Play all audios:
ABSTRACT Over the past decade, the synthesis of superparamagnetic nanoparticles, especially iron-oxide nanoparticles (IONPs), has been researched intensively for many high-technology
applications, including enhanced storage media, biosensing and medical applications. In medicine, IONPs are used as contrast agents in magnetic resonance imaging and in hyperthermia therapy,
and can also be exploited in drug or gene delivery as they are relatively non-toxic. However, their usage _in vivo_ is limited by their agglomeration in biological fluids, induced by their
high surface energies and tendency to adsorb proteins. The addition of a polymer layer to the surface of IONPs can stabilize these nanoparticles, giving well-dispersed individual
nanoparticles in biological fluids for _in vitro_ and _in vivo_ applications, thereby increasing the blood circulation half-life. Moreover, the polymer layer can endow the IONPs with
functionality, providing a scaffold for the encapsulation or attachment of therapeutic agents (drugs or genes), targeting agents and permeation enhancers. This review examines recent
advancements in the use of IONPs in medicine, a field that has been particularly active in the last few years. You have full access to this article via your institution. Download PDF SIMILAR
CONTENT BEING VIEWED BY OTHERS DESIGN OF OXIDE NANOPARTICLES FOR BIOMEDICAL APPLICATIONS Article 29 January 2025 MAGNETO-PLASMONIC NANOSTARS FOR IMAGE-GUIDED AND NIR-TRIGGERED DRUG DELIVERY
Article Open access 22 June 2020 STUDY OF BIOPOLYMER ENCAPSULATED EU DOPED FE3O4 NANOPARTICLES FOR MAGNETIC HYPERTHERMIA APPLICATION Article Open access 29 April 2024 MAIN Iron-oxide
nanoparticles (IONPs) represent a significant class of inorganic nanomaterial that is contributing to the current revolution in nanomedicine1,2,3]. Their unique physical properties,
including high surface area to volume ratios and superparamagnetism, confer useful attributes for medical applications such as magnetic resonance imaging (MRI), drug and gene delivery,
tissue engineering and bioseparation3. Two distinct classes of superparamagnetic IONP-based materials are currently used for medical applications: superparamagnetic iron-oxide (SPIO)
nanoparticles with a mean particle diameter of 50–100 nm, and ultra-small superparamagnetic iron-oxide (USPIO) nanoparticles with a size below 50 nm. These two classes of IONPs have been
studied widely for medical applications, particularly as the next (potential) generation of MRI contrast agents. They are also seen as potential vectors for drug and gene delivery. The
biodistribution of these nanoparticles can be altered by the application of an external magnetic field; they also have potential applications in hyperthermia therapy as some magnetic
particles can heat up under the influence of a localized high-frequency magnetic field. The intent of this review is to present recent advances in the synthesis of IONPs and their subsequent
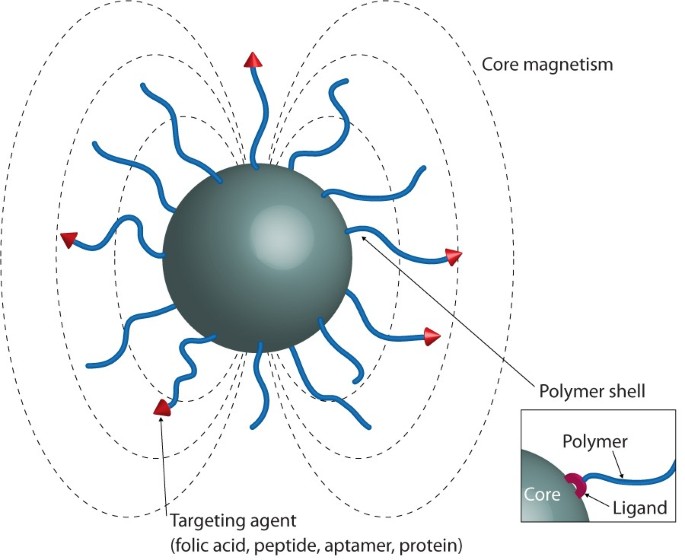
stabilization in biological fluids using polymers, focusing on the current strategies used to graft polymers onto IONPs surfaces (see Figure 1) and the different types of polymers used. The
additional properties conferred by the polymers, such as targeting, effects on biodistribution and pharmacokinetics, are also discussed, and the main applications of IONPs are reviewed.
SYNTHESIS AND PROPERTIES OF IRON-OXIDE NANOPARTICLES SPIO and USPIO nanoparticles are the most extensively studied magnetic nanoparticles for biomedical applications as they are both
biocompatible and easy to synthesize. Both are composed of ferrite nanocrystallites of magnetite (Fe3O4) or maghemite (Fe2O3, γ). Over the last ten years, there has been an explosion of
interest in these materials and this has been reflected in a large number of recent publications describing the synthesis and modification of hybrid IONPs. In this review, we focus on the
polymer modification of the nanoparticles, and provide only minimal information on the inorganic synthesis of IONPs. For more detailed information on IONP synthesis, readers are referred to
other reviews3,4. A concise description of IONP synthesis follows. The co-precipitation process is the simplest and most widely employed chemical route for the synthesis of IONPs. Briefly,
IONPs are prepared by aging a stoichiometric mixture of ferrous and ferric salts in aqueous media under basic conditions to yield magnetite in the absence of oxygen. However, this oxidation
state is unstable and can quickly transform to maghemite in air, or under acidic conditions in the absence of oxygen. The main advantage of this approach is that it produces a large amount
of material, with control over particle size (2–20 nm) and shape afforded by adjusting pH, ionic strength and the concentration of the growth solution. In addition, the nanoparticles can be
functionalized _in situ_ using additives such as organic compounds (_e.g._ sodium citric) or polymers (_e.g._ dextran, polyvinyl alcohol). The magnetic properties of the nanoparticles can
also be tailored. For instance, the saturation magnetization varies with the concentration of salt (NaCl) used in the synthesis (63–71 emu/g for magnetite). Another method, high-temperature
decomposition of organo-metallic precursors (_e.g._ Fe(CO)5) in organic solvents, offers improved control over the size and shape of IONPs5. The IONP size (3–19 nm) can be tuned by the
choice of precursor and temperature. Shape control (_i.e._ yielding spherical particles) is also enhanced using the decomposition process. The IONPs produced by decomposition are coated with
hydrophobic compounds to facilitate stabilization in organic solvents, however this reduces their stability in aqueous biological environments. The magnetic properties of IONPs can be tuned
by the incorporation of other metals, such as cobalt, nickel and manganese, into the material6. Another way to synthesize IONPs is by flame spray pyrolysis7, yielding a wide range of IONPs,
such as magnetite, maghemite and wustite (FeO) particles. The iron oxidation state can be controlled by varying the fuel-to-air ratio during combustion, as well as by varying the valence
state of the iron precursor (Fe(CO)5, Fe(NO3)3, etc.). Flame spray pyrolysis presents useful advantages: it can produce fine particles (6–50 nm) on a large scale and can be easily scaled up
using industrial plant (several grams per hour), it limits the presence of impurities on the nanoparticles, and it can be extended to produce different nanoparticles (TiO2, ZrO2, silica,
etc.) as well as hybrid particles (_e.g._ silica-IONPs)8. At low temperature, IONPs self-organize in solution; all magnetic spins align resulting in ferromagnetism. When the temperature is
sufficiently high, thermal energy can invert the direction of magnetic spin, disrupting spin alignment. Above this temperature (the blocking temperature), the nanoparticle ensemble loses its
magnetic properties. The application of an external magnetic field induces the spontaneous reorganization of magnetic spin directions and re-magnetizes the IONPs. If the magnetic field is
removed, the particles lose their spin alignment, liberating heat. Ferromagnetism is essential for the synthesis of stable colloidal IONP suspensions, as the non-alignment of spin limits the
inter-particle attraction that would otherwise occur. Superparamagnetism is exhibited by very small particles. The saturation magnetization or magnetization (_i.e._ _M_s, units of emu/g),
defined by the alignment of all magnetic spins in a sample, decreases with IONP size9. The magnetic spins of atoms close to the surface are less well organized than for atoms in the bulk of
the particle near the core (Figure 2). This phenomenon is referred to as ‘spin canting’. In addition, high crystallinity results in higher saturation magnetization9. To improve _M_s, the
addition of dopants, such as manganese, has been reported6. Magnetite (92 emu/g) has a higher _M_s value than maghemite (78 emu/g) for a similar particle size10. An important property of
IONPs, or magnetic nanoparticles in general, is their ability to accelerate the MRI relaxation processes of surrounding water protons, resulting in enhanced MRI contrast. Briefly, in MRI, an
external magnetic field is applied to the IONPs, resulting in the alignment of nanoparticle magnetic spin, inducing a magnetic dipole moment. The protons of water molecules within the
magnetic field of the nanoparticles have a magnetic relaxation time significantly different to that of water molecules outside the IONPs’ magnetic field. An accumulation of IONPs at a
specific site therefore produces a significant enhancement in contrast in an MR image. Magnetism can also be exploited to exert control over the biodistribution of IONPs. Several successful
examples of magnetic targeting have been reported in the literature, as discussed later. MODIFICATION OF IONPS ‘Naked’ IONPs are not stable in water (at neutral pH) or in physiological
fluids, tending to agglomerate and precipitate quickly. Either steric or electrostatic stabilization of the IONPs is required to ensure stable aqueous dispersions. A range of different
functionalities and approaches can be used to attain colloidal stability. ANCHORING FUNCTIONALITY The presence of hydroxyl groups, such as Fe-OH, on the IONP surfaces provides a versatile
synthetic handle allowing attachment of different functionalities. A range of chemistries can be used to stabilize metal nanoparticles, exploiting electrostatic, hydrophobic, chelating and
covalent interactions (Figure 3). One of the most commonly used surface modification techniques is the attachment of alkoxysilane compounds. Silane can be covalently attached onto IONP
surfaces by reaction of the surface Fe-OH group with the Si-OCH3 moiety11. Further cross-linking events produce a thin inorganic silica layer around the particles12. However, during this
cross-linking process, the irreversible formation of aggregates can be observed, limiting the use of the resultant IONPs. The functionalization of IONPs using silane chemistry is versatile,
involving the use of many functional silanes available commercially, such as alcohol, amine and thiol, which are useful for further biofunctionalization using small biocompounds12 and
carbohydrates13. Another important and widely employed functionality for the modification of IONP surfaces is the carboxylic acid group, which can interact with the surface of IONPs by
coordination processes. The –COOH group has been employed for IONP synthesis in organic solvents (oleic acid). Citric acid has been used commercially for the stabilization of IONPs, such as
in the MRI contrast agent VSOP C184. However, the –COOH/IONP coordination bond is labile and can be broken easily by increasing temperature or by exchange with another carboxylic acid
compound. Phosphonic acid also shows a strong affinity for IONP surfaces through the formation of Fe–O–P– bonds14. These bonds are more stable than the carboxylic acid bond and have shown
stability for several weeks at neutral pH. Phosphonic acid also shows a higher grafting density than the carboxylic acid group14. Finally, dopamine can coordinate to the IONP surfaces as a
result of improved orbital overlap of the five-member ring15. This approach has been widely used to attach a range of biologically important molecules, such as peptides and amino acids, to
IONPs16. However, problems with the stability of this bond in water and biological fluids have been reported by Carpenter _et al._ after long exposure periods17. Phosphonic acid and dopamine
groups appear to improve IONP stability over a larger range of pH and temperature compared with carboxylic acid groups. STABILIZATION OF IONPS USING POLYMER CHAINS For many applications in
medicine, a polymer coating on the IONPs is preferred over simple functionalization with small organic compounds. A polymer coating provides colloidal stability in water through steric
stabilization, and can provide surface functionality allowing the possibility of designing hybrid particles with capacity for multimodal tracking, targeting, delivery and stimulated release
of therapeutic agents such as peptides, proteins and DNA/RNA. GRAFTING ‘ONTO’ VERSUS ‘FROM’. Polymer attachment to the IONP surface can be achieved via two alternative approaches: grafting
‘onto’ and ‘from’. In the case of grafting ‘from’, an initiator is fixed to the surface of the IONPs and the polymer is grown from the surface, while in the grafting ‘onto’ approach, a
functional, pre-formed polymer is grafted onto IONPs _in situ_. Each of these approaches has advantages and disadvantages. It is well known that grafting ‘from’ yields a higher grafting
density than the grafting ‘onto’ approach. However, grafting ‘onto’ allows control of polymer architecture and functionality, and is therefore more versatile than the grafting ‘from’ method.
In addition, grafting ‘from’ can present difficulties in maintaining the integrity of the hybrid nanoparticles in organic solvents. However, the use of living radical polymerization, with a
carefully designed protocol, can facilitate the modification of IONPs using the grafting ‘from’ approach. For example, Hatton _et al._18 grafted several polymers from IONPs coated with an
atom transfer radical polymerization (ATRP) initiator yielding polymer/IONP hybrids. Ring-opening polymerization (ROP) has also been employed to obtain IONPs coated with linear biodegradable
poly(esters)19 or hyper-branched polymers20. Finally, a grafting ‘through’ method has been described using IONPs coated with methacrylic bonds to yield well-dispersed particle solutions21.
STABILIZATION OF IONPS USING MONOFUNCTIONAL POLYMER CHAINS VIA EXCHANGE CHEMISTRY. The attachment of polymer chains onto IONPs can be achieved using polymers with functionality capable of
binding to the IONP surface. For example, the Centre for Advanced Macromolecular Design has developed an anti-fouling polymer-coated maghemite made by grafting ‘onto’ of phosphonic
acid-terminated poly(oligoethylene glycol-acrylate) (poly(OEG-A)), yielding hybrid organic/inorganic nanoparticles that form stable dispersions in both water and fetal bovine serum22.
Stayton, Hoffman and co-workers23 used a terminal carboxylic acid group on telechelic poly(_N_-isopropylacrylamide) to modify IONPs. In a similar approach, Gao _et al._24 used
cysteine-terminated polyethylene glycol (PEG) anchored to the surface of IONPs. The presence of both carboxylic acid and thiol groups allows the anchoring of the polymers and the
simultaneous cross-linking of these polymers around the nanoparticles. This was achieved though oxidation of the thiol groups to disulfide crosslinking groups. The great advantage of this
route is that it yields nanoparticles that are stable in biological fluid and addresses the problem of the labile and unstable nature of the interaction between carboxylic acid and IONPs.
Kohler _et al._25 proposed the use of a telechelic polymer having a terminal trimethylsilyl group able to react onto IONPs and a peptide able to confer targeting properties coupled to the
other terminus of the polymer. De Palma12 showed that the silane approach allows the preparation of stable hybrid nanoparticles over a large range of pH, resulting from strong covalent
FeO–Si– bonds. Dopamine has also been used as the ligand to attach polymers onto IONPs. Dopamine-terminated PEG was fixed onto IONPs to stabilize these particles in biological fluids. The
polymer also had a terminal biotin group. The presence of biotin was exploited for the attachment of antibodies using the well-known biotin–neutravidin recognition reaction26. The recent
development of high-yield ‘click’ chemistries, such as the azide–alkyne reaction27, now provides alternative routes to stabilized IONPs. For example, Turro _et al._28 described the
stabilization of Fe2O3 nanoparticles using alkyne-terminated organophosphate or carboxylic acid groups to exchange with oleic acid on the Fe2O3 surface. The IONPs were subsequently
covalently attached to poly(_tert_-butyl acrylate) via click reactions using CuSO4. STABILIZATION OF IONPS USING FUNCTIONAL DIBLOCK POLYMERS. The attachment of polymer chains to IONPs can
also be achieved via multiple interactions between the polymer chains and the particle surface. This multiple attachment strategy should enhance the stability of the resultant hybrid
nanoparticles, and can be achieved through the use of block or random copolymers. The insertion of several functional groups along the copolymer backbone increases the number of possible
anchoring points on the IONP surface. The control of polymer architecture is a crucial factor influencing polymer aggregation. When statistical copolymers are used, the presence of multiple
groups on the backbone can result in interactions with several particles leading to flocculation. In the case of di- and multi-block polymers, the functional block should be kept relatively
short (several units) to avoid flocculation. The introduction of several attachment points on a single chain can reduce the packing density. Using this multi-interaction approach, many
different types of functionalities have been used, such as dopamine29, carboxylic acid30 and trimethyl silane31, yielding stable nanoparticles. The acid groups, not used for IONP
stabilization, were exploited for the attachment of Cy5.5 dye11 and small-molecule drugs such as doxorubicin32. MICELLE AND LIPOSOME ENCAPSULATION. Stabilization of IONPs using micelles and
liposomes has also been described in the literature. Liposomes are large, closed, tertiary structures consisting of phospholipid bilayers with sizes in the range 100–5,000 nm. IONPs can be
stabilized by hydrophobic interactions involving the phospholipids. In addition, a single liposome can encapsulate several IONPs within its hydrophobic layer. Park _et al._33 studied the
stabilization of IONPs using amphiphilic copolymers, including a triblock copolymer (Pluronic F127, BASF), a commercially available PEG functionalized with a phospholipid moiety (DSGPE-mPEG
2000, Avanti Polar) and a random terpolymer containing dodecyl methacrylate, PEG methacrylate, and methacrylic acid. These three different approaches yielded very stable inorganic/organic
IONPs with sizes below 30 nm. Nasongkia _et al._34 reported the stabilization of IONPs using micelles constructed from an amphiphilic block polymer (PEG-P(D,L-lactide)). POLYMER PROPERTIES
_In vivo_, the polymer provides an interface with biological media, dictating the stability, compatibility and system circulation time and cell uptake of the hybrid IONPs. The first
generations of polymers used for IONP stabilization were based on natural polymers, such as dextran or carbohydrate derivatives (Figure 4). These biopolymers were adopted for their ability
to interact with IONP surfaces whilst simultaneously conferring stability in blood plasma (low-fouling surfaces). Presently, all of the commercially available MRI agents are based on natural
carbohydrate polymers, such as Ferumoxtran-10 and other ferumoxides. These IONPs are referred to as monocrystalline iron-oxide nanoparticles. A limitation of using physically adsorbed
polymers to disperse IONPs is stability. Attempts to address stability issues have involved cross-linking to form cross-linked iron-oxide (CLIO) nanoparticles. The chemical modification of
CLIO nanoparticles using ammonia produces amine-functional dextran suitable for attaching biomolecules such as proteins and peptides, or hydrophobic drugs such as doxorubicin32. However,
after cross-linking the polymer shell, exhaustive purification is required to ensure complete elimination of the reactant before use _in vivo_ or _in vitro_. Polyvinylpyrrolidone (PVP),
polyvinyl alcohol (PVA) and PEG have also been used to stabilize IONPs3,21. These polymers are widely termed ‘biocompatible’, however both PVA and PVP can adsorb proteins through
hydrogen-bonding interactions35. In contrast, PEG forms anti-fouling surfaces. An alternative to the use of linear PEG is the utilization of polymers (and copolymers) of poly(oligoethylene
oxide (meth)acrylate)22,36. These brush-like polymers, short PEG chains grafted onto an acrylic backbone, offer comparable anti-fouling properties and blood compatibility to linear PEG36
with an additional synthetic advantage, as the polymers can be made using living radical polymerization. Cationically charged polymers such as polyethyleneimine (PEI)37 have also been used
for the stabilization of IONPs. However, as they interact strongly with proteins and are known to be cytotoxic, these polymers are generally not suitable for _in vivo_ applications. IONPs
stabilized with cationic polymers can, however, be used to deliver DNA or RNA38. Transfection using these IONPs can be improved using a magnetic field (magnetofection)39. PHARMACOKINETICS
AND BIODISTRIBUTION TOXICITY OF IONPS IONPs generally show low toxicity as they degrade into Fe2+ and Fe3+40. However, magnetite and maghemite nanoparticles show different toxicities41.
Magnetite has been found to display cytotoxicity as it can degrade via the Fenton reaction: H2O2 + Fe2+ → Fe3+ + HO− + HO•− generating a reactive free radical. In addition, an excess of iron
in the body is undesirable and is associated with a number of diseases; for example, in the brain, iron can induce neurodegenerative disorders (Alzheimer’s and Parkinson’s diseases)42.
However, _in vivo_ studies have shown that IONPs are relatively safe as they do not accumulate in the vital organs and are rapidly eliminated from the body. The presence of a polymer
coating, such as PEG, can also mediate IONP toxicity, as demonstrated for human fibroblasts41,43. RELATIONSHIP BETWEEN PHYSICOCHEMICAL PROPERTIES OF POLYMERS AND BLOOD CIRCULATION HALF-LIFE
Nanocarriers and MRI contrast agents need to have long blood circulation times and must evade the reticuloendothelial system to accumulate in target tissues44. The physicochemical properties
of IONPS, such as size, charge, polymer density and morphology, play a crucial role in determining their blood half-life45. Several studies have described the effect of size on the
stability of IONPs _in vivo_. Particles with sizes in the range 10–100 nm are optimal for long circulation times _in vivo_. Large particles (> 200 nm) are readily sequestered by the
phacocytic cells in the spleen46 or by the macrophage cells present in blood, while very small IONPs (< 10 nm) are rapidly removed by the renal clearance process38. Perrault and
co-workers47 examined the effects of core (hard part) size and the polymer (PEG, soft part) chain length on the biodistribution of IONPs, and found that the blood half-life decreased as the
core diameter increased; an increase in PEG molecular weight was found to cause a significant increase in circulation time. Shape is a factor in determining the stability and biodistribution
of nanoparticles48. Indeed, Decuzzi and Ferrari49 established via simulation that oblate spheroid nanoparticles show a longer circulation time than spherical nanoparticles. Recently, this
theoretical result has been validated by several studies. For instance, Muro _et al._50 compared the biostability of spheres of various diameters versus elliptical discs, showing that
elliptical discs have longer circulation times. A similar result has been reported for spherical gold nanoparticles and nanorods51; cell uptake was found to be three times higher for
spherical gold nanoparticles. Spherical particles need to have sizes smaller than 200 nm to pass through the spleen; in contrast, elliptical nanoparticles (discs) with sizes greater than
1,000 nm can pass through49. This unexpected result has been explained by auto-organization (alignment or tumbling) of non-spherical nanoparticles under the influence of flow49. However,
there is still much work needed to fully understand the role of nanoparticle shape on both their biodistribution and cell uptake. The texture of hybrid nanoparticles, including the
mechanical properties of the polymer coating, has a major role in determining protein adsorption. Ideally, the polymer layer is at the interface of the IONP surface interacting with the
biological medium, mediating protein adsorption35. Charged polymers can cause colloidal instability in biological fluids, such as plasma, through protein adsorption. Investigations into the
effect of nanoparticle surface charge (_i.e._ zeta-potential) on stability in biological media have shown that an increase in plasma protein adsorption occurs when the surface charge density
is increased. The level of protein adsorption, for any given nanoparticle charge density, will be dependent on the protein structure. Negatively charged nanoparticles bind with proteins
having isoelectric points greater than 5.5, such as IgG. In contrast, positively charged particles bind to proteins having isoelectric points less than 5.5, such as albumin35. Hydrophobic
polymers bound to nanoparticle surfaces can also interact with biological media through specific hydrophobic interactions. The amount and nature of protein adsorption to hybrid nanoparticles
is strongly influenced by the hydrophobicity of the polymer52. Nanoparticles with hydrophobic surfaces exhibit more susceptibility to opsonization, a process whereby the body tags particles
for ingestion and destruction by phagocytes, than more hydrophilic nanoparticles. Cedervall _et al._53 quantified both the nature and the amount of protein adsorbed to nanoparticles with
varying hydrophobicity, establishing that hydrophilic particles present a relatively strongly anti-fouling surface, binding only to albumin; in contrast, hydrophobic surfaces were found to
bind to several proteins, including albumin, IgG, apoliproteins and fibrinogen. One of the most efficient polymers employed in preventing protein binding on surfaces is PEG, which has been
used to limit interactions between proteins and nanoparticles surfaces54. There are two significant factors that are known to influence the efficiency of PEG-amelioration of protein binding
to surfaces: molecular weight and grafting density. Increasing the molecular weight or density of PEG chains causes a decrease in protein adsorption55. PEGylated nanoparticles show a lower
cell uptake rate by macrophages (polymorphonuclear cells), resulting in an increase in the blood circulation time31,33,36. Biocirculation of nanoparticles can thus be adversely affected by
specific protein binding. However, in some cases, the presentation of specific proteins on nanoparticle surfaces can be useful for aiding passage through biological barriers; the presence of
apolipoprotein on nanoparticles, for instance, can facilitate passage through the blood–brain barrier56. FUNCTIONALIZATION STRATEGIES PASSIVE TARGETING. Long blood-circulation time is known
to be beneficial for promoting the accumulation of nanoparticles smaller than 500 nm in tumors and at inflammatory and infection sites due to the enhanced permeability and retention (EPR)
effect, offering a mechanism for passive targeting. EPR is caused by increased permeability of the vascular system close to tumor sites and inefficient lymphatic drainage. Passive targeting
has been demonstrated for nanoparticles with sizes ranging from 10 to 500 nm57. Perrault _et al._47 studied the effect of nanoparticle size (20–100 nm) on accumulation at tumor sites and
found that particle accumulation (40–100 nm) depends only on the blood residence half-life and is independent of nanoparticle size; in contrast, for smaller particles (around 20 nm), the
accumulation depends on both factors. However, small particles have a relatively short residence time at the tumor site when compared with larger particles (> 40 nm). Small nanoparticles
therefore arrive rapidly at the tumor site, but also have a short residence time, whereas larger nanoparticles take longer to reach the tumor sites, but reside for longer in the blood. The
residence time is a key parameter for therapeutic efficiency, as the nanoparticles are initially transported by the blood, followed by passive diffusion from the blood vessel to the tumor
periphery. The distribution (and therefore efficiency) of the nanoparticles depends on both size and residence time in the vicinity of the tumor47. A different passive targeting technique
has been developed for IONPs using reticuloendothelial system clearance to facilitate imaging of specific organs, such as the spleen or liver. This targeting method relies on the rapid
uptake of IONPs by macrophages. The first commercial contrast agents (Feridex IV) exploited this targeting method to image the presence of infected tissue in the liver58. The rapid uptake of
IONPs by Kupffer cells in the liver allows differentiation of healthy tissues from tumor cells using MRI. However, this macrophage-assisted targeting method is limited to specific organs
such as the liver and spleen (both rich in macrophages) or inflamed tissues. ACTIVE TARGETING. Improved MRI resolution can also be envisaged by adopting an active targeting IONP strategy
using biological recognition events. A range of targeting compounds have been described in the literature, including peptides (RGD, NGR)22, proteins (monoclonal antibodies)59, aptamers60,
carbohydrates61 and small molecules (folic acid)24. This active-targeting approach has the potential to reduce the IONP concentration required for clinical use whilst still maintaining
sufficient MRI resolution. The attachment of targeting functionality to IONPs has been achieved using a number of synthetic strategies, including carboxylic acid-amine reactions62, click
chemistry (_i.e._ CuAAC)63, pyridyl disulfide–thiol exchange and thiol–ene reactions64. The CuAAC approach suits polymers synthesized via atom transfer radical polymerization (ATRP), while
thiol–ene or pyridyl–disulfide exchange reactions are more appropriate for the functionalization of polymers obtained using reversible addition-fragmentation transfer polymerization
(RAFT)65. RAFT polymers can be converted easily to terminal thiol66, and are amenable to subsequent diverse reactions, such as thiol–ene and pyridyl–disulfide exchange reactions66. The
accessibility of the targeting groups on IONP surfaces is important, as shown by Martin _et al._61, who found that differences in accessibility induced different biological responses.
Targeting moieties (_i.e._ lactose) fixed on a dendrimer presented improved exposure and binding (by 1–2 orders of magnitude) compared to monofunctional lactose terminated polymers. An
enhanced availability of dendritic molecules on the surface prevents interment of the lactose within the polymer coating. In addition, the fixed presentation of the targeting moieties by the
dendritic structure enhances binding via a concentration effect known as multivalency. The study by Martin _et al._61 proves that the binding affinity of carbohydrate ligands when presented
on polymer surfaces can be significantly enhanced through the use of dendritic scaffolds. However, the importance of multivalent interactions depends to some extent on the specific
biological binding event, and therefore some caution should be exercised in extending the conclusions from the study of Martin _et al._ to all targeting interactions. Combining a targeting
agent with a fluorescence label on nanoparticles produces multi-functional and multi-modal nanoparticles that have both a ‘tracking signal’ and a high affinity for specific targeted cell
types. For example, Wang _et al._67 modified IONPs with a poly(amidoamine) dendrimer, following by the attachment of folic acid, producing IONPs that were readily taken up by KB tumor cells
with folic acid receptor expression (KB-HFAR). APPLICATIONS IONPs can be employed in many applications, and in medicine are primarily applied as contrast agents in MRI and in drug and gene
delivery68. IONPs are also used in hyperthermia therapy, a treatment by which body tissue is exposed to (locally) high temperatures thereby damaging and killing cancer cells. The fundamental
magnetic properties of IONPs are pivotal for all these applications. The use of IONPs in MRI and drug and gene delivery is described in more detail below. MAGNETIC RESONANCE IMAGING MRI was
developed in 1973 and has since become one of the most powerful non-invasive techniques used in clinical medicine, allowing the internal structure and function of the body to be
visualized69. MRI utilizes the magnetic spins of hydrogen nuclei aligned by a powerful external magnetic field. A radiofrequency pulse disturbs the spin alignment from equilibrium, and the
spin relaxation back to equilibrium is monitored at high temporal resolution. Two independent relaxation processes occur in MRI: longitudinal relaxation denoted _T_1, and transverse
relaxation denoted _T_2. These two relaxation processes can be monitored independently to generate different MR images, such as _T_1-, _T_2- and _T_2*-weighted images. Local fluctuations in
proton spin density, mainly attributed to water molecules and caused by variations in the biological environment, affect the relaxation responses from which images can be constructed.
Improvement in MRI contrast can be effected using magnetic nanoparticles to shorten the relaxation times (_T_1 and _T_2). IONPs influence the _T_2 time for water, while gadolinium-modified
nanoparticles (another type of contrast agent for MRI) influence the longitudinal relaxation (_T_1)70. These two relaxation modes yield different MR images. The efficacy of a nanoparticle
contrast agent can be characterized by measuring the relaxivity, _R_1 and _R_2, of water protons surrounding the nanoparticle. The relaxivity is inversely proportional to the individual
measured relaxation rates (_R_1 = 1/_T_1, _R_2 = 1/_T_2) over a range of contrast concentrations. The magnetic properties of the iron cores (magnetic saturation) influence the values of both
_R_1 and _R_2. A higher _R_2/_R_1 ratio improves the contrast. Iron-oxide contrast agents such as Resovist and Endorem have relaxivities in the range of 150–160 s−1 per millimole. The
quality of a contrast agent (_in vivo_) depends on the IONP core, and also on its ability to evade the reticuloendothelial system clearance mechanism, its stability in biological fluids and
its ability to concentrate at the target area. The polymer coating plays a significant role in tuning the properties of IONPs thereby mediating the interface between the IONP surface and the
biological medium. The ways in which the design of the polymer layer can affect the MRI properties of the hybrid nanoparticles are discussed below. The nature of the ligand, the linker
between IONPs and polymer, can affect the organization of magnetic spins in the IONPs, resulting in modified magnetic properties71. Daou and co-workers72 compared the effect of two different
ligand types, phosphonate and carboxylate, on the magnetic properties of IONPs, showing that phosphonate ligands yielded superior magnetic properties due to the absence of spin canting in
the IONP layer close to the interface. The chain length of polymers may also have an effect on the relaxivity properties of the nanoparticles. The magnitude of MRI relaxivity is dependent on
the number of water molecules disturbed by the magnetic field generated by the IONPs, which may be influenced by the thickness and nature of the polymer layer. Laconte _et al._73 reported a
decrease in _R_2 with increasing chain length (or molecular weight) of the polymer coating. Duan _et al._74 examined the effect of polymer hydrophobicity on the magnetic properties
(relaxivities) of IONPs, and found that the hydrophobic polymers diminished the relaxivity (_R_2) behavior. In contrast, IONPs with a core size of approximately 10 nm coated with PEI, a
hydrophilic charged polymer, presented significantly enhanced relaxivity. A similar result was also observed for IONPs with a core size greater than 30 nm, although the difference was less
significant. DRUG AND GENE DELIVERY Several reports have been published on the use of IONPs as nano-carriers for drug and gene delivery75. The presence of a magnetic core offers the promise
of targeting specific organs within the body76. Magnetic focusing can be exploited to concentrate the IONPs in the desired area such that the accumulation of IONPs can exaggerate the EPR
effect. In addition, the iron-oxide core can be engineered to liberate toxic organic compounds by the introduction of platinum inside the IONP cores77. The polymer coating can also now be
exploited, not just for stabilizing the IONPs in biological media, but also as a scaffold (reservoir) for the drug or gene cargo. The loading and release of bioactive materials from the
polymer coating then becomes a significant parameter dictating the efficiency of IONPs as nano-carriers. Therapeutics can be conjugated to the polymer chains using a number of alternative
approaches: covalent coupling, charge complexation, hydrogen bonding or hydrophobic/hydrophilic interactions. The therapeutic agent can also be attached directly onto the IONP surface.
Gendeli and coworkers78 covalently attached an anti-cancer drug (amptothecin) to USPIOs (core diameter, 9–10 nm; hydrodynamic diameter, 52 nm) coated with polyvinylalcohol/polyvinylamine
using a biodegradable linkage. The magnetic properties of the nanoparticles were exploited to enhance cell uptake. Yu _et al._32 developed IONPs bearing (poly(OEG-A)) that are able to
conjugate doxorubicin via a pH-sensitive bond. Doxorubicin release could be mediated by manipulating pH, whilst the magnetic properties of the IONPs were completely preserved. Hydrophobic
interactions between polymers and drugs have also been exploited, as demonstrated by Nasongkia and co-workers34, who loaded doxorubicin into hydrophobic cores stabilized with a hydrophilic
shell and decorated with targeting agents (RGD peptide). Drug release was controlled by degradation of the poly(D,L-lactide) cores and pH control. The presence of a targeting agent was shown
to improve the delivery of doxorubicin to specific cells (Figure 5). Gong _et al._79 created microcapsules of around 50 μm in size using a microfluidic process, yielding shells comprised of
IONPs embedded in a dextran layer surrounding a core loaded with aspirin. On application of an alternating magnetic field, the shell was deformed, releasing the aspirin. Liu _et al._80
exploited the ability of IONPs to generate heat when they are subjected to a high-frequency oscillating magnetic field. In their work, IONPs were coated with a thermo-responsive anti-fouling
polymer (pluronic PEG-_b_-PEO-_b_-PEG), which was cross-linked using gelatin. Drugs were encapsulated in the polymer shell using hydrophobic interactions and then released by induced
heating stimulated by the magnetic field. DNA- and siRNA-based therapies have shown great promise for the treatment of disease81. However, realizing the potential in practice is not
straightforward as the nucleotide therapeutics need to be protected from enzymatic degradation during circulation. Various carriers have been proposed, including viruses, polymers
nanoparticles, bioconjugates and organic/ inorganic particles82. The development of nano-carriers for siRNA has received particular attention82. In 2009, several publications described the
use of organic/inorganic nanoparticles (_e.g._ gold and IONPs) for siRNA delivery. The application of hybrid metal nanoparticles to gene therapy has some advantages over other delivery
methods. Firstly, fixed shapes and topologies can be engineered, and secondly, the presence of inorganic cores with magnetic (_e.g._ IONPs) or optical (gold nanoparticles) properties permits
tracking of hybrid nanoparticles _in vitro_. The conjugation of oligonucleotides to carrier nanoparticles can be achieved using electrostatic interactions with cationic polymers or by
covalent attachment either to the polymer83 or directly to the nanoparticle84. A number of cationic polymers (_e.g._ poly(ethylene imine)85 and poly(dimethyaminoethyl (meth)acrylate)86) have
been grafted onto IONPs and used for conjugation with siRNA via electrostatic interactions. Pan _et al._85 developed PEI-coated IONPs for the encapsulation of genes and demonstrated
successful _in vitro_ delivery. Several other studies have also demonstrated the feasibility of using hybrid nanoparticles for the delivery of DNA or siRNA _in vitro_. Magnetic cores have
been exploited for enhanced transfection under a magnetic field (magnetofection)37. Kamau _et al._86 functionalized IONPs with PEI for gene delivery applications and studied their
transfection efficiency under an applied magnetic field _in vitro_. On exposure to permanent and oscillating magnetic fields, the _in vitro_ transfection efficiency was 40 times higher than
in the absence of a magnetic field. However, the presence of cationic polymer on the surface of these nanoparticles may cause severe problems if the nanoparticles were to be used _in vivo_,
as significant protein adsorption would occur in biological media. An enhanced design of IONPs for gene delivery has been proposed by the Centre for Advanced Macromolecular Design based on a
mixed polymer layer approach using both charged (cationic poly(dimethylaminoethyl acrylate)) and neutral (poly(OEG-A)) polymers co-grafted onto IONPs to yield uncharged particles (Figure
6)87. The presence of positive charges within the polymer layer facilitates complexation with siRNA, and the siRNA/particle complexes display excellent stability in fetal bovine serum and
have an effect on transfection _in vitro_, both with and without a magnetic field. Medarova _et al._83 used a different strategy to deliver siRNA with IONPs based on the use of multimodal
IONPs. First, IONPs were coated with a dextran polymer layer bearing amine groups. A peptide (myristoylated polyarginine peptide) serving as a membrane translocation module and an optical
label (Cy5.5, used for near-infrared _in vivo_ optical imaging) were then fixed on the dextran by traditional coupling chemistry. The residual amines not used for the coupling process were
then accessed and used for the attachment of siRNA. This platform allows the simultaneous delivery of siRNA and particle tracking _in vivo_ through a combination of near-infrared optical
imaging and MRI. Lee _et al._84 attached siRNA directly onto IONP surfaces using disulfide bonds. To enhance the solution stability of these nanoparticles _in vivo_, a thiol-functionalized
PEG was also grafted to the nanoparticle surface. Targeting and enhanced tracking were achieved using a targeting agent (RGD peptide) and a label (Cy5.5 for near-infrared _in vivo_ optical
imaging). CONCLUSION This review presented an overview of the synthesis, surface modification and use of IONPs for biological applications. Extremely versatile and multi-functional
nanoparticles can be made by combining the favorable properties of the metal cores with the functionality of polymer shells. The polymers can confer many excellent properties to the IONPs,
including stabilization in biological fluids, targeting and drug and gene portability and delivery, and these benefits can be combined with the MRI properties of the iron cores. REFERENCES *
M. De, P. S. Ghosh, V. M. Rotello, _Adv. Mater._ 20, 4225 ( 2008 ). Article CAS Google Scholar * Y.-W. Jun, J.-H. Lee, J. Cheon, _Angew. Chem. Int. Ed._ 47, 5122 ( 2008 ). Article CAS
Google Scholar * S. Laurent et al., _Chem. Rev._ 108, 2064 ( 2008 ). Article CAS Google Scholar * J. Gao, H. Gu, B. Xu, _Acc. Chem. Res._ 42, 1097 ( 2009 ). Article CAS Google Scholar
* S. Peng, C. Wang, J. Xie, S. Sun, _J Am. Chem Soc._ 128, 10676 ( 2006 ). Article CAS Google Scholar * S. Sun et al., _J. Am. Chem. Soc._ 126, 273 ( 2004 ). Article CAS Google
Scholar * W. Y. Teoh et al., _NSTI Nanotech 2007_ 4, 187 ( 2007 ). CAS Google Scholar * D. Li et al., _Chem. Mater._ 18, 6403 ( 2006 ). Article CAS Google Scholar * J.-W. Jun et al.,
_J. Am. Chem. Soc._ 127, 5732 ( 2005 ). Article CAS Google Scholar * A. K. Gupta, M. Gupta, _Biomater._ 26, 3995 ( 2005 ). Article CAS Google Scholar * H. Lee et al., _J. Am. Chem.
Soc._ 129, 12739 ( 2007 ). Article CAS Google Scholar * R. De Palma et al., _Chem. Mater._ 19, 1821 ( 2007 ). Article CAS Google Scholar * K. El-Boubbou, C. Gruden, X. Huang, _J. Am.
Chem. Soc._ 129, 13392 ( 2007 ). Article CAS Google Scholar * Y. Sahoo et al., _Langmuir_ 17, 7907 ( 2001 ). Article CAS Google Scholar * L. Wang et al., _J. Am. Chem. Soc._ 128, 13358
( 2006 ). Article CAS Google Scholar * K. Cheng, S. Peng, C. Xu, S. Sun, _J. Am. Chem. Soc._ 131, 10637 ( 2009 ). Article CAS Google Scholar * M. D. Shultz, J. U. Reveles, S. N.
Khanna, E. E. Carpenter, _J. Am. Chem. Soc._ 129, 2482 ( 2007 ). Article CAS Google Scholar * M. Lattuada, T. A. Hatton, _Langmuir_ 23, 2158 ( 2007 ). Article CAS Google Scholar * J.
Tian, Y. K. Feng, Y. S. Xu, _Macromol. Res._ 14, 209 ( 2006 ). Article CAS Google Scholar * L. Wang, K. G. Neoh, E. T. Kang, B. Shuter, S.-C. Wang, _Adv. Funct. Mater._ 19, 2615 ( 2009 ).
Article CAS Google Scholar * C. Flesch et al., _Macromol. Rapid Commun._ 26, 1494 ( 2005 ). Article CAS Google Scholar * C. Boyer et al., _J. Mater. Chem._ 19, 111 ( 2009 ). Article
CAS Google Scholar * R. Narain, M Gonzales, A. S. Hoffman, P. S. Stayton, K. M. Krishnan, _Langmuir_ 23, 6299 ( 2007 ). Article CAS Google Scholar * G. Huang et al., _J. Mater. Chem._
19, 6367 ( 2009 ). Article CAS Google Scholar * N. Kohler, G. E. Fryxell, M. Zhang, _J. Am. Chem. Soc._ 126, 7206 ( 2004 ). Article CAS Google Scholar * E. Amstad et al., _Small_ 5,
1334 ( 2009 ). Article CAS Google Scholar * C. D. Hein, X.-M. Liu, D. Wang, _Pharm. Res._ 25, 2216 ( 2008 ). Article CAS Google Scholar * M. A. White, J. A. Johnson, J. T. Koberstein,
N. J. Turro, _J. Am. Chem. Soc._ 128, 11356 ( 2006 ). Article CAS Google Scholar * M. I. Shukoor et al., _Chem. Mater._ 20, 3567 ( 2008 ). Article CAS Google Scholar * F Zhang, C.-C.
Wang, _Langmuir_ 25, 8255 ( 2009 ). Article CAS Google Scholar * H. Lee et al., _J. Am. Chem. Soc._ 128, 7383 ( 2006 ). Article CAS Google Scholar * M. K. Yu et al., _Angew. Chem. Int.
Ed._ 47, 5362 ( 2008 ). Article CAS Google Scholar * J. Park et al., _J. Mater. Chem._ 19, 6412 ( 2009 ). Article CAS Google Scholar * N. Nasongkla et al., _Nano Lett._ 6, 2427 ( 2006
). Article CAS Google Scholar * P. Aggarwala, J. B. Halla, C. B. McLelanda, M. A. Dobrovolskaia, S. E. McNeila, _Adv. Drug Deliv. Rev._ 61, 428 ( 2009 ). Article CAS Google Scholar *
G. Prencipe et al., _J. Am. Chem. Soc._ 131, 4783 ( 2009 ). Article CAS Google Scholar * X. Wang, L. Zhou, Y. Ma, X. Li, H. Gu, _Nano Res._ 2, 365 ( 2009 ). Article CAS Google Scholar
* H. S. Choi et al., _Nature Biotechnol._ 25, 1165 ( 2007 ). Article CAS Google Scholar * O. Mykhaylyk, Y. S. Antequera, D. Vlaskou, C. Plank, _Nature Protoc._ 2, 2391 ( 2007 ). Article
CAS Google Scholar * A. Nel, T. Xia, L. Mädler, N Li, _Science_ 311, 622 ( 2006 ). Article CAS Google Scholar * A. K. Gupta, A. S. G. Curtis, _J. Mat. Sci.: Mat. Med._ 15, 493 ( 2004 ).
CAS Google Scholar * P. M. Doraiswamy, A. E. Finefrock, _Lancet Neurol._ 3, 431 ( 2004 ). Article CAS Google Scholar * Y. Wang et al., _Adv. Funct. Mater._ 18, 308 ( 2008 ). Article
CAS Google Scholar * S. M. Moghimi, A. C. Hunter, J. C. Murray, _Pharmacol. Rev._ 53, 283 ( 2001 ). CAS Google Scholar * F. Alexis, E. Pridgen, L. K. Molnar, O. C. Farokhzad, _Mol.
Pharmaceutics_ 5, 505 ( 2008 ). Article CAS Google Scholar * Y. Tabata, Y Ikada, _Adv. Polym. Sci._ 94, 107 ( 1990 ). Article CAS Google Scholar * S. D. Perrault, C. Walkey, T.
Jennings, H. C. Fischer, W. C. W. Chan, _Nano Lett._ 9, 1909 ( 2009 ). Article CAS Google Scholar * S. Mitragotri, _Pharm. Res._ 26, 232 ( 2009 ). Article CAS Google Scholar * P.
Decuzzi, M. Ferrari, _Biomater._ 27, 5307 ( 2006 ). Article CAS Google Scholar * S. Muro et al., _Mol. Therap._ 16, 1450 ( 2008 ). Article CAS Google Scholar * B. D. Chithrani, A. A.
Ghazani, W. C. W. Chan, _Nano Lett._ 6, 662 ( 2006 ). Article CAS Google Scholar * D. E. Owens, N. A. Peppas, _Int. J. Pharm._ 307, 93 ( 2006 ). Article CAS Google Scholar * T.
Cedervall et al., _Angew. Chem. Int. Ed._ 46, 5754 ( 2007 ). Article CAS Google Scholar * H. R. Kim et al., _Electrophoresis_ 28, 2252 ( 2007 ). Article CAS Google Scholar * R. Gref et
al., _Colloids Surf., B_ 18, 301 ( 2000 ). Article CAS Google Scholar * T. M. Göppert, R. H. Müller, _J. Drug Target._ 13, 179 ( 2005 ). Article CAS Google Scholar * D. Peer et al.,
_Nature Nanotech._ 2, 751 ( 2007 ). Article CAS Google Scholar * D. D. Stark et al., _Radiology_ 168, 297 ( 1988 ). Article CAS Google Scholar * P. Carter, _Nature Rev. Cancer_ 1, 118
( 2001 ). Article CAS Google Scholar * F. Gu et al., _PNAS_ 105, 2586 ( 2008 ). Article CAS Google Scholar * A. L. Martin, B. Li, E. R. Gillies, _J. Am. Chem. Soc._ 131, 734 ( 2009 ).
Article CAS Google Scholar * X. Shi, T. P. Thomas, L. A. Myc, A. Kotlyar, J. R. Baker, _Phys. Chem. Chem. Phys._ 9, 5712 ( 2007 ). Article CAS Google Scholar * R. A. Evans, _Aust. J.
Chem._ 60, 384 ( 2007 ). Article CAS Google Scholar * K. L. Killops, L. M. Campos, C. J. Hawker, _J. Am. Chem. Soc._ 130, 5062 ( 2008 ). Article CAS Google Scholar * C. Boyer et al.,
_Chem. Rev._ 109, 5402 ( 2009 ). Article CAS Google Scholar * C. Boyer, V. Bulmus, T. P. Davis, _Macromol. Rapid Commun._ 30, 493 ( 2009 ). Article CAS Google Scholar * S. H. Wang et
al., _Adv. Funct. Mater._ 17, 3043 ( 2007 ). Article CAS Google Scholar * M. Namdeo et al., _J. Nanosci. Nanotech._ 8, 3247 ( 2008 ). Article CAS Google Scholar * K. N. Raymond, V. C.
Pierre, _Bioconjugate Chem._ 16, 3 ( 2005 ). Article CAS Google Scholar * Y.-X. Wang, S. M. Hussain, G. P. Krestin, _Eur. Radiol._ 11, 2319 ( 2001 ). Article CAS Google Scholar * C. R.
Vestal, Z. J. Zhang, _J. Am. Chem. Soc._ 125, 9828 ( 2003 ). Article CAS Google Scholar * T. J. Daou et al., _Chem. Mater._ 20, 5869 ( 2008 ). Article CAS Google Scholar * L. E. W.
LaConte et al., _J. Magn. Res. Imag._ 26, 1634 ( 2007 ). Article Google Scholar * H. Duan et al., _J. Phys. Chem. C_ 112, 8127 ( 2008 ). Article CAS Google Scholar * J. Dobson, _Gene
Therapy_ 13, 283 ( 2006 ). Article CAS Google Scholar * L. Zhang et al., _Clin. Pharmacol. Ther._ 83, 761 ( 2008 ). Article CAS Google Scholar * J. Gao et al., _J. Am. Chem. Soc._ 130,
11828 ( 2008 ). Article CAS Google Scholar * F. Cengelli et al., _ChemMedChem_ 4, 988 ( 2009 ). Article CAS Google Scholar * X. Gong, S. Peng, W. Wen, P. Sheng, W. Li, _Adv. Funct.
Mater._ 19, 292 ( 2009 ). Article CAS Google Scholar * T.-Y. Liu, K.-H. Liu, D.-M. Liu, S.-Y. Chen, I.-W. Chen, _Adv. Funct. Mater._ 19, 616 ( 2009 ). Article CAS Google Scholar * D.
S. Shewach, R. D. Kuchta, _Chem. Rev._ 109, 2859 ( 2009 ). Article CAS Google Scholar * A. Akinc et al., _Nature Biotech._ 26, 561 ( 2008 ). Article CAS Google Scholar * Z. Medarova,
W. Pham, C. Farrar, V. Petkova, A. Moore, _Nature Med._ 13, 372 ( 2007 ). Article CAS Google Scholar * J.-H. Lee et al., _Angew. Chem. Int. Ed._ 48, 4174 ( 2009 ). Article CAS Google
Scholar * B. Pan et al., _Cancer Res._ 67, 8156 ( 2007 ). Article CAS Google Scholar * S. W. Kamau et al., _Nucl. Acids Res._ 34, e 40 ( 2006 ). Article CAS Google Scholar * C. Boyer
et al., _J. Mater. Chem._ 20, 255 ( 2010 ). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by an Australian Research Council Discovery grant
(DP1092640) (T. P. Davis, C. Boyer), and a Federation Fellowship (T. P. Davis). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Centre for Advanced Macromolecular Design, School of Chemical
Sciences and Engineering, University of New South Wales, Sydney, NSW 2052, Australia Cyrille Boyer, Michael R Whittaker, Jingquan Liu & Thomas P Davis * School of Biotechnology and
Biomolecular Sciences, University of New South Wales, Sydney, NSW 2052, Australia Volga Bulmus Authors * Cyrille Boyer View author publications You can also search for this author inPubMed
Google Scholar * Michael R Whittaker View author publications You can also search for this author inPubMed Google Scholar * Volga Bulmus View author publications You can also search for this
author inPubMed Google Scholar * Jingquan Liu View author publications You can also search for this author inPubMed Google Scholar * Thomas P Davis View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Cyrille Boyer or Thomas P Davis. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Boyer, C., Whittaker, M., Bulmus, V. _et al._ The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. _NPG Asia Mater_ 2, 23–30
(2010). https://doi.org/10.1038/asiamat.2010.6 Download citation * Published: 21 January 2010 * Issue Date: January 2010 * DOI: https://doi.org/10.1038/asiamat.2010.6 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative