Play all audios:
The Follicular Lymphoma International Prognostic Index (FLIPI) and the Follicular Lymphoma International Prognostic Index-2 (FLIPI-2) have been widely used as models for predicting outcomes
in follicular lymphoma (FL) based on clinical parameters.1, 2 However, host immunity and tumor microenvironment are not taken into account by either FLIPI or FLIPI-2, which have been
demonstrated to remarkably influence the clinical outcomes of patients with FL. Thus, a series of studies have focused on the search for simple and effective surrogate biomarkers that are
immunologically relevant and can serve as prognostic factors. Natural killer (NK) cells are important components of the innate immune response with crucial roles in eliminating viruses,
regulating dendritic cells, and killing malignant cells.3 NK cell count is a surrogate marker of host immune status. Previously, Plonquet _et al._4 reported that the peripheral blood NK cell
count was associated with clinical outcomes of diffuse large B-cell lymphoma patients with age-adjusted International Prognostic Index scores of 2 or 3. To our knowledge, researches
regarding prognostic value of peripheral blood NK cell counts in FL are not very well established. Shafer _et al._5 found that low NK cell counts in the blood (0.15 × 109/l) as suggested in
earlier reports were associated with inferior OS by univariate analysis (_P_=0.02) and trended toward significance by multivariate analysis (_P_=0.08). To reevaluate the role of NK cell
counts in the prognosis of FL, we established this cohort study. One hundred and thirty-two patients with FL were admitted to the First Affiliated Hospital of Nanjing Medical University,
Jiangsu Province Hospital between January 2001 and October 2015, but five of them were lost to follow-up. The diagnostic criteria and clinical management strategies did not change much
during the follow-up times. All cases were pathologically confirmed as FL according to 2008 WHO classification. Complete blood cell (CBC) data were collected in the remaining 127 FL patients
upon diagnosis following an informed consent. However, only 114 patients’ peripheral blood flow cytometry (PBFCM) records at diagnosis for NK cell markers were available. Therefore, we
retrospectively reviewed these 114 patients in this study. The counts of peripheral blood NK cells were calculated from the percentages obtained by flow cytometry. NK cells were referred to
CD3-CD16+ and/or CD56+ lymphocytes. Baseline clinical characteristics were totally available, including age, gender, pathological grade, the number of nodal sites involved, bulky lesion,
bone marrow involvement, Ann Arbor stage, B symptoms, serum lactate dehydrogenase (LDH) and serum beta-2 microglobulin (β2-MG) (Table 1a). The FLIPI and FLIPI-2 were used for prognostic
stratification. High FLIPI scores (high risk) or high FLIPI-2 scores (high risk) were denoted as score ⩾3. Among the patients, 97 (85.1%) cases were treated with rituximab-containing
therapy, 9 cases (7.9%) with chemotherapy and 2 cases (1.7%) with radiotherapy. A watch and wait approach was performed at diagnosis for remaining cases (5.3%). CBC and PBFCM analysis
indicated that the median NK cell counts at diagnosis were 0.17 × 109/l (range, 0.03 × 109/l−5.08 × 109/l). All _P_-values represented were two-sided, and statistical significance was
declared at _P_<0.05. The patients’ clinical parameters were analyzed for possible interactions with the level of NK cell counts at diagnosis by using Mann–Whitney _U_-test, but no
significant correlation was observed among any groups (Table 1a). Until December 2015, with a median follow-up of 23 months (range, 1–116 months), the median progression-free survival (PFS)
and overall survival (OS) were not reached. The correlation between clinical features and PFS or OS has been analyzed by univariate and multivariate analyses. In this cohort, we analyzed
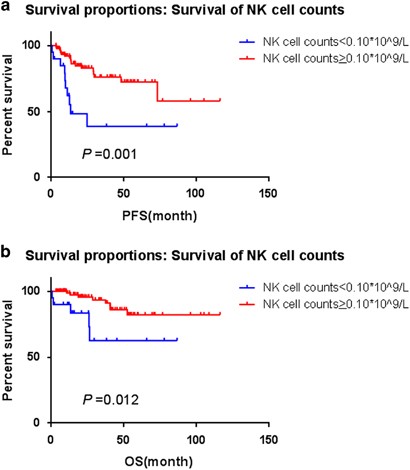
different NK cell counts cutoff points by X-tile. The most discriminative cutoff point was determined to be 0.10 × 109/l for FL because it yielded the greatest difference in PFS and OS.
After dichotomization by the optimal cutoff levels, NK cell counts <0.10 × 109/l (low group) predicted for shorter time to progression (_P_=0.001) (Figure 1a) and worse OS (_P_=0.012) in
Kaplan–Meier method (Figure 1b). The median PFS was 13.3 months in patients with low NK cell counts group (<0.10 × 109/l), but not reached in those with high NK cell counts group (⩾0.10 ×
109/l), whereas the median OS were not reached in both groups. There were five deaths per group (low versus high NK cells), which may be a coincidence owing to either small sample size or
relatively short follow-up. The causes of death mainly lay in lymphoma progression, severe infection, hepatic failure, respiratory failure, hemorrhage, pleura effusion, seroperitoneum
effusion and extensive involved sites. As a dichotomised variable, low NK cell counts (<0.10 × 109/l) had an association with inferior PFS and poor OS by univariate Cox regression
analysis. The analysis showed that other discrete variables were also related to lower PFS (Table 1b) or shorter OS (Table 1b). Considering FLIPI and FLIPI-2 are widely used prognostic
indices of the baseline characteristics of FL, covering proven prognostic factors such as LDH, Hb and β2-MG, only low NK cell counts, FLIPI (high vs low/int.) or FLIPI-2 (high vs low/int.)
were entered into the multivariate models, which further revealed that both low NK cell counts (<0.10 × 109/l) and high FLIPI-2 scores (⩾3), as dichotomised variables, maintained their
prognostic value for PFS and OS (Table 1c). As we know, FL cells express high levels of HLA-class I,6 which may protect themselves from being recognized by NK cells owing to HLA matching.5
Nevertheless, this NK cell-mediated cytotoxicity can be repaired partially by expression of NKG2D ligands on HLA-class I-positive cells. Therefore, there is a possibility that NK cells would
have a role in the antitumor efficacy of HLA-class I-positive malignancies including FL,5 in accordance with the result of low NK cell levels correlating with inferior outcome in FL. In
this study, NK cell counts were defined as CD3-CD16+ and/or CD56+ lymphocytes. Actually, circulating NK cells can be divided into two main subsets. They are CD56dim and CD56bright cells,
respectively. CD56bright cells do not express cytotoxicity markers, but CD56dim cells do.7 Besides, peripheral NK cells diversely express functional receptors, combination of which might
determine the antitumor ability.8 A better knowledge of these various NK cell subsets may help to deepen the understanding of crosstalks between the immune system and follicular lymphoma.
Moreover, rituximab has shown excellent antitumor activity in malignant B-cell lymphomas such as FL. The mechanism of rituximab is currently believed to act through four signaling pathways:
antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity, direct signaling triggering apoptosis, and increased sensitivity to chemotherapy.9 Most researches
exploring ADCC and rituximab have pointed toward interactions of rituximab with CD16 on NK cells.10 In this signaling pathway, rituximab recruits NK cells towards malignant B cells via CD16,
and the NK cells subsequently eliminate the malignant rituximab-coated cells. It is feasible that CD16 has a role in activating NK cells locally, and that the resulting cytokines produced
by NK cells enhances ADCC mediated by other receptors and other cells.10 Thus, this privileged mechanism of action of rituximab supports that lower NK cell counts may link to worse outcome
with a defected NK cell activity and a decreased rituximab-dependent cellular cytotoxicity.11 The results may provide potential of treatment targeting the activation of NK cells, including
rituximab, lenalidomide or their combination. Lenalidomide has already shown notable activities in relapsed and refractory FL.12 It has a profound effect on NK cells. Through expanding NK
cell numbers and enhancing NK cells activity as well as NK-mediated ADCC, the mechanism of lenalidomide action comprises both acquired and innate antitumor immune response.13 Moreover,
considerable attention has been given to cell therapy. NK-92 cells are reported to have high-selective killing effects against various cancer cells, including myeloma, leukemia, melanoma and
breast cancer, in preclinical or clinical setting.14, 15 Whether it would have efficacy or not in FL remains unknown. According to traditional prognostic scoring, FLIPI was significantly
associated with shorter survival by univariate analysis, but not maintained the prognostic values in multivariate analysis, which was probably due to the small sample size of the present
study, relatively short follow-up or their not reflecting immune systemic mechanisms and the microenvironment. In conclusion, the baseline peripheral blood NK cell count obtained at
diagnosis may represent as an effective biomarker in clinical practice for host immune homeostasis and the tumor microenvironment in FL. Furthermore, this could become the foundation for
development of novel therapeutic agents targeting the activation of NK cells. REFERENCES * Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R _et al_. Follicular
lymphoma international prognostic index. _Blood_ 2004; 104: 1258–1265. Article Google Scholar * Federico M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, Vitolo U _et al_.
Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. _J Clin
Oncol_ 2009; 27: 4555–4562. Article Google Scholar * McDaniel JM, Pinilla-Ibarz J, Epling-Burnette PK . Molecular action of lenalidomide in lymphocytes and hematologic malignancies. _Adv
Hematol_ 2012; 2012: 513702. Article Google Scholar * Plonquet A, Haioun C, Jais JP, Debard AL, Salles G, Bene MC _et al_. Peripheral blood natural killer cell count is associated with
clinical outcome in patients with aaIPI 2-3 diffuse large B-cell lymphoma. _Ann Oncol_ 2007; 18: 1209–1215. Article CAS Google Scholar * Shafer D, Smith MR, Borghaei H, Millenson MM, Li
T, Litwin S _et al_. Low NK cell counts in peripheral blood are associated with inferior overall survival in patients with follicular lymphoma. _Leuk Res_ 2013; 37: 1213–1215. Article
Google Scholar * Schultze JL, Cardoso AA, Freeman GJ, Seamon MJ, Daley J, Pinkus GS _et al_. Follicular lymphomas can be induced to present alloantigen efficiently: A conceptual model to
improve their tumor immunogenicity. _Proc Natl Acad Sci USA_ 1995; 92: 8200–8204. Article CAS Google Scholar * Ferlazzo G, Thomas D, Lin SL . The abundant NK cells in human secondary
lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. _J Immunol_ 2004; 172: 1455–1462. Article CAS Google Scholar * Moretta L, Moretta A .
Unravelling natural killer cell function: triggering and inhibitory human NK receptors. _EMBO J_ 2004; 23: 255–259. Article CAS Google Scholar * Plosker GL, Figgitt DP . Rituximab: a
review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. _Drugs_ 2003; 63: 803–843. Article CAS Google Scholar * Weiner GJ . Rituximab: mechanism of action.
_Semin Hematol_ 2010; 47: 115–123. Article CAS Google Scholar * Kim JK, Chung JS, Shin HJ, Song MK, Yi JW, Shin DH _et al_. Influence of NK cell count on the survival of patients with
diffuse large B-cell lymphoma treated with R-CHOP. _Blood Res_ 2014; 49: 162–169. Article Google Scholar * Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G _et al_. Lenalidomide
oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. _J Clin Oncol_ 2009; 27: 5404–5409. Article CAS Google Scholar * Wu L, Adams
M, Carter T, Chen R, Muller G, Stirling D _et al_. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor
cells. _Clin Cancer Res_ 2008; 14: 4650–4657. Article CAS Google Scholar * Tonn T, Becker S, Esser R, Schwabe D, Seifried E . Cellular immunotherapy of malignancies using the clonal
natural killer cell line NK-92. _J Hematother Stem Cell Res_ 2001; 10: 535–544. Article CAS Google Scholar * Yan Y, Steinherz P, Klingemann HG, Dennig D, Childs BH, McGuirk J _et al_.
Antileukemia activity of a natural killer cell line against human leukemias. _Clin Cancer Res_ 1998; 4: 2859–2868. CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This
study was supported by National Natural Science Foundation of China (30971296, 81170485, 81170488, 81370657 and 81470328), Key Projects of the Health Department of Jiangsu Province
(K201108), Jiangsu Province’s Medical Elite Programme (RC2011169), the National Public Health Grand Research Foundation (201202017), a project funded by the Priority Academic Programme
Development of Jiangsu Higher Education Institute (JX10231801), Project of National Key Clinical Specialty, the National Science & Technology Pillar Programme (2014BAI09B12) and a
project funded by Jiangsu Provincial Special Programme of Medical Science (BL2014086). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Hematology, the First Affiliated Hospital
of Nanjing Medical University, Jiangsu Province Hospital, Nanjing, China L He, H-Y Zhu, S-C Qin, Y Li, Y Miao, J-H Liang, Y Xia, Y Wang, Y-J Wu, L Wang, L Fan, J-Y Li & W Xu *
Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, China L He, H-Y Zhu, S-C Qin, Y Li, Y Miao, J-H Liang, Y Xia, Y Wang, Y-J Wu, L Wang, L
Fan, J-Y Li & W Xu Authors * L He View author publications You can also search for this author inPubMed Google Scholar * H-Y Zhu View author publications You can also search for this
author inPubMed Google Scholar * S-C Qin View author publications You can also search for this author inPubMed Google Scholar * Y Li View author publications You can also search for this
author inPubMed Google Scholar * Y Miao View author publications You can also search for this author inPubMed Google Scholar * J-H Liang View author publications You can also search for this
author inPubMed Google Scholar * Y Xia View author publications You can also search for this author inPubMed Google Scholar * Y Wang View author publications You can also search for this
author inPubMed Google Scholar * Y-J Wu View author publications You can also search for this author inPubMed Google Scholar * L Wang View author publications You can also search for this
author inPubMed Google Scholar * L Fan View author publications You can also search for this author inPubMed Google Scholar * J-Y Li View author publications You can also search for this
author inPubMed Google Scholar * W Xu View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to W Xu. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the
Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE He, L., Zhu, HY., Qin, SC. _et al._ Low natural killer (NK) cell counts in
peripheral blood adversely affect clinical outcome of patients with follicular lymphoma. _Blood Cancer Journal_ 6, e457 (2016). https://doi.org/10.1038/bcj.2016.67 Download citation *
Published: 12 August 2016 * Issue Date: August 2016 * DOI: https://doi.org/10.1038/bcj.2016.67 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative