, smoking history, height and weight, history of colorectal cancer screening, first-degree
family history of cancer and demographic factors, such as age and race. For all women, only potential exposures that occurred before a reference date, approximately 2 years before diagnosis,
were considered in the analysis. The interview collected information on type and duration of NSAID use (aspirin or ibuprofen) and HT use (oestrogen only or combined oestrogen and
progestin).
For NSAIDs, regular use was defined as use at least twice per week for 1 month or greater. Ever use was defined as regular use of any NSAID type at any point in time before the reference
date. Never users reported no use or less than the defined regular use threshold before the reference date. Duration of NSAID use was calculated using the reported years of regular use of
any type of NSAID medication; information on the reported frequency of use, measured in pills per day on average, was also collected. An NSAID-dose variable used in Cox models was created
using both the available duration and frequency information and included the following categories: ⩽1 time per day for ⩽2 years (dose 1); >1 time per day for ⩽2 years (dose 2); ⩽1 time per
day for >2 years (dose 3); and >1 time per day for >2 years (dose 4).
For HT, ever use was defined as use of any preparation type for at least 6 consecutive months at any time before the reference date. Never users reported no use or less than the defined ever
use threshold before the reference date. Duration of HT use was calculated using the reported years of use of any type of HT preparation.
Vital status on all enrolled cancer cases was determined through linkages to the National Death Index to obtain date and cause of death; cause of death was classified using ICD10 coding
conventions. The National Death Index identifies known deaths with a high degree of sensitivity, validity and completeness (Fillenbaum et al, 2009). The primary outcome of interest was death
due to colorectal cancer. Time to death was evaluated from SEER-recorded date of colorectal cancer diagnosis and National Death Index-recorded date of death. Patients alive at the time of
their last known vital assessment were censored at that date, with the most recent vital status linkage occurring in December 2009. Patients dying of causes other than colorectal cancer were
censored at their recorded date of death.
Sub-site and stage of the colorectal tumour at diagnosis were defined using SEER records. Advanced disease was defined as colorectal cancer with distant metastasis at diagnosis; non-advanced
disease included local and regional stage disease at diagnosis. Sub-site of disease was categorized using ICD10 codes: proximal disease (C18.0–C18.5); distal disease (C18.6–C18.7); and
rectal disease (C19.9–C20.9).
Kaplan–Meier survival curves were generated for both NSAID use (ever vs never) and HT use (ever vs never). The proportional hazards assumption was evaluated graphically as well as
statistically through inclusion of interaction terms between exposures and current time in Cox regression models (Ahmed et al, 2007). For both exposures investigated, the proportional
hazards assumption was not statistically violated for colorectal cancer-specific mortality.
Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between pre-diagnostic NSAID use, HT use and
colorectal cancer-specific mortality. To increase comparability to previous studies that excluded metastatic disease (Chan et al, 2009), analyses were restricted to cases diagnosed with
local or regional disease (n=933). Cox regression models included the following list of covariates selected a priori: age at cancer diagnosis, body mass index at reference date, smoking
status, family history of colorectal cancer, history of preventive screening and stage of disease at diagnosis. Additionally, the regression models investigating each medication were
adjusted for pre-diagnostic use of the other medication (i.e., NSAID use adjusted for pre-diagnostic HT use). Preventive screening was defined as sigmoidoscopy or colonoscopy (endoscopy)
screening that was received at least 2 years before the diagnosis of colorectal cancer. Categories of body mass index were defined as the following in units of kg m−2: not overweight