Play all audios:
ABSTRACT Programmed cell death (PCD) is an integral part of plant development and of responses to abiotic stress or pathogens. Although the morphology of plant PCD is, in some cases, well
characterised and molecular mechanisms controlling plant PCD are beginning to emerge, there is still confusion about the classification of PCD in plants. Here we suggest a classification
based on morphological criteria. According to this classification, the use of the term ‘apoptosis’ is not justified in plants, but at least two classes of PCD can be distinguished: vacuolar
cell death and necrosis. During vacuolar cell death, the cell contents are removed by a combination of autophagy-like process and release of hydrolases from collapsed lytic vacuoles.
Necrosis is characterised by early rupture of the plasma membrane, shrinkage of the protoplast and absence of vacuolar cell death features. Vacuolar cell death is common during tissue and
organ formation and elimination, whereas necrosis is typically found under abiotic stress. Some examples of plant PCD cannot be ascribed to either major class and are therefore classified as
separate modalities. These are PCD associated with the hypersensitive response to biotrophic pathogens, which can express features of both necrosis and vacuolar cell death, PCD in starchy
cereal endosperm and during self-incompatibility. The present classification is not static, but will be subject to further revision, especially when specific biochemical pathways are better
defined. SIMILAR CONTENT BEING VIEWED BY OTHERS DYING IN SELF-DEFENCE: A COMPARATIVE OVERVIEW OF IMMUNOGENIC CELL DEATH SIGNALLING IN ANIMALS AND PLANTS Article Open access 04 October 2022
AC-DEVD-CHO (CASPASE-3/DEVDASE INHIBITOR) SUPPRESSES SELF-INCOMPATIBILITY–INDUCED PROGRAMMED CELL DEATH IN THE POLLEN TUBES OF PETUNIA (_PETUNIA HYBRIDA_ E. VILM.) Article Open access 30
January 2024 ORGANIZED DISASSEMBLY OF PHOTOSYNTHESIS DURING PROGRAMMED CELL DEATH MEDIATED BY LONG CHAIN BASES Article Open access 25 June 2020 MAIN Research on plant cell death has grown
considerably in the past few years, owing to the importance of cell death for plant development and defense. Just as animal cells engage several mechanisms leading to death, the road to cell
demise in plants can also vary. The long evolutionary distance and distinct cellular architecture between the two kingdoms may account for the differences between the mechanisms of plant
and animal cell death. It is therefore appropriate to assess the relevance of animal cell death nomenclature1 to plants. At present, there is confusion in cell death terminology in plant
biology, which drives our attempt to formulate a more logical classification. Although our molecular understanding of plant cell death regulation and execution is insufficient to create
definitive classifications based on precise biochemical pathways, it is possible to begin classifying plant cell death scenarios based on morphological criteria, as was initially the case in
animal cell death research2, 3 and is still used for the classification of cell death in animal science.1 This document attempts to provide a classification of plant cell death. We urge
authors, reviewers and editors to follow this classification to facilitate communication between scientists and accelerate research in this field. ABSENCE OF APOPTOSIS IN PLANTS Apoptosis is
one of the three major types of cell death found in animals. Compared with the other two – autophagic cell death and necrosis – apoptosis is much better understood, both cytologically and
biochemically.1, 4 Apoptosis is accompanied by rounding up of the cell, reduction of cellular volume, chromatin condensation, nuclear segmentation and very little ultrastructural
modification of cytoplasmic organelles. Its hallmark is blebbing of the plasma membrane (which maintains its integrity until the final stages of apoptosis), followed by fragmentation of the
cell into smaller parcels called apoptotic bodies. Finally, the apoptotic bodies are engulfed by phagocytes and degraded by lysosomal enzymes. This is critical to prevent subsequent
induction of inflammation due to leakage of dead cell contents. The term ‘apoptosis’ should be applied exclusively to cell death that manifests these morphological features. Although
apoptosis is often associated with activation of caspases and oligonucleosomal fragmentation of DNA, these processes can also take place during non-apoptotic cell death, and are thus
insufficient criteria for assignment.1 Plant cells do not exhibit ‘classic’ apoptosis for the following reasons. First, rigid cell walls preclude the necessity for breakdown of the plant
cells into apoptotic bodies. Second, there are no phagocytic cells. A considerable number of articles describing ‘plant apoptosis’ or ‘apoptotic-like programmed cell death (PCD)’ have
nevertheless been published. Critical analysis of this literature reveals three major points that indicate misuse of the term ‘apoptosis’. First, chromatin condensation and DNA fragmentation
are often quoted as apoptotic features. However, neither is specific to apoptosis, because they can also be observed during necrosis and autophagic death.5, 6, 7 Second, stress treatments
often induce shrinkage of the plant protoplast, but not of the cell itself, which can be morphologically reminiscent of apoptotic cell shrinkage. However, animal cells that shrink during
apoptosis maintain their plasma membrane integrity to form apoptotic bodies,8 whereas plant protoplasts that shrink in response to stress usually have damaged plasma membranes and do not
fragment further into discrete bodies.9 Third, increased caspase-like proteolytic activities (in most cases unlinked to specific proteases) in dying plant cells have been used as an argument
for the existence of plant apoptosis. This is an insufficient criterion because activation of caspases _per se_ does not always lead to apoptosis in animal cells.7 Furthermore, the
activation of plant proteases that possess caspase-like activity has not been shown to lead to apoptotic morphology.10, 11, 12 DEFINITION OF ‘VACUOLAR’ PLANT CELL DEATH Plants have elaborate
vacuolar systems that, in contrast to animal lysosomes, can occupy most of the plant cell volume.13 Similar to the roles of lysosomes in animals, plants also use lytic vacuoles to recycle
parts of their cells during normal development and during nutritional stress.14 These lytic vacuoles acquire an important function in one major class of plant cell death, which we recommend
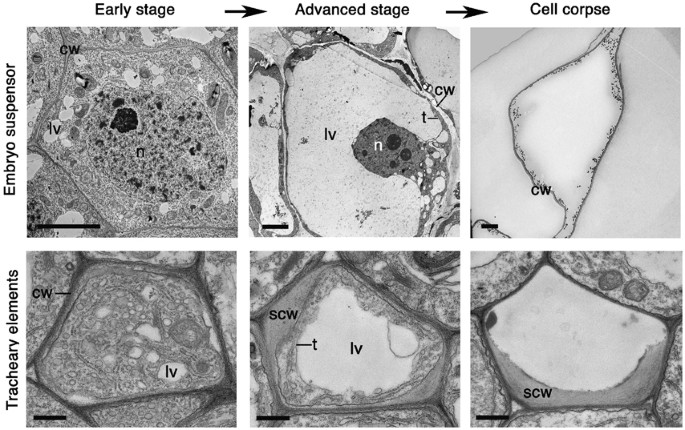
be termed ‘vacuolar cell death’.15 Vacuolar cell death is often manifested by a gradual decrease in the volume of the cytoplasm and a concomitant increase in the volume occupied by lytic
vacuoles (Figure 1). Engulfment of the cytoplasm by lytic vacuoles with subsequent cargo degradation is a major mechanism of cell dismantling during vacuolar cell death. Electron micrographs
often show invaginations in the vacuolar membrane (tonoplast) and fusion of vesicles with the vacuole, followed by uptake and degradation of portions of the cytoplasm in the vacuolar lumen.
This process resembles micro- or macro-autophagy.16, 17, 18, 19, 20 The final step in the execution of vacuolar cell death is rupture of the tonoplast, and a massive release of vacuolar
hydrolases. These rapidly destroy the entire protoplast or in some cases even the entire cell including the cell wall. Other morphological events during vacuolar cell death include formation
of actin cables, nuclear envelope disassembly and, in some examples, nuclear segmentation. The remaining mitochondria and other organelles, as well as the plasma membrane, remain
morphologically intact until rupture of the tonoplast (Table 1; Figure 1). A robust approach to diagnose vacuolar cell death would combine electron microscopy (EM) with the analysis of
autophagic activity, requirement for vacuolar processing enzymes (VPE) and cytoskeletal changes (Table 2). Execution of vacuolar cell death may be a slow process that can take several days
until the rupture of the tonoplast that accomplishes protoplast clearance.18, 19, 20, 21 Depending on the system, the cell wall can be largely degraded, as for example during aerenchyma
formation, leaf perforations in the lace plant and petal senescence22, 23, 24 or can remain intact, for example, during xylem differentiation in vascular plants or leaf remodelling in
_Monstera_ (Figure 1).24, 25, 26 Examples of vacuolar cell death are found during embryo, organ and tissue morphogenesis and senescence, and include, in addition to those mentioned above,
the formation of embryo-suspensor, pollen, ovary, ducts and laticifers.19 Knockout of _ATG_ genes was shown to accelerate _Arabidopsis_ leaf senescence,27, 28 and _ATG5_ has recently been
found to be required for vacuolar cell death of _Arabidopsis_ tracheary elements.29 More extensive work is still needed to determine whether or not _ATG_-dependent autophagic pathways are
required for the execution of vacuolar cell death. DEFINITION OF ‘NECROTIC’ PLANT CELL DEATH Necrosis of animal cells is defined morphologically by the lack of apoptotic or autophagic
features, and positively by the frequent occurrence of an initial gain in cell volume, swelling of various organelles, early rupture of the plasma membrane and loss of intracellular
content.1, 30 Although it is no longer considered to be an unprogrammed process, necrosis remains poorly characterised at the biochemical and genetic levels, so there are as yet no molecular
markers for it. In animal systems, necrosis is often preceded by an increase in cytosolic calcium ion concentration ([Ca2+]cyt), lipid degradation and activation of calpain family
proteases. Mitochondria and lysosomes have been implicated in the downstream events. Mitochondrial changes include uncoupling of respiration, the production of reactive oxygen species (ROS)
and nitrogen species (RNS), a drop in ATP level and mitochondrial membrane permeabilisation (MMP). Lysosomal events include ROS production and permeabilisation of the lysosomal membrane
causing release of active cathepsin proteases to the cytosol.1, 31 Cell death with many of the above characteristics occurs widely in plants. It is induced by a range of abiotic stresses and
by successful recognition of a pathogen during the hypersensitive response (HR). It is also found in the cells challenged by necrotrophic pathogens (they are called necrotrophic because
they kill host cells to derive nutrients). However, in the case of the HR, necrotic features are often accompanied by the features of vacuolar cell death (see below). Cytological hallmarks
that distinguish plant necrosis from vacuolar cell death include mitochondrial swelling, the absence of the growing lytic vacuoles and an early rupture of the plasma membrane leading to
shrinkage of the protoplast (Table 1; Figure 2).9, 19, 32, 33 Because there are no lytic vacuoles that clear the cytoplasm during necrosis, the corpses of necrotic cells remain largely
unprocessed. A shrunken protoplast is one of the most easily detected features of plant necrotic cells (Figure 2). Time-course analysis of animal necrosis has revealed that the initial gain
in cell volume (swelling) as a result of ion pump failure is followed by cell shrinkage.30 Plant cells have a cell wall that should counteract swelling of the protoplast at early stages of
necrosis, which would therefore escape detection.32 However, an early loss of plasma membrane integrity can result in readily detectable protoplast shrinkage. Necrosis is typically an acute
cell death response that develops rapidly and takes from several minutes (toxic treatments) to up to a day, as seen in the HR. A recommended approach to diagnose plant necrosis is by
combining EM analysis with the assessment of mitochondrial dysfunction (MMP and decreased levels of both oxygen consumption and ATP production) and both ROS and RNS accumulation (Table 2).
MIXED AND ATYPICAL MODALITIES OF PLANT CELL DEATH HR WITH SOME FEATURES OF VACUOLAR CELL DEATH It has been long known that a programmed, localised cell death connected with the HR occurs at
the site of successful recognition of biotrophic pathogens. Whether this cell death is the cause of restricted pathogen replication or a consequence thereof has been debated for decades.34
The nature of the HR cell death with respect to its morphology has also been debated.35, 36, 37, 38, 39 Most recently, HR cell death and pathogen replication restriction have been de-coupled
by manipulation of metacaspase expression, showing that, for at least the pathogens tested, the elimination of the host cell death response does not lead to pathogen proliferation.40 HR
cell death usually exhibits all characteristics of plant cell necrosis (Tables 1 and 2). However, HR cell death is at the same time often accompanied by the growth of lytic vacuoles and
tonoplast rupture, which can require VPE from the vacuole in some cases.10, 41 In addition, increased autophagic activity before HR cell death is apparently controlled by _ATG_ genes,42
although the precise role of autophagy may differ depending on the particular HR cell death pathway being studied.43, 44 Although autolytic components appear to be important for the HR cell
death in some cases that have been studied, collapse of lytic vacuoles during the HR does not lead to complete clearance of the protoplast, as it does in vacuolar PCD.39, 45 When discussing
the relationship of the HR cell death to its correlated cytological features, and ultimately to the restriction of pathogen success, it is important to consider where the pathogens
proliferate: for example, bacterial pathogens proliferate in the apoplast, outside the cell, while viruses proliferate within cells. Thus, vacuolar collapse can be effective to restrict
viral pathogens,10 while discharge of defense proteins into the apoplast, accompanied by fusion of the tonoplast and plasma membrane, slows bacterial pathogens outside the cells.11 SHRUNKEN
PROTOPLAST AND INTACT PLASMA MEMBRANE DURING VICTORIN-INDUCED CELL DEATH A particular cell death, evoked by the fungal toxin victorin, is important because it has evolved to use the host HR
as a means to kill cells, which are then ‘digested’ by the necrotrophic pathogen. Furthermore, similar to classic pathogen-induced HR, victorin sensitivity is dependent on an NB-LRR immune
receptor.46 Although victorin-induced cell death in oat plants exhibits hallmarks of necrosis such as protoplast shrinkage and MMP, the shrunken protoplast is surrounded by an intact plasma
membrane and the tonoplast retains its integrity.47 This suggests that initiation of the HR-related cell death can sometimes occur without the loss of membrane integrity.48 MIXTURE OF
VACUOLAR AND NECROTIC HALLMARKS DURING SELF-INCOMPATIBILITY RESPONSE During self-incompatibility (SI) response in _Papaver_, an incompatible pollen tube is stopped by interactions with the
pistil S-determinant that trigger a network of signalling events that converge to mediate PCD.49 SI cell death exhibits some characteristics of vacuolar cell death, including alterations to
the actin cytoskeleton, organelle engulfment and loss of vacuolar integrity. SI also has features of necrosis including swelling of the mitochondria and increase in [Ca2+]cyt.49, 50 A LONG
TIME GAP BETWEEN CELL DEATH AND CORPSE PROCESSING IN CEREAL STARCHY ENDOSPERM The cereal endosperm consists of the starchy endosperm surrounded by the aleurone cell layer. Cells of the
starchy endosperm accumulate storage reserves and die during seed maturation, but their corpses remain unprocessed until germination. Upon seed germination, aleurone cells secrete hydrolytic
enzymes that break down and mobilise the reserves accumulated in the dead starchy endosperm.51, 52 RECOMMENDATIONS TO AUTHORS, REVIEWERS AND EDITORS OF SCIENTIFIC JOURNALS Arbitrary and
sometimes contradictory usage of terminology has been a problem in the field of plant cell death research. Here we have grouped together morphological characteristics that distinguish two
major classes of cell death occurring in plants (Table 1). On the basis of this simplistic grouping, we suggest that terms ‘vacuolar cell death’ and ‘necrotic cell death’ (or ‘necrosis’) are
used when referring to corresponding classes of cell death in plants. Because the HR cell death with autolytic features, victorin-induced cell death, and both starchy endosperm and SI cell
death do not neatly fall into these two categories, we suggest that they are left as separate cell death modalities. The present classification is of course not static, but will be subjected
to further revisions, especially when specific biochemical pathways and molecular identity of mediators for plant PCD are better defined. We recommend that plant cell death researchers
abandon terms such as ‘apoptosis’ or ‘apoptotic-like’. We think such terminology is incorrect and misleading, because the features often cited are also found in other types of PCD, whereas
the _bona fide_ cytological characteristics of apoptosis (formation of apoptotic bodies and phagocytosis) are absent in plants. Adequate choices of analytical methods are required to
correctly diagnose a type of plant cell death (Table 2). Being a classic, cytological method, EM analysis provides excellent descriptive data on the changes in the dying cell for the initial
classification of the particular morphotype. We encourage the use of EM in showing the temporal details of the cell death under study, such as the structure of organelles, formation of
autophagosome-like structures, nuclear events and early detachment of plasma membrane from the cell wall (beginning of protoplast shrinkage). We would furthermore advise that experiments
with protoplasts are not used in isolation, but are supported by tests having a direct relationship to the physiologically relevant cell death in the model system. Several of the
recommendations formulated in the classification of animal cell death1 are also relevant to studies of plant cell death. For example, the term ‘dead cell’ should only be used for cells that
are shown to be dead by specific staining, such as fluorescein diacetate or Evan's blue. We thus encourage the use of quantification of cell death along with the necessary statistical
treatments to show significance for the reported data. CONCLUSION We recognise two major classes of cell death occurring in plant biology: (i) vacuolar cell death and (ii) necrotic cell
death. Vacuolar cell death occurs during plant tissue and organ formation and elimination, although necrosis is typically found under abiotic stress, some forms of the HR-related cell death
and cell death induced by necrotrophic pathogens. A few examples of cell death cannot be ascribed to either major class and therefore classified as separate modalities. This category
includes HR cell death with autolytic features and victorin-induced cell death, as well as cell death occurring in starchy cereal endosperm and during SI response. Further studies using
tools of genetics, biochemistry and cell biology are required to understand molecular mechanisms underlying variability of plant cell death morphology. ABBREVIATIONS * GFP: green fluorescent
protein * EM: electron microscopy * IF microscopy: immunofluorescent microscopy * HR: hypersensitive response * JC-1: JC-1 mitochondrial membrane potential detection kit based on
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide * MDC: monodansylcadaverine * MMP: mitochondrial membrane permeabilisation * PCD: programmed cell death * RNS:
reactive nitrogen species * ROS: reactive oxygen species * SI: self-incompatibility * TMRE: tetramethyl-rhodamine ethyl ester * VPE: vacuolar processing enzyme REFERENCES * Kroemer G,
Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH _et al_. Classification of cell death. Recommendations of the Nomenclature Committee on Cell Death 2009. _Cell Death Differ_
2009; 16: 3–11. Article CAS PubMed Google Scholar * Kerr JF, Wyllie AH, Currie AR . Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. _Br J
Cancer_ 1972; 26: 239–257. Article CAS PubMed PubMed Central Google Scholar * Clarke PGH . Developmental cell death: morphological diversity and multiple mechanisms. _Anat Embryol_
1990; 181: 195–206. Article CAS Google Scholar * Leist M, Jäättelä M . Four deaths and a funeral: from caspases to alternative mechanisms. _Nat Rev Mol Cell Biol_ 2001; 2: 589–598.
Article CAS PubMed Google Scholar * Lee CY, Baehrecke EH . Steroid regulation of autophagic programmed cell death during development. _Development_ 2001; 128: 1443–1455. CAS PubMed
Google Scholar * Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jäättelä M . Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death.
_Cell Death Differ_ 2005; 12: 1297–1309. Article CAS PubMed Google Scholar * Fink SL, Cookson BT . Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying
eukaryotic cells. _Infect Immun_ 2005; 73: 1907–1916. Article CAS PubMed PubMed Central Google Scholar * Nunez R, Sancho-Martinez SM, Novoa JML, Lopez-Hernandez FJ . Apoptotic volume
decrease as a geometric determinant for cell dismantling into apoptotic bodies. _Cell Death Differ_ 2010; 17: 1665–1671. Article CAS PubMed Google Scholar * Heath MC . Hypersensitive
response-related death. _Plant Mol Biol_ 2005; 44: 321–334. Article Google Scholar * Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M _et al_. A plant vacuolar protease, VPE,
mediates virus-induced hypersensitive cell death. _Science_ 2004; 305: 855–858. Article CAS PubMed Google Scholar * Hatsugai N, Iwasaki S, Tamura K, Kondo M, Fuji K, Ogasawara K _et al_.
A novel membrane fusion-mediated plant immunity against bacterial pathogens. _Genes Dev_ 2009; 23: 2496–2506. Article CAS PubMed PubMed Central Google Scholar * Chichkova NV, Shaw J,
Galiullina RA, Drury GE, Tuzhikov AI, Kim SH _et al_. Phytaspase, a relocalisable cell death promoting plant protease with caspase specificity. _EMBO J_ 2010; 29: 1149–1161. Article CAS
PubMed PubMed Central Google Scholar * Marty F . Plant vacuoles. _Plant Cell_ 1999; 11: 587–599. Article CAS PubMed PubMed Central Google Scholar * Müntz K . Protein dynamics and
proteolysis in plant vacuoles. _J Exp Bot_ 2007; 58: 2391–2407. Article PubMed Google Scholar * Jones AM . Programmed cell death in development and defense. _Plant Physiol_ 2001; 125:
95–97. Article Google Scholar * Beers EP . Programmed cell death during plant growth and development. _Cell Death Differ_ 1997; 4: 649–661. Article CAS PubMed Google Scholar * Filonova
LH, Bozhkov PV, Brukhin VB, Daniel G, Zhivotovsky B, von Arnold S . Two waves of programmed cell death occur during formation of development of somatic embryos in the gymnosperm, Norway
spruce. _J Cell Sci_ 2000; 113: 4399–4411. CAS PubMed Google Scholar * Bozhkov PV, Filonova LH, Suarez MF . Programmed cell death in plant embryogenesis. _Curr Top Dev Biol_ 2005; 67:
135–179. Article CAS PubMed Google Scholar * van Doorn WG, Woltering EJ . Many ways to exit? Cell death categories in plants. _Trends Plant Sci_ 2005; 10: 117–122. Article CAS PubMed
Google Scholar * Avci U, Petzold HE, Ismail IO, Beers EP, Haigler CH . Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in
_Arabidopsis_ roots. _Plant J_ 2008; 56: 303–315. Article CAS PubMed Google Scholar * Bethke PC, Lonsdale JE, Fath A, Jones RL . Hormonally regulated programmed cell death in barley
aleurone cells. _Plant Cell_ 1999; 11: 1033–1046. Article CAS PubMed PubMed Central Google Scholar * Drew MC, He CJ, Morgan PW . Programmed cell death and aerenchyma formation in roots.
_Trends Plant Sci_ 2000; 5: 123–127. Article CAS PubMed Google Scholar * Rubinstein B . Regulation of cell death in flower petals. _Plant Mol Biol_ 2000; 44: 303–318. Article CAS
PubMed Google Scholar * Gunawardena AHLAN . Programmed cell death and tissue remodeling in plants. _J Exp Bot_ 2008; 59: 445–451. Article CAS PubMed Google Scholar * Fukuda H .
Xylogenesis: initiation, progression, and cell death. _Annu Rev Plant Physiol Plant Mol Biol_ 1996; 47: 299–345. Article CAS PubMed Google Scholar * Courtois-Moreau CL, Pesquet E, Sjödin
A, Muñiz L, Bollhöner B, Kaneda M _et al_. A unique program for cell death in xylem fibers of _Populus_ stem. _Plant J_ 2009; 58: 260–274. Article CAS PubMed Google Scholar * Doelling
JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD . The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in _Arabidopsis thaliana_. _J Biol Chem_
2002; 277: 33105–33114. Article CAS PubMed Google Scholar * Hanaoka H, Noda T, Shirano Y, Kato T, Hayashi H, Shibata D _et al_. Leaf senescence and starvation-induced chlorosis are
accelerated by the disruption of an _Arabidopsis_ autophagy gene. _Plant Physiol_ 2002; 129: 1181–1193. Article CAS PubMed PubMed Central Google Scholar * Kwon SI, Cho HJ, Jung JH,
Yoshimoto K, Park OK . 2010. The RabGTPase RabG3b functions in autophagy and contributes to tracheary element differentiation in _Arabidopsis_. _Plant J_ 2010; 64: 151–164. CAS PubMed
Google Scholar * Majno G, Joris I . Apoptosis, oncosis, and necrosis. An overview of cell death. _Am J Pathol_ 1995; 146: 3–15. CAS PubMed PubMed Central Google Scholar * Christofferson
DE, Yuan J . Necroptosis as an alternative form of programmed cell death. _Curr Opin Cell Biol_ 2010; 22: 263–268. Article CAS PubMed PubMed Central Google Scholar * Jones AM . Does
the plant mitochondrion integrate cellular stress and regulate programmed cell death? _Trends Plant Sci_ 2000; 5: 225–230. Article CAS PubMed Google Scholar * Scott I, Logan DC .
Mitochondrial morphology transition is an early indicator of subsequent cell death in _Arabidopsis_. _New Phytol_ 2008; 177: 90–101. CAS PubMed Google Scholar * Kiraly Z, Barna B, Ersek T
. Hypersensitivity as a consequence, not cause, of plant resistance to infection. _Nature_ 1972; 239: 456–458. Article Google Scholar * Jones AM, Dangl JL . Logjam at the Styx: programmed
cell death in plants. _Trends Plant Sci_ 1996; 1: 114–119. Article Google Scholar * Greenberg JT . Programmed cell death in plant–pathogen interactions. _Annu Rev Plant Physiol Plant Mol
Biol_ 1997; 48: 525–545. Article CAS PubMed Google Scholar * Richberg MH, Aviv DH, Dangl JL . Dead cells do tell tales. _Curr Opin Plant Biol_ 1998; 1: 480–485. Article CAS PubMed
Google Scholar * Lam E, Kato N, Lawton M . Programmed cell death, mitochondria and the plant hypersensitive response. _Nature_ 2001; 411: 848–853. Article CAS PubMed Google Scholar *
Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E . The hypersensitive response; the centenary is upon us but how much do we know? _J Exp Bot_ 2008; 59: 501–520. Article CAS PubMed Google
Scholar * Coll NS, Vercammen D, Smidler A, Clover C, van Breusegem F, Dangl JL _et al_. _Arabidopsis_ type I metacaspases control cell death. _Science_ 2010; 330: 1393–1397. Article CAS
PubMed Google Scholar * Rojo E, Martin R, Carter C, Zouhar J, Pan S, Plotnikova J _et al_. VPE_γ_ exhibits a caspase-like activity that contributes to defense against pathogens. _Curr
Biol_ 2004; 14: 1897–1906. Article CAS PubMed Google Scholar * Hofius D, Schultz-Larsen T, Joensen J, Tsitsigiannis DI, Petersen NHT, Mattson O _et al_. Autophagic components contribute
to hypersensitive cell death in _Arabidopsis_. _Cell_ 2009; 137: 773–783.193. Article CAS PubMed Google Scholar * Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar SP .
Autophagy regulates programmed cell death during the plant innate immune response. _Cell_ 2005; 121: 567–577. Article CAS PubMed Google Scholar * Yoshimoto K, Jikumaru Y, Kamiya Y,
Kusano M, Consonni C, Panstruga R _et al_. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response
in _Arabidopsis_. _Plant Cell_ 2009; 21: 2914–2927. Article CAS PubMed PubMed Central Google Scholar * Beers EP, McDowell JM . Regulation and execution of programmed cell death in
response to pathogens, stress and developmental cues. _Curr Opin Plant Biol_ 2001; 4: 561–567. Article CAS PubMed Google Scholar * Lorang J, Cuesta-Marcos A, Hayes PM, Wolpert TJ .
Identification and mapping of adult-onset sensitivity to victorin in barley. _Mol Breeding_ 2010; 26: 545–550. Article CAS Google Scholar * Curtis MJ, Wolpert TJ . The victorin induced
mitochondrial permeability transition precedes cell shrinkage and biochemical markers of cell death and shrinkage occurs without loss of membrane integrity. _Plant J_ 2004; 38: 244–259.
Article CAS PubMed Google Scholar * Krzymowska M, Konopka-Postupolska D, Sobczak M, Macioszek V, Ellis BE, Hennig J . Infection of tobacco with different _Pseudomonas syringae_ pathovars
leads to distinct morphotypes of programmed cell death. _Plant J_ 2007; 50: 253–264. Article CAS PubMed Google Scholar * Bosch M, Franklin-Tong VE . Self-incompatibility in Papaver:
signalling to trigger PCD in incompatible pollen. _J Exp Bot_ 2008; 59: 481–490. Article CAS PubMed Google Scholar * Geitmann A, Franklin-Tong VE, Emons AC . The self-incompatibility
response in _Papaver rhoeas_ pollen causes early and striking alterations to organelles. _Cell Death Differ_ 2004; 11: 812–822. Article CAS PubMed Google Scholar * Fath A, Bethke P,
Lonsdale J, Meza-Romero R, Jones R . Programmed cell death in cereal aleurone. _Plant Mol Biol_ 2000; 44: 255–266. Article CAS PubMed Google Scholar * Young TE, Gallie DR . Programmed
cell death during endosperm development. _Plant Mol Biol_ 2000; 44: 283–301. Article CAS PubMed Google Scholar * Gao M, Showalter AM . Yariv reagent induces programmed cell death in
_Arabidopsis_ cell cultures and implicates arabinogalactan protein involvement. _Plant J_ 1999; 19: 321–331. Article CAS PubMed Google Scholar * Faoro F, Iriti M . Plant cell death and
cellular alterations induced by ozone: key studies in Mediterranean conditions. _Environ Pollut_ 2009; 157: 1470–1477. Article CAS PubMed Google Scholar Download references AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Plant Sciences, Mann Laboratory, University of California, Davis, 95616, CA, USA W G van Doorn * Department of Horticulture, Virginia
Polytechnic Institute and State University, Blacksburg, 24061, VA, USA E P Beers * Department of Biology and Carolina Center of Genome Science, University of North Carolina, Chapel Hill,
27599, NC, USA J L Dangl * School of Biosciences, College of Life and Environmental and Life Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK, V E Franklin-Tong *
Faculty of Life Sciences, University of Manchester, Manchester, M13 9PT, UK P Gallois * Department of Biological Science, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto
606-8502, Japan, I Hara-Nishimura * Departments of Biology and Pharmacology, University of North Carolina, Chapel Hill, 27599, NC, USA A M Jones * Department of Environmental Science and
Technology, Institute for Environmental Science and Technology, Saitama University, Saitama, 338-8570, Japan M Kawai-Yamada * Department of Plant Biology and Pathology, Rutgers the State
University of New Jersey, 59 Dudley Road, New Brunswick, 08901, NJ, USA E Lam * Department of Biology, University of Copenhagen, Ole Maaloes Vej 5, 220 Copenhagen, Denmark, J Mundy & M
Petersen * Aberystwyth University, Institute of Biological, Environmental and Rural Sciences, Aberystwyth, SY23 3DA, Wales, UK L A J Mur * The Integrative Cell Biology Laboratory, School of
Biological and Biomedical Sciences, University of Durham, South Road, DH1 3LE, Durham, UK A Smertenko * Plant Pathology Programme, Scottish Crop Research Institute, Invergowrie, Dundee DD2
5DA, UK, M Taliansky * VIB Department of Plant Systems Biology and Department of Plant Biotechnology and Genetics, University of Ghent, Technologiepark 927, Ghent 9052, Belgium, F Van
Breusegem * Department of Botany and Plant Pathology, Center for Genome Research and Biocomputing, Oregon State University, Corvallis, 97330, OR, USA T Wolpert * Horticultural Supply Chains,
Droevendaalsesteeg 1, and Food and Biobased Research, Bornse Weilanden 9, Wageningen University and Research Center, Wageningen, 6708, The Netherlands E Woltering * Division of Toxicology,
Institute of Environmental Medicine, Karolinska Institutet, Box 210, Stockholm171 77, Sweden, B Zhivotovsky * Department of Plant Biology and Forest Genetics, Uppsala BioCentre, Swedish
University of Agricultural Sciences, Box 7080, Uppsala 75007, Sweden, P V Bozhkov Authors * W G van Doorn View author publications You can also search for this author inPubMed Google Scholar
* E P Beers View author publications You can also search for this author inPubMed Google Scholar * J L Dangl View author publications You can also search for this author inPubMed Google
Scholar * V E Franklin-Tong View author publications You can also search for this author inPubMed Google Scholar * P Gallois View author publications You can also search for this author
inPubMed Google Scholar * I Hara-Nishimura View author publications You can also search for this author inPubMed Google Scholar * A M Jones View author publications You can also search for
this author inPubMed Google Scholar * M Kawai-Yamada View author publications You can also search for this author inPubMed Google Scholar * E Lam View author publications You can also search
for this author inPubMed Google Scholar * J Mundy View author publications You can also search for this author inPubMed Google Scholar * L A J Mur View author publications You can also
search for this author inPubMed Google Scholar * M Petersen View author publications You can also search for this author inPubMed Google Scholar * A Smertenko View author publications You
can also search for this author inPubMed Google Scholar * M Taliansky View author publications You can also search for this author inPubMed Google Scholar * F Van Breusegem View author
publications You can also search for this author inPubMed Google Scholar * T Wolpert View author publications You can also search for this author inPubMed Google Scholar * E Woltering View
author publications You can also search for this author inPubMed Google Scholar * B Zhivotovsky View author publications You can also search for this author inPubMed Google Scholar * P V
Bozhkov View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to W G van Doorn or P V Bozhkov. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by V De Laurenzi RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE van Doorn, W., Beers, E., Dangl, J. _et al._ Morphological classification of plant cell deaths. _Cell Death Differ_ 18, 1241–1246 (2011). https://doi.org/10.1038/cdd.2011.36 Download
citation * Received: 09 February 2011 * Accepted: 07 March 2011 * Published: 15 April 2011 * Issue Date: August 2011 * DOI: https://doi.org/10.1038/cdd.2011.36 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative KEYWORDS * apoptosis * autophagy * cell wall * hypersensitive response * necrosis * vacuolar cell death