Play all audios:
ABSTRACT Hepatic veno-occlusive disease (VOD) remains one of the most severe complications of hematopoietic SCT (HSCT). Anticoagulation and thrombolytic therapies using tissue-plasminogen
activator (t-PA) have been used, but are reported to be ineffective and are associated with significant bleeding complications. We analyzed 56 moderate-to-severe post HSCT hepatic VOD cases
treated with t-PA. We analyzed clinical outcomes according to the maximal daily dose of t-PA (t-PAmax) and the severity of VOD. Patients were stratified by t-PAmax⩽10 mg (_n_=37) vs
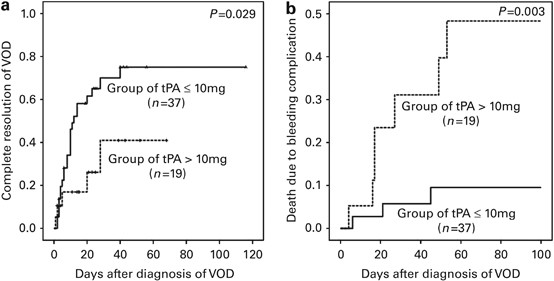
t-PAmax>10 mg (_n_=19). A higher t-PAmax was associated with increased mortality. Bleeding complications were more likely at higher t-PAmax in both moderate and severe VOD (_P_=0.036,
0.063), especially if patients had concomitant use of anticoagulants (36.4% vs 13.3%). In moderate VOD, the response rate was 86.4% for t-PAmax⩽10 mg/day and 80% for t-PAmax>10 mg
compared with 33.3% and 7.1%, respectively, for severe VOD (_P_=0.106). The 5-year OS in moderate and severe VOD was 49% and 7%, respectively, and it was 32% for t-PAmax⩽10 mg and 18% for
t-PAmax>10 mg. Our data demonstrate that lower bleeding complications and bleeding-related deaths may result from strict limitations on the t-PAmax without concomitant use of
anticoagulation therapy. However, the overall response and survival outcomes should be re-evaluated by a well-validated study in the future. Access through your institution Buy or subscribe
This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access
$259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are
calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
EFFICACY OF LOW DOSE AND SHORT DURATION DEFIBROTIDE PROPHYLAXIS FOR HEPATIC VENO-OCCLUSIVE DISEASE AFTER AUTOLOGOUS HAEMATOPOIETIC STEM CELL TRANSPLANTATION Article 24 August 2020 LOW-DOSE
UNFRACTIONATED HEPARIN PROPHYLAXIS IS A SAFE STRATEGY FOR THE PREVENTION OF HEPATIC SINUSOIDAL OBSTRUCTION SYNDROME AFTER MYELOABLATIVE ADULT ALLOGENIC STEM CELL TRANSPLANT Article 27 April
2022 A MULTICENTRE, MULTINATIONAL, PROSPECTIVE, OBSERVATIONAL REGISTRY STUDY OF DEFIBROTIDE IN PATIENTS DIAGNOSED WITH VENO-OCCLUSIVE DISEASE/SINUSOIDAL OBSTRUCTION SYNDROME AFTER
HAEMATOPOIETIC CELL TRANSPLANTATION: AN EBMT STUDY Article 31 May 2021 REFERENCES * Reiss U, Cowan M, McMillan A, Horn B . Hepatic venoocclusive disease in blood and bone marrow
transplantation in children and young adults: incidence, risk factors, and outcome in a cohort of 241 patients. _J Pediatr Hematol Oncol_ 2002; 24: 746–750. Article Google Scholar * Cesaro
S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G _et al_. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after
hematopoietic stem cell transplantation. _Haematologica_ 2005; 90: 1396–1404. PubMed Google Scholar * McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M _et al_.
Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. _Ann Intern Med_ 1993; 118: 255–267. Article CAS Google
Scholar * Bearman SI, Lee JL, Baron AE, McDonald GB . Treatment of hepatic venocclusive disease with recombinant human tissue plasminogen activator and heparin in 42 marrow transplant
patients. _Blood_ 1997; 89: 1501–1506. CAS PubMed Google Scholar * Schriber J, Milk B, Shaw D, Christiansen N, Baer M, Slack J _et al_. Tissue plasminogen activator (tPA) as therapy for
hepatotoxicity following bone marrow transplantation. _Bone Marrow Transplant_ 1999; 24: 1311–1314. Article CAS Google Scholar * Ho VT, Linden E, Revta C, Richardson PG . Hepatic
veno-occlusive disease after hematopoietic stem cell transplantation: review and update on the use of defibrotide. _Semin Thromb Hemost_ 2007; 33: 373–388. Article Google Scholar *
Corbacioglu S, Greil J, Peters C, Wulffraat N, Laws HJ, Dilloo D _et al_. Defibrotide in the treatment of children with veno-occlusive disease (VOD): a retrospective multicentre study
demonstrates therapeutic efficacy upon early intervention. _Bone Marrow Transplant_ 2004; 33: 189–195. Article CAS Google Scholar * Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z,
Kurtzberg J _et al_. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding
trial. _Biol Blood Marrow Transplant_ 2010; 16: 1005–1017. Article CAS Google Scholar * Richardson PG, Murakami C, Jin Z, Warren D, Momtaz P, Hoppensteadt D _et al_. Multi-institutional
use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk
population and factors predictive of outcome. _Blood_ 2002; 100: 4337–4343. Article CAS Google Scholar * Kim HJ, Min WS, Eom KS, Park SJ, Park YH, Kim DW _et al_. Autologous stem cell
transplantation using modified TAM or combination of triple-alkylating agents conditioning regimens as one of the post-remission treatments in patients with adult acute myeloid leukemia in
first complete remission. _Bone Marrow Transplant_ 2004; 34: 215–220. Article CAS Google Scholar * Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M _et al_.
Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. _Biol Blood
Marrow Transplant_ 2009; 15: 367–369. Article Google Scholar * Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V _et al_. Defining the intensity of conditioning regimens: working
definitions. _Biol Blood Marrow Transplant_ 2009; 15: 1628–1633. Article Google Scholar * McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED . Venocclusive disease of the liver
after bone marrow transplantation: diagnosis, incidence, and predisposing factors. _Hepatology_ 1984; 4: 116–122. Article CAS Google Scholar * Jones RJ, Lee KS, Beschorner WE, Vogel VG,
Grochow LB, Braine HG _et al_. Venoocclusive disease of the liver following bone marrow transplantation. _Transplantation_ 1987; 44: 778–783. Article CAS Google Scholar * Miano M, Faraci
M, Dini G, Bordigoni P . Early complications following haematopoietic SCT in children. _Bone Marrow Transplant_ 2008; 41 (Suppl 2): S39–S42. Article Google Scholar * Forrest DL, Thompson
K, Dorcas VG, Couban SH, Pierce R . Low molecular weight heparin for the prevention of hepatic veno-occlusive disease (VOD) after hematopoietic stem cell transplantation: a prospective phase
II study. _Bone Marrow Transplant_ 2003; 31: 1143–1149. Article CAS Google Scholar * Gray RJ . A class of k-sample tests for comparing the cumulative incidence of a competing risk.
_Annals of Statistics_ 1988; 16: 1141–1154. Article Google Scholar * Ho VT, Revta C, Richardson PG . Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: update on
defibrotide and other current investigational therapies. _Bone Marrow Transplant_ 2008; 41: 229–237. Article CAS Google Scholar * Kulkarni S, Rodriguez M, Lafuente A, Mateos P, Mehta J,
Singhal S _et al_. Recombinant tissue plasminogen activator (rtPA) for the treatment of hepatic veno-occlusive disease (VOD). _Bone Marrow Transplant_ 1999; 23: 803–807. Article CAS Google
Scholar * Lee JH, Lee KH, Choi JS, Zang DY, Kim SB, Kim SW _et al_. Veno-occlusive disease (VOD) of the liver in Korean patients following allogeneic bone marrow transplantation (BMT):
efficacy of recombinant human tissue plasminogen activator (rt-PA) treatment. _J Korean Med Sci_ 1996; 11: 118–126. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This
study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (1020370). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS
* Department of Hematology, Catholic Blood and Marrow Transplantation Center, Seoul St Mary’s Hospital, The Catholic University of Korea, Seoul, Korea J-H Yoon, W-S Min, H-Je Kim, J-H Kim,
S-H Shin, S-A Yahng, S-E Lee, B-S Cho, K-S Eom, Y-J Kim, S Lee, C-K Min, S-G Cho, D-W Kim, J-W Lee & C-W Park Authors * J-H Yoon View author publications You can also search for this
author inPubMed Google Scholar * W-S Min View author publications You can also search for this author inPubMed Google Scholar * H-Je Kim View author publications You can also search for this
author inPubMed Google Scholar * J-H Kim View author publications You can also search for this author inPubMed Google Scholar * S-H Shin View author publications You can also search for
this author inPubMed Google Scholar * S-A Yahng View author publications You can also search for this author inPubMed Google Scholar * S-E Lee View author publications You can also search
for this author inPubMed Google Scholar * B-S Cho View author publications You can also search for this author inPubMed Google Scholar * K-S Eom View author publications You can also search
for this author inPubMed Google Scholar * Y-J Kim View author publications You can also search for this author inPubMed Google Scholar * S Lee View author publications You can also search
for this author inPubMed Google Scholar * C-K Min View author publications You can also search for this author inPubMed Google Scholar * S-G Cho View author publications You can also search
for this author inPubMed Google Scholar * D-W Kim View author publications You can also search for this author inPubMed Google Scholar * J-W Lee View author publications You can also search
for this author inPubMed Google Scholar * C-W Park View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to W-S Min. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yoon, JH., Min, WS., Kim,
HJ. _et al._ Experiences of t-PA use in moderate-to-severe hepatic veno-occlusive disease after hematopoietic SCT: is it still reasonable to use t-PA?. _Bone Marrow Transplant_ 48, 1562–1568
(2013). https://doi.org/10.1038/bmt.2013.101 Download citation * Received: 19 March 2013 * Revised: 18 June 2013 * Accepted: 18 June 2013 * Published: 29 July 2013 * Issue Date: November
2013 * DOI: https://doi.org/10.1038/bmt.2013.101 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * veno-occlusive disease * hematopoietic SCT *
bleeding * complication * t-PA