Play all audios:
ABSTRACT We previously showed that minimal residual disease (MRD) detection pre-hematopoietic cell transplant (HCT) and acute GvHD (aGvHD) independently predicted risk of relapse in
pediatric ALL. In this study we further define risk by assessing timing of relapse and the effects of leukemia risk category and post-HCT MRD. By multivariate analysis, pre-HCT MRD <0.1%
and aGvHD by day +55 were associated with decreased relapse and improved event-free survival (EFS). Intermediate leukemia risk status predicted decreased relapse, and improved EFS and
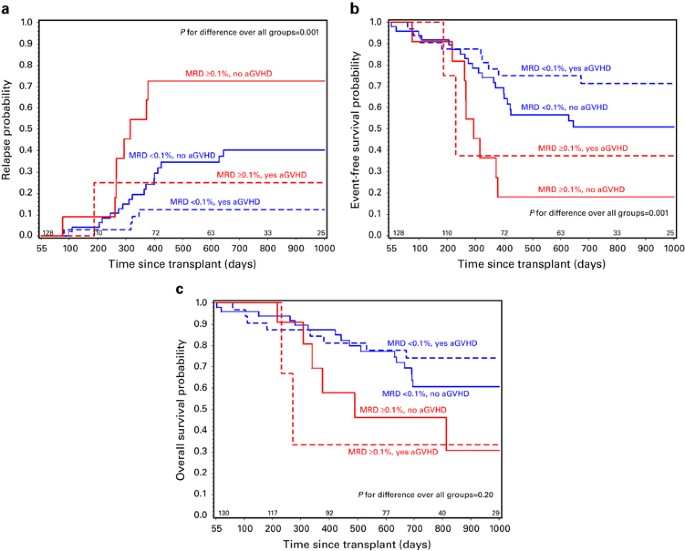
overall survival (OS). Patients with pre-HCT MRD ⩾0.1% who did not develop aGvHD compared with those with MRD <0.1% who did develop aGvHD had much worse survival (2 years EFS 18% vs 71%;
_P_=0.001, 2 years OS 46 vs 74%; _P_=0.04). Patients with pre-HCT MRD <0.1% who did not experience aGvHD had higher rates of relapse than those who did develop aGvHD (40% vs 13%; _P_=
0.008). Post-HCT MRD led to a substantial increase in relapse risk (HR=4.5, _P_<0.01). Patients at high risk of relapse can be defined after transplant using leukemia risk category,
presence of MRD pre or post HCT, and occurrence of aGvHD. An optimal window to initiate intervention to prevent relapse occurs between day +55 and +200 after HCT. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print
issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS PROGNOSTIC FACTORS AND CLINICAL OUTCOMES IN PATIENTS WITH RELAPSED ACUTE LEUKEMIA AFTER ALLOGENEIC HEMATOPOIETIC STEM CELL TRANSPLANTATION Article 29 April 2023
RELATIONSHIP BETWEEN MORPHOLOGIC REMISSION WITH OR WITHOUT HEMATOLOGIC RECOVERY AND OUTCOME AFTER ALLOGENEIC HEMATOPOIETIC CELL TRANSPLANTATION IN ADULT ACUTE MYELOID LEUKEMIA Article 29
August 2024 RELAPSE OF ACUTE MYELOID LEUKEMIA AFTER ALLOGENEIC HEMATOPOIETIC CELL TRANSPLANTATION: CLINICAL FEATURES AND OUTCOMES Article 02 December 2020 REFERENCES * Pulsipher MA, Peters
C, Pui CH . High-risk pediatric acute lymphoblastic leukemia: to transplant or not to transplant? _Biol Blood Marrow Transplant_ 2011; 17: S137–S148. Article PubMed PubMed Central Google
Scholar * Locatelli F, Schrappe M, Bernardo ME, Rutella S . How I treat relapsed childhood acute lymphoblastic leukemia. _Blood_ 2012; 120: 2807–2816. Article CAS PubMed Google Scholar
* Borgmann A, von Stackelberg A, Hartmann R, Ebell W, Klingebiel T, Peters C _et al_. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute
lymphoblastic leukemia in a second remission: a matched-pair analysis. _Blood_ 2003; 101: 3835–3839. Article CAS PubMed Google Scholar * Schrauder A, von Stackelberg A, Schrappe M,
Cornish J, Peters C . Allogeneic hematopoietic SCT in children with ALL: current concepts of ongoing prospective SCT trials. _Bone Marrow Transplant_ 2008; 41: S71–S74. Article PubMed
Google Scholar * Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV _et al_. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia
(ALL R3): an open-label randomised trial. _Lancet_ 2010; 376: 2009–2017. Article CAS PubMed PubMed Central Google Scholar * Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick
NJ _et al_. Reinduction platform for children with first marrow relapse in acute lymphoblastic lymphoma. _J Clin Oncol_ 2008; 26: 3971–3978. Article CAS PubMed PubMed Central Google
Scholar * Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ _et al_. Graft-versus-leukemia reactions after bone marrow transplantation. _Blood_ 1990; 75: 555–562. CAS PubMed
Google Scholar * Cornelissen JJ, Carston M, Kollman C, King R, Dekker AW, Lowenberg B _et al_. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic
leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. _Blood_ 2001; 97: 1572–1577. Article CAS PubMed Google Scholar * Passweg JR, Tiberghien P, Cahn JY,
Vowels MR, Camitta BM, Gale RP _et al_. Graft-versus-leukemia effects in T lineage and B lineage acute lymphoblastic leukemia. _Bone Marrow Transplant_ 1998; 21: 153–158. Article CAS
PubMed Google Scholar * Lee S, Cho BS, Kim SY, Choi SM, Lee DG, Eom KS _et al_. Allogeneic stem cell transplantation in first complete remission enhances graft-versus-leukemia effect in
adults with acute lymphoblastic leukemia: antileukemic activity of chronic graft-versus-host disease. _Biol Blood Marrow Transplant_ 2007; 13: 1083–1094. Article PubMed Google Scholar *
Nordlander A, Mattsson J, Ringden O, Leblanc K, Gustafsson B, Ljungman P _et al_. Graft-versus-host disease is associated with a lower relapse incidence after hematopoietic stem cell
transplantation in patients with acute lymphoblastic leukemia. _Biol Blood Marrow Transplant_ 2004; 10: 195–203. Article PubMed Google Scholar * Zikos P, Van Lint MT, Lamparelli T,
Gualandi F, Occhini D, Bregante S _et al_. Allogeneic hemopoietic stem cell transplantation for patients with high risk acute lymphoblastic leukemia: favorable impact of chronic
graft-versus-host disease on survival and relapse. _Haematologica_ 1998; 83: 896–903. CAS PubMed Google Scholar * Gustafsson Jernberg A, Remberger M, Ringden O, Winiarski J .
Graft-versus-leukaemia effect in children: chronic GVHD has a significant impact on relapse and survival. _Bone Marrow Transplant_ 2003; 31: 175–181. Article CAS PubMed Google Scholar *
Locatelli F, Zecca M, Messina C, Rondelli R, Lanino E, Sacchi N _et al_. Improvement over time in outcome for children with acute lymphoblastic leukemia in second remission given
hematopoietic stem cell transplantation from unrelated donors. _Leukemia_ 2002; 16: 2228–2237. Article CAS PubMed Google Scholar * Dini G, Zecca M, Balduzzi A, Messina C, Masetti R,
Fagioli F _et al_. No difference in outcome between children and adolescents transplanted for acute lymphoblastic leukemia in second remission. _Blood_ 2011; 118: 6683–6690. Article CAS
PubMed Google Scholar * Teachey DT, Sheen C, Hall J, Ryan T, Brown VI, Fish J _et al_. mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute
lymphoblastic leukemia. _Blood_ 2008; 112: 2020–2023. Article CAS PubMed PubMed Central Google Scholar * Brown VI, Fang J, Alcorn K, Barr R, Kim JM, Wasserman R _et al_. Rapamycin is
active against B-precursor leukemia _in vitro_ and _in vivo_, an effect that is modulated by IL-7-mediated signaling. _Proc Natl Acad Sci USA_ 2003; 100: 15113–15118. Article CAS PubMed
PubMed Central Google Scholar * Teachey DT, Obzut DA, Cooperman J, Fang J, Carroll M, Choi JK _et al_. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical
models of primary adult human ALL. _Blood_ 2006; 107: 1149–1155. Article CAS PubMed PubMed Central Google Scholar * Pulsipher MA, Wall DA, Grimley M, Goyal RK, Boucher KM, Hankins P _et
al_. A phase I/II study of the safety and efficacy of the addition of sirolimus to tacrolimus/methotrexate graft versus host disease prophylaxis after allogeneic haematopoietic cell
transplantation in paediatric acute lymphoblastic leukaemia (ALL). _Br J Haematol_ 2009; 147: 691–699. Article CAS PubMed PubMed Central Google Scholar * Pulsipher MA, Langholz B, Wall
DA, Schultz KR, Bunin N, Carroll WL _et al_. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children's Oncology Group/Pediatric
Blood and Marrow Transplant Consortium trial. _Blood_ 2014; 123: 2017–2025. Article CAS PubMed PubMed Central Google Scholar * Locatelli F, Zecca M, Rondelli R, Bonetti F, Dini G, Prete
A _et al_. Graft versus host disease prophylaxis with low-dose cyclosporine-A reduces the risk of relapse in children with acute leukemia given HLA-identical sibling bone marrow
transplantation: results of a randomized trial. _Blood_ 2000; 95: 1572–1579. CAS PubMed Google Scholar * Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL _et al_. The
addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase III COG/PBMTC trial. _Blood_ 2014; 123: 2017–2025. Article CAS PubMed PubMed Central
Google Scholar * Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A _et al_. Prognostic value of minimal residual disease quantification before allogeneic stem-cell
transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. _J Clin Oncol_ 2009; 27: 377–384. Article PubMed Google Scholar * Borowitz MJ, Wood BL,
Devidas M, Loh M, Raetz E, Nachman JB _et al_. Improved Post-Induction Chemotherapy Does Not Abrogate Prognostic Significance of Minimal Residual Disease (MRD) for Children and Young Adults
with High Risk Acute ymphoblastic Leukemia (ALL). A Report From Children's Oncology Group (COG) Study AALL0232. _Blood_ 2011; 18: 625. Google Scholar * Dworzak MN, Froschl G, Printz D,
Mann G, Potschger U, Muhlegger N _et al_. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. _Blood_
2002; 99: 1952–1958. Article CAS PubMed Google Scholar * Borowitz MJ, Pullen DJ, Shuster JJ, Viswanatha D, Montgomery K, Willman CL _et al_. Minimal residual disease detection in
childhood precursor-B-cell acute lymphoblastic leukemia: relation to other risk factors. A Children's Oncology Group study. _Leukemia_ 2003; 17: 1566–1572. Article CAS PubMed Google
Scholar * Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. _J Am Stat Assoc_ 1958; 53: 457–481. Article Google Scholar * Cox D . Regression models and life
tables. _J R Stat Soc B_ 1972; 34: 187–202. Google Scholar * Aalen O, Johansen S . An empirical transition matrix for nonhomogeneous Markov chains based on censored observations. _Scand J
Statist_ 1978; 5: 141–150. Google Scholar * Bader P, Kreyenberg H, von Stackelberg A, Eckert C, Salzmann-Manrique E, Meisel R _et al_. Monitoring of minimal residual disease after
allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 Trial. _J Clin
Oncol_ 2015; 33: 1275–1284. Article CAS PubMed Google Scholar * Bruggemann M, Schrauder A, Raff T, Pfeifer H, Dworzak M, Ottmann OG _et al_. Standardized MRD quantification in European
ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18-20 September 2008. _Leukemia_ 2010; 24: 521–535. Article CAS PubMed Google Scholar *
Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E _et al_. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. _Blood_ 2012; 120:
5173–5180. Article CAS PubMed PubMed Central Google Scholar * Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M _et al_. Phase I trial of anti-CD22 recombinant
immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. _J Clin Oncol_ 2012; 30: 1822–1828. Article CAS PubMed PubMed Central Google Scholar *
Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK . Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of
acute lymphoblastic leukemia. _Leukemia_ 2007; 21: 2240–2245. Article CAS PubMed Google Scholar * Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S _et al_. Targeted therapy
with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and
prolonged leukemia-free survival. _J Clin Oncol_ 2011; 29: 2493–2498. Article CAS PubMed Google Scholar * Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A _et al_. T cells with
chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. _Sci Transl Med_ 2011; 3: 95ra73. Article CAS PubMed PubMed Central
Google Scholar * Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR _et al_. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. _N Engl J Med_ 2013; 368:
1509–1518. Article CAS PubMed PubMed Central Google Scholar * Grupp SA, June CH . Adoptive cellular therapy. _Curr Top Microbiol Immunol_ 2011; 344: 149–172. CAS PubMed Google Scholar
* Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P _et al_. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute
lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? _J Clin Oncol_ 2004; 22: 1696–1705. Article PubMed Google Scholar *
Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B _et al_. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed
chimerism after allogeneic stem cell transplantation. _Blood_ 2011; 118: 5681–5688. Article CAS PubMed Google Scholar * Lankester AC, Bierings MB, van Wering ER, Wijkhuijs AJ, de Weger
RA, Wijnen JT _et al_. Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell
transplantation. _Leukemia_ 2010; 24: 1462–1469. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study was supported in part by N01
HC-45220/HHSN268200425220C, U10 CA098543 and R01CA1116660. PBMTC activities were supported by 2U01HL069254 and a consortium grant from the St Baldrick’s Foundation. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Division of Hematology and Hematological Malignancies, Huntsman Cancer Institute/University of Utah School of Medicine, Primary Children’s Hospital, Salt Lake
City, UT, USA M A Pulsipher & E Raetz * Department of Preventive Medicine, USC Keck School of Medicine, Los Angeles, CA, USA B Langholz * Manitoba Blood and Marrow Transplant Program,
Winnepeg, Manitoba, Canada D A Wall * Department of Pediatrics University of BC, BC Children's Hospital, Vancouver, British Columbia, Canada K R Schultz * Division of Oncology,
Children's Hospital of Philadelphia, Philadelphia, PA, USA N Bunin, D Teachey & S A Grupp * NYU Department of Pediatrics and Cancer Institute, NYU Langone Medical Center, New York,
NY, USA W Carroll & S Gardner * Division of Blood and Marrow Transplantation and Cellular Therapies, Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center,
Pittsburgh, PA, USA R K Goyal * Department of Pathology and Laboratory Medicine, Nationwide Children’s Hospital, Columbus, OH, USA J Gastier-Foster * Department of Pathology, The Ohio State
University College of Medicine, Columbus, OH, USA J Gastier-Foster * Department of Pediatrics, The Ohio State University College of Medicine, Columbus, OH, USA J Gastier-Foster * Department
of Pathology, Johns Hopkins Medical Institutions, Baltimore, MD, USA M Borowitz * Department of Pathology, Children's Hospital of Philadelphia, Philadelphia, PA, USA S A Grupp Authors *
M A Pulsipher View author publications You can also search for this author inPubMed Google Scholar * B Langholz View author publications You can also search for this author inPubMed Google
Scholar * D A Wall View author publications You can also search for this author inPubMed Google Scholar * K R Schultz View author publications You can also search for this author inPubMed
Google Scholar * N Bunin View author publications You can also search for this author inPubMed Google Scholar * W Carroll View author publications You can also search for this author
inPubMed Google Scholar * E Raetz View author publications You can also search for this author inPubMed Google Scholar * S Gardner View author publications You can also search for this
author inPubMed Google Scholar * R K Goyal View author publications You can also search for this author inPubMed Google Scholar * J Gastier-Foster View author publications You can also
search for this author inPubMed Google Scholar * M Borowitz View author publications You can also search for this author inPubMed Google Scholar * D Teachey View author publications You can
also search for this author inPubMed Google Scholar * S A Grupp View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to M
A Pulsipher. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION This study was presented in part at the American Society of
Hematology Meeting, December 2012 and the Second International Workshop: Biology Prevention and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation, November 5–6,
2012, Bethesda MD, Plenary Oral Presentation, Best Abstracts Session. Supplementary Information accompanies this paper on Bone Marrow Transplantation website SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION (DOC 83 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pulsipher, M., Langholz, B., Wall, D. _et al._ Risk factors and
timing of relapse after allogeneic transplantation in pediatric ALL: for whom and when should interventions be tested?. _Bone Marrow Transplant_ 50, 1173–1179 (2015).
https://doi.org/10.1038/bmt.2015.103 Download citation * Received: 17 December 2014 * Revised: 17 February 2015 * Accepted: 11 March 2015 * Published: 11 May 2015 * Issue Date: September
2015 * DOI: https://doi.org/10.1038/bmt.2015.103 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative