Play all audios:
ABSTRACT A pilot study was undertaken to determine the feasibility of infusing 131I labelled monoclonal antibodies (MoAbs) into either the cavity remaining after resection of malignant
glioma or into glioma cysts. Of the seven patients recruited into the study, two had cystic lesions and five resection cavities. Six of the seven were treated after relapse from primary
therapy. All patients apart from one, were given a single injection of 131I conjugated to a MoAb (ERIC-1) recognising the human neural cell adhesion molecule (NCAM). One patient received a
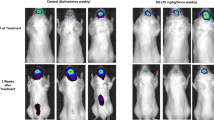
further injection of 131I-MoAb after regrowth of their disease. Pharmacokinetic studies revealed that the MoAb remained predominantly in the tumour cavity with little leakage into the
systemic compartment. This resulted in a high calculated dose of radiation being delivered to the tumour cells either lining or within close proximity to the cavity/cyst wall. In such a
small study, it is not possible to determine accurately response rates, but individual patient responses were observed. This, along with the low toxicity noted, demonstrates the feasibility
of using 131I-MoAbs in this way. With 131I, radiation dose is deposited in tissue to a depth of 1 mm from the source. The possibility of applying isotopes such as 90Yttrium which will
irradiate tumour/tissue to a greater depth (6 mm) is discussed in context with the biology of glioma infiltration into normal brain parenchyma. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 24 print issues and online
access $259.00 per year only $10.79 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
SACITUZUMAB GOVITECAN IN PATIENTS WITH BREAST CANCER BRAIN METASTASES AND RECURRENT GLIOBLASTOMA: A PHASE 0 WINDOW-OF-OPPORTUNITY TRIAL Article Open access 07 August 2024 CONVECTION
ENHANCED DELIVERY OF RHENIUM (186RE) OBISBEMEDA (186RNL) IN RECURRENT GLIOMA: A MULTICENTER, SINGLE ARM, PHASE 1 CLINICAL TRIAL Article Open access 07 March 2025 NEW APPROACHES TO TARGETED
DRUG THERAPY OF INTRACRANIAL TUMORS Article Open access 20 March 2025 AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Imperial Cancer Research Fund, Frenchay Hospital, Bristol, UK V
Papanastassiou Authors * V Papanastassiou View author publications You can also search for this author inPubMed Google Scholar * BL Pizer View author publications You can also search for
this author inPubMed Google Scholar * HB Coakham View author publications You can also search for this author inPubMed Google Scholar * J Bullimore View author publications You can also
search for this author inPubMed Google Scholar * T Zananiri View author publications You can also search for this author inPubMed Google Scholar * JT Kemshead View author publications You
can also search for this author inPubMed Google Scholar RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Papanastassiou, V., Pizer, B., Coakham, H. _et
al._ Treatment of recurrent and cystic malignant gliomas by a single intracavity injection of 131I monoclonal antibody: feasibility, pharmacokinetics and dosimetry. _Br J Cancer_ 67, 144–151
(1993). https://doi.org/10.1038/bjc.1993.25 Download citation * Issue Date: 01 January 1993 * DOI: https://doi.org/10.1038/bjc.1993.25 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
