Play all audios:
ABSTRACT The regulation of mitochondrial quality has emerged as a central issue in neurodegeneration, diabetes, and cancer. We utilized repeated low-dose applications of the complex I
inhibitor 1-methyl-4-phenylpyridinium (MPP+) over 2 weeks to study cellular responses to chronic mitochondrial stress. Chronic MPP+ triggered depletion of functional mitochondria resulting
in diminished capacities for aerobic respiration. Inhibiting autophagy/mitophagy only partially restored mitochondrial content. In contrast, inhibiting activation of extracellular
signal-regulated protein kinases conferred complete cytoprotection with full restoration of mitochondrial functional and morphological parameters, enhancing spare respiratory capacity in
MPP+ co-treated cells above that of control cells. Reversal of mitochondrial injury occurred when U0126 was added 1 week after MPP+, implicating enhanced repair mechanisms. Chronic MPP+
caused a >90% decrease in complex I subunits, along with decreases in complex III and IV subunits. Decreases in respiratory complex subunits were reversed by co-treatment with U0126,
ERK1/2 RNAi or transfection of dominant-negative MEK1, but only partially restored by degradation inhibitors. Chronic MPP+ also suppressed the _de novo_ synthesis of mitochondrial
DNA-encoded proteins, accompanied by decreased expression of the mitochondrial transcription factor TFAM. U0126 completely reversed each of these deficits in mitochondrial translation and
protein expression. These data indicate a key, limiting role for mitochondrial biogenesis in determining the outcome of injuries associated with elevated mitophagy. SIMILAR CONTENT BEING
VIEWED BY OTHERS MEKK3-MEK5-ERK5 SIGNALING PROMOTES MITOCHONDRIAL DEGRADATION Article Open access 20 October 2020 DELE1 TRACKS PERTURBED PROTEIN IMPORT AND PROCESSING IN HUMAN MITOCHONDRIA
Article Open access 06 April 2022 TETRACYCLINES PROMOTE SURVIVAL AND FITNESS IN MITOCHONDRIAL DISEASE MODELS Article 18 January 2021 MAIN Mitochondrial dysfunction has long been implicated
in Parkinson’s disease (PD) pathogenesis.1 Reduced mitochondrial complex I activity is observed in the substantia nigra of patients with PD. Neurotoxins, such as rotenone or
1-methyl-4-phenylpyridinium (MPP+), which inhibit complex I of the mitochondrial respiratory chain, cause degeneration of dopaminergic neurons. Recently, diverse proteins involved in
familial PD, including _α_-synuclein, Parkin, DJ-1, and PTEN-induced kinase 1, have been implicated in regulating mitochondrial dynamics and quality control.1 A key element in mitochondrial
quality control involves autophagic targeting of mitochondria for lysosomal degradation. Recent studies implicate induction of the autophagy-lysosome pathway in genetic and toxin models of
PD.2, 3, 4, 5 Decreases in chaperone-mediated autophagy,6 elicit further increases in macroautophagy (hereafter autophagy), but this compensatory increase may ultimately prove detrimental.7
Although autophagosomes are observed in substantia nigra neurons of PD and Lewy body dementia patients,8, 9 whether autophagy is protective or detrimental may depend on interactions of this
pathway with other metabolic or reparative pathways. We previously demonstrated that acute MPP+ or 6-hydroxydopamine (6-OHDA) treatments induce autophagy and mitochondrial degradation in
SH-SY5Y cells, mediated by activation of extracellular signal-regulated protein kinases (ERK).2, 4 Given that chronic or repetitive micro-insults have been proposed as possible contributing
factors for human neurodegenerative diseases,10 and chronic PD models may more fully mimic _α_–synuclein aggregation and formation of Lewy body-like structures,11, 12 we modified our MPP+
injury model to cause gradual cell death over a 2-week period to study the potential roles of compensatory or repair pathways. Retinoic acid-differentiated SH-SY5Y cells were treated with
multiple low doses of MPP+, resulting in striking changes in mitochondrial morphology, function and autophagy, with depletion of nuclear DNA-encoded mitochondrial proteins and impaired
translation of mitochondrial DNA (mtDNA)-encoded proteins. Similar to the acute model, inhibition of autophagy using siRNA to the essential autophagy proteins Atg7 or microtubule-associated
protein 1 light chain 3 (LC3) conferred partial protection against autophagic cell death. In contrast to the acute model, however, the MPP+-induced disruption in mitochondrial morphology,
protein expression, and function was completely reversed by inhibition of ERK1/2 activation. These data suggest that in the chronic setting, blunting the rapid mitochondrial turnover induced
by MPP+ serves to prevent autophagic cell death, while allowing compensatory/reparative biogenesis responses to develop. RESULTS CHARACTERIZATION OF A 2-WEEK MODEL OF PROGRESSIVE MPP+
TOXICITY Retinoic acid-differentiated SH-SY5Y cells were treated with lower doses of MPP+ (in fresh medium) three times a week, resulting in time- and dose-related neuronal cell death over 2
weeks (Supplementary Figure S1A). Chronic MPP+ treatment caused fragmentation of the reticular mitochondrial network observed in control cells, with isolated, sometimes enlarged,
mitochondria (Figure 4b), reductions in mitochondrial membrane potential (Supplementary Figure S1B), and numerous ultrastructural alterations (Figure 4d). The mitochondrial matrix was pale
with disorganized cristae often restricted to the periphery. Quantitative ultrastructural analysis revealed significant increases in the percentage of both the smallest (≤0.2 _μ_m2) and
largest (>3.0 _μ_m2) mitochondrial profiles, with increased circularity of those >1 _μ_m2 of area, consistent with swelling (Supplementary Figures S1C–E). CHRONIC MPP+ INCREASED
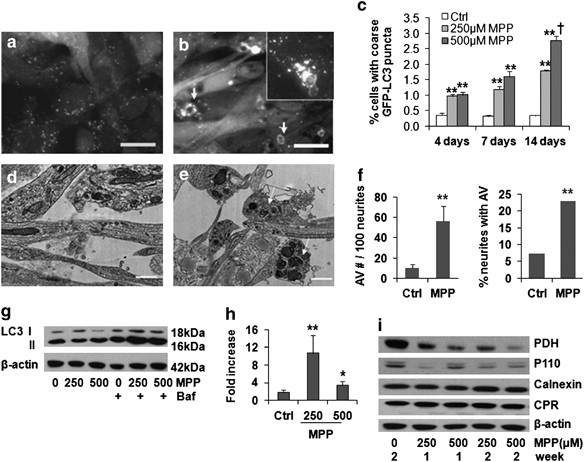
AUTOPHAGIC FLUX IN SH-SY5Y CELLS GFP-LC3 is an established marker of pre-autophagosomal membranes (phagophores) and early autophagic vacuoles (AVs). In stable GFP-LC3-expressing,
neuronal-differentiated SH-SY5Y cells, chronic MPP+ treatment led to a reduction in very fine GFP-LC3 granules and an increase in coarse GFP-LC3 puncta, often with ring-like morphology
(Figures 1a–c). Ultrastructural analysis confirmed increased AVs in the cell body and in neurites (Figures 1d–f). Chronic MPP+ treatment also significantly increased the average size of
monodansylcadavarine fluorescent puncta (Supplementary Figures S2A and B), consistent with fusion and maturation to autolysosomes. Treatment with bafilomycin A1, an inhibitor of early AV
acidification and fusion to lysosomes, was used to arrest degradation of LC3 II, the form of LC3 that is covalently bound to autophagic membranes. The difference in LC3 II levels in the
presence and absence of bafilomycin reflects the degradative flux of autophagosomes in that time frame.13 In contrast to acute MPP+ toxicity, chronic MPP+ caused a slight decrease in the
steady-state LC3 II level. As reported in other cell types, induction of autophagy can cause a decrease in LC3 II levels due to increased turnover of LC3 protein.13 The addition of
bafilomycin A1 to MPP+-treated cells caused a greater increase in LC3 II levels than that observed in control cells, indicating an increase in LC3 turnover in cells treated chronically with
MPP+, particularly at the lower dose (Figures 1g and h). There was mitochondrial protein loss, but no marked change in two endoplasmic reticulum proteins (Figure 1i), indicating induction of
selective mitophagy. AUTOPHAGY DOES NOT HAVE A MAJOR, DETERMINING ROLE FOR CELL FATE DURING CHRONIC MPP+ TOXICITY In the acute MPP+ model, inhibition of autophagy does not prevent
mitochondrial damage, but reduces autophagic cell death.4 In the chronic model, RNAi of Atg7 or LC3 attenuated the increase in LC3 II and reduced the number of autophagic vacuoles (Figures
2a and b), but resulted in only modest protection (Supplementary Figure S2C). Interestingly, Atg7 knockdown protected MPP+-treated cells against mitochondrial swelling (Figure 2b), inhibited
the loss of mitochondrial protein p60 and PDH (Figure 2c; Supplementary Figure S2D) and conferred partial improvement in maximal oxygen consumption rates (OCR) (Figure 2d), which were not
observed in the acute model.4 These data suggest that limiting the autophagic response may unmask other compensatory responses in the chronic MPP+ model. INHIBITION OF ERK1/2 ACTIVATION
REVERSED STRUCTURAL DAMAGE TO MITOCHONDRIA AND PRESERVED LEVELS OF MITOCHONDRIAL COMPLEX PROTEINS IN CHRONIC MPP+-TREATED CELLS ERK1/2 has been implicated in the regulation of autophagy and
mitophagy,2, 4, 14 showing a time-related 40–50% increase, peaking and then decreasing after each MPP+ pulse (Figures 3a and b). Increased ERK1/2 activity was observed using an _in vitro_
kinase pull-down assay (Figure 3c) and a luciferase assay detecting the _in situ_ activity of transfected ERK2 or its kinase-dead control (Figure 3d). Inhibiting the upstream MAPK/ERK1/2
kinase (MEK) by adding U0126 with each dose of MPP+ conferred nearly complete protection against MPP+-induced cell death (Figure 4a). This protection was not due to decreased expression of
dopamine transporter, increased expression of vesicular monoamine transporter, or interference with the acute effects of MPP+ on mitochondrial respiration (Supplementary Figures S3A and B).
Instead, U0126 reversed the mitochondrial morphology changes (Figure 4b) and the loss of mitochondrial proteins (Figure 4c; Figure 5c) observed in cells chronically stressed with MPP+.
U0126/MPP+ co-treated cells displayed relatively uniform mitochondria with intact inner and outer membranes and preserved cristae (Figure 4d). The degree of protection was much greater than
that observed by inhibiting autophagy alone, implicating other mechanisms to promote the integrity of chronically injured mitochondria. MPP+ can directly inhibit complex I (NADH-ubiquinone
oxidoreductase) activity of the mitochondrial electron transport chain although other mechanisms of toxicity have also been implicated.15 MPP+ caused >90% decrease in the nuclear-encoded
alpha subunit 9 (NDUFA9), with complete reversal by U0126 (Figures 5a and b). Similar results were observed for the complex I subunit NDUFB8 and for proteins in complex IV (Figure 5c), with
a lesser effect on complex III and no effect on complex V-alpha. The decreased respiratory protein expression could be partially attributed to the combined activities of the proteasome,
autophagy-lysosome system and the mitochondrial LON peptidase 1 (Supplementary Figures S3C–F). As the U0126 data implicated the MEK/ERK signaling pathway, we transfected SH-SY5Y cells with
either siRNA targeting ERK1/2 (Figure 5g; Supplementary Figure S3G) or dominant-negative MEK1 (DN-MEK1; Supplementary Figures S4A and B). Either of these methods also attenuated the
MPP+-elicited decrease in mitochondrial respiratory complex proteins. AMELIORATION OF MPP+ INDUCED STRUCTURAL CHANGES IS ASSOCIATED WITH RESTORATION OF BASAL FUNCTION AND ENHANCED SPARE
RESPIRATORY CAPACITY Analysis of live cell aerobic respiration revealed that U0126 not only reversed the MPP+-induced decrease in basal respiration (the mean of the first four time points in
Figure 5d), but also caused a further increase in FCCP-induced maximal mitochondrial respiration (Figures 5d and e). If cells were cultured in galactose for 24 h prior to analysis to induce
dependence on mitochondrial respiration,16 MPP+ reduced cellular ATP concentrations, and this effect was also rescued by U0126 (Figure 5f). Moreover, the spare respiratory capacity17 or
mitochondrial reserve capacity (RC), defined as the difference between maximal FCCP-induced respiration and basal respiration, was increased above the baseline control cell level by
approximately six-fold in MPP+-injured cells rescued by U0126 (Figure 5e). To distinguish prevention of injury from enhanced compensation or recovery, we conducted experiments in which the
MEK1/2 inhibitor was added 1 week after beginning MPP+ treatments (Supplementary Figures S4C–F). At 1 week, there are already significant alterations in mitochondrial structure and function.
Addition of U0126 during the second week of continued MPP+ treatments significantly restored mitochondrial structure and function (Supplementary Figure S4F), implicating the involvement of
reparative pathways. INHIBITING MEK/ERK ACTIVATION RESTORES INDICES OF MITOCHONDRIAL BIOGENESIS IN MPP+-TREATED CELLS Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
(PGC-1_α_), nuclear respiratory factor 1 (NRF-1), and mitochondrial transcription factor A (TFAM) regulate mitochondrial biogenesis. Whereas the former two factors act as co-regulators of
nuclear transcription, PGC-1_α_ also functions in the mitochondria,18 and TFAM has an important role in mtDNA stability and the biosynthesis of the 13 mtDNA-encoded respiratory chain
subunits. There were no significant changes in the levels of PGC-1_α_ or NRF-1 (Figure 6a; Supplementary Figures S5A and B), although subcellular localization of PGC-1_α_ was altered. In
control cells, PGC-1_α_ was colocalized with mitochondrial marker p60, as described in other systems.18 Significant nuclear signal was not observed, consistent with its constitutive nuclear
export unless phosphorylated.19 MPP+ elicited dissociation of cytoplasmic PGC-1_α_ granules from mitochondria (Figures 6b and c; Supplementary Figure S5C), which was opposed by U0126. There
were no significant changes in PGC-1_α_ mRNA levels (Supplementary Figure S5D). In contrast, TFAM protein was significantly decreased after chronic MPP+ treatment (Figures 6d and e). TFAM
mRNA levels were not significantly decreased (Supplementary Figure S5D), whereas inhibition of the mitochondrial Lon peptidase 1 produced restoration of TFAM levels in MPP+-treated cells
(Supplementary Figure S3F), implicating a degradative process. As mitochondrially localized PGC-1_α_ binds the D-loop region of mtDNA, where it interacts with TFAM,18 and TFAM is central to
transcription of mitochondrial genes, we directly studied mitochondrial protein synthesis by [35S]-methionine pulse labeling. Whereas a single dose of 250 _μ_M MPP+ had little effect on
mitochondrial translation, chronic administration of MPP+ over 1 week significantly inhibited biosynthesis of the 13 mtDNA-encoded respiratory chain subunits (Figures 7a and c). However, it
has no apparent effect on nuclear DNA-encoded protein translation (Figure 7b). The MEK inhibitor U0126 prevented the decrease in TFAM protein levels in MPP+-treated cells (Figures 6d and e),
and restored translation of the mtDNA-encoded proteins (Figures 7a and c). ERK1/2 siRNA protected TFAM levels in MPP+-treated cells (Figure 5g; Supplementary Figure S3G). Furthermore,
overexpression of TFAM-HA reversed MPP+-induced decreases in respiratory complex I, III, and IV subunits (Figure 6f, Supplementary Figure S5E). DISCUSSION Low dose, repeated MPP+
administration caused significant loss of functional mitochondria, accompanied by increased mitophagy, alterations in nuclear-encoded biogenesis proteins and suppression of mitochondrial
translation. While suppressing autophagy conferred only partial restoration of mitochondrial content, the MEK inhibitor U0126 conferred complete restoration of mitochondrial protein content,
morphology, and function. Similar protective effects were observed after use of ERK1/2 siRNA or DN-MEK1 to downregulate the ERK1/2 signaling pathway. These changes were accompanied by
recovery of TFAM levels and restored translation of mtDNA-encoded respiratory subunits, indicating that suppression of mitochondrial biogenesis has an important role in the loss of
mitochondrial function accompanying chronic, low-dose exposures to MPP+. Mitochondrial respiration is tightly linked to ATP production. Neurons and differentiated neuronal cells are largely
dependent upon oxidative phosphorylation and usually exhibit a substantial mitochondrial RC (also known as spare respiratory capacity), to rapidly meet the requirements of increased
functional demand and stress.17 Indeed, the RC is a major determinant of the outcome of neuronal excitotoxicity, ischemia, and oxidative stress.17, 20 Our data indicate that chronic MPP+
exposure not only inhibited basal mitochondrial respiration, but also diminished the RC. Notably, injury combined with inhibition of ERK1/2 activation resulted in a greater RC than observed
basally in uninjured cells, suggesting a biogenesis-related adaptation. Although a single low dose of MPP+ was sufficient to inhibit oxygen consumption (Supplementary Figure S3B), presumably
through direct inhibition of complex I activity, it was insufficient to cause either mitochondrial translation deficits or cell death. With repeated, chronic exposures, a significant
decrease in the expression of mitochondrial respiratory complex proteins, particularly complex I, IV, and III subunits, contributed to a more permanent loss of respiratory function. These
data indicate that additional cell biological mechanisms may be more important to MPP+ toxicity than simply its direct, immediate effect on complex I activity. In addition to autophagy and
biogenesis, as observed in this study, microtubule-dependent trafficking could also impact functional levels of mitochondria in neurons, and several complex I inhibitors have been shown to
impair microtubule function.15 Blocking different protease degradation pathways partially prevented the MPP+-induced decreases in mitochondrial respiratory complex proteins, suggesting that
degradation is only one of the mechanisms leading to decreased expression levels. Indeed, after 1 week of MPP+ treatment, there was substantial inhibition of mtDNA-, but not nuclear DNA-,
encoded protein synthesis. It is possible that decreases in expression of mtDNA-encoded proteins can impair the overall stability of mitochondrial complexes, resulting in enhanced
degradation of nuclear DNA-encoded subunits. Although we didn’t observe notable changes in nuclear DNA-encoded protein synthesis, nor were there significant changes in the mRNA levels of
several nuclear-encoded subunits, the possibility of post-transcriptional or translational regulation of these proteins remains. Increasing evidence indicates that ERK1/2 signaling has a
central role in regulating mitochondrial function.21, 22 ERK1/2 activation leads to the loss of mitochondrial membrane potential and mitochondrial swelling in renal proximal tubular cells,23
promotes ROS production and mitochondrial calcium elevations in ischemia,24 and increases Bax protein expression and release of cytochrome _c_.25 The degree of mitophagy elicited by ERK
activation is directly correlated with its mitochondrial localization.2 As with acute exposures to MPP+ or 6-OHDA,2, 4 ERK1/2 was activated, albeit to a lower level, in the chronic MPP+
model. It is known that the effects of ERK signaling in mediating multiple cellular functions are related not only to the magnitude of activation, but also to subcellular
compartmentalization and temporal patterns of activation.26 In the chronic MPP+ model, MPP+ was administrated three times per week for 2 weeks and caused multiple cycles of periodic
activation. This pattern of ERK activation might be associated with distinct effects on regulating mitochondrial turnover versus biogenesis between acute and chronic MPP+ models, and explain
why multiple administrations of MPTP are typically needed to elicit parkinsonian injury in mice. At this point, it is unknown whether the effects of ERK on mitochondrial biogenesis is
mediated through cytoplasmic, nuclear or mitochondrial signaling, as ERK is known to traffic to each of these compartments with different temporal patterns of activation. In addition to its
previously described role in promoting mitophagy,2 the current study reveals a novel role for ERK1/2 in modulating mitochondrial protein synthesis (Figure 7). Both effects would synergize in
reducing levels of functional mitochondria. Studies in other cell types implicate ERK1/2 in promoting glycolytic metabolism to provide intermediates compatible with rapid growth,27 while
decreasing mitochondrial function.22 Although decreases in mitochondrial biogenesis may confer growth advantages for transformed cells, the loss of mitochondrial content likely contributes
to the toxic effects of MPP+ in neuronal cells. Notably, protection afforded by U0126, which inhibited mitophagy as well as reversing the biogenesis deficit, was much more complete than
inhibiting degradation alone. Moreover, even when ERK1/2 inhibition was delayed to the second week of MPP+ treatments following development of significant mitochondrial injury, there was
restoration of mitochondrial structure and function, indicating a key role for reparative mitochondrial biogenesis in determining successful adaptation to chronic intoxication. Mitochondrial
biogenesis is regulated by PGC-1_α_, NRF-1, and TFAM.28 TFAM is responsible for initiating synthesis of mtDNA-encoded respiratory chain proteins. TFAM knockout mice exhibit respiratory
chain deficits and reduced mtDNA in midbrain DA neurons,29 whereas overexpression of TFAM elicits an increase in mtDNA and mitochondrial respiratory chain proteins.30 We found that low dose,
repetitive exposures to MPP+ significantly decreased TFAM protein expression, reversed by co-treatment with U0126- or ERK1/2-targeted siRNA. Although siRNA knockdown of the mitochondrial
LON peptidase 1 partially restored TFAM and mitochondrial respiratory protein levels, the more complete reversal observed with U0126 implicate additional mechanisms, potentially related to
protein synthesis. Depending on the cellular context, ERK1/2 may either destabilize31 or stabilize32 mRNA species. Thus, the effects of ERK1/2 inhibition on biogenesis may depend upon
whether basal or injury-stimulated contexts are studied. U0126 shows no basal effects on TFAM or mitochondrial respiratory protein levels, whereas showing striking effects in reversing the
MPP+-induced changes in protein translation and degradation. The ability of overexpressed TFAM to reverse the MPP+-induced decreases in mitochondrial respiratory complex proteins confirmed
the importance of TFAM modulation in chronic MPP+ toxicity. Although the total level of PGC-1_α_ was not affected by chronic MPP+, a decreased mitochondrial localization of PGC-1 _α_ was
observed. PGC-1_α_ is not only located in the nucleus, but also in the mitochondria where it interacts with TFAM to regulate mitochondrial biogenesis.18 Thus, altered localization of
PGC-1_α_ may cooperate with the loss of TFAM expression to impair mitochondrial biogenesis. Inhibiting ERK1/2 activation also restored mitochondrial distribution of PGC-1_α_. Although there
were no changes in NRF-1 expression observed in our chronic MPP+ setting, the possibility of functional alterations due to altered phosphorylation remains.33 MPP+ sensitivity in cells is
correlated with DAT expression and inversely correlated with expression of vesicular monoamine transporter 2 (VMAT2).34, 35 Repeated administration of U0126 caused an increase in DAT and a
decrease in VMAT2, indicating that the protection elicited by U0126 was not due to altered transport of MPP+. Moreover, U0126 did not affect the kinetics of acute respiratory responses to
MPP+, and conferred protection even when administered only during the second week of toxicity. Therefore, the major protective effect of U0126 in the chronic MPP+ model is likely to involve
restoration of mitochondrial protein synthesis in conjunction with partial reduction of mitochondrial degradation. The autophagy-lysosomal degradation pathway is recognized as a key adaptive
response in the pathogenesis of many neurodegenerative diseases including PD.36 In acute injury, autophagy-lysosomal dysfunction has been demonstrated, but the role of autophagy may be
influenced by additional compensatory mechanisms in a chronic low-dose toxicity setting. Although the current data demonstrate that ERK1/2-dependent autophagic cell death is also implicated
in chronic MPP+ toxicity, there are several significant differences between acute and chronic systems. In the acute model, inhibiting MEK-ERK1/2 signaling prevents autophagy/mitophagy and
reduces cell death, but has no significant effect on mitochondrial morphology4 or mitochondrial translation (Supplementary Figure S6). In the chronic model, the MEK inhibitor not only
inhibited cell death but also resulted in restoration of mitochondrial structure and function, even when applied 1 week after initiation of MPP+ injury. This difference may be attributed to
the greater capacity for repair in less severely, but chronically injured cells. In the acute model, a large percentage of mitochondria are damaged, which may exceed the ability of the cell
to successfully repair/regenerate sufficient functional mitochondria. However, in the chronic MPP+ model, the turnover of damaged mitochondria, particularly when slowed by inhibiting ERK1/2,
may occur at a rate more compatible with mitochondrial biogenesis. It is interesting that inhibition of autophagy by Atg7 siRNA also seemed to alleviate some of the mitochondrial
morphological and functional abnormalities caused by MPP+. Taken together with the U0126 data, these results suggest that blunting the robust mitochondrial degradation response induced by
MPP+, which is associated with autophagic cell death in several neuronal injury models,2, 4, 37 allows for eventual structural and functional mitochondrial recovery in the context of chronic
low-dose injury. In summary, we discovered a key role for mitochondrial biogenesis in adaptation to chronic parkinsonian neuronal injury elicited by repeated administration of MPP+ in
RA-differentiated SH-SY5Y cells. These data not only strengthen a growing body of data implicating dysregulation of the autophagy-lysosome system in PD pathogenesis but also highlight a
novel role for impaired mitochondrial biogenesis in determining the outcome of autophagy induction. The ERK1/2 signaling pathway has a central role in the regulation of chronic MPP+
toxicity, with effects on mitochondrial content, quality, and function. Although autophagy and mitochondrial dysfunction are implicated in both acute and chronic MPP+ neurotoxin models,
there were striking differences in the reversibility of mitochondrial structural and functional parameters. These data indicate that the chronic MPP+ model may be particularly useful for
studying mechanisms that regulate localized repair and restoration of mitochondrial function in response to damaging Parkinsonian stresses. MATERIALS AND METHODS CELL LINES AND TREATMENT
SH-SY5Y cells (ATCC, Manassas, VA, USA), maintained in antibiotic-free Advanced Dulbecco’s modified Eagle’s medium with 5% heat-inactivated fetal calf serum (BioWhittaker, Walkersville, MD,
USA), 2 mM glutamine and 10 mM HEPES, were used at passages 30–45. Cells were plated at 3 × 104/cm2 in 6-, 24-, 48-well plates or LabTek II coverglass chamber slides (Nalge Nunc
International/Thermo Fisher, Pittsburgh, PA, USA) and treated with 10 _μ_M retinoic acid (RA) to induce neuronal differentiation for 72 h prior to and during each experiment. RA inhibits
proliferation38 and these differentiated SH-SY5Y cells can be maintained for weeks. Cells were treated with 250 or 500 _μ_M MPP+ three times per week for up to 2 weeks. Some cultures also
received bafilomycin-A (5 nM; Calbiochem, San Diego, CA, USA), 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene (U0126) (5 _μ_M; Promega, Madison, WI, USA) or E64D (10 _μ_M,
Calbiochem). A GFP-LC3-expressing stable SH-SY5Y cell line was used to assess MPP+-induced LC3 puncta formation.2 Cell viability was measured using Alamar Blue (Trek Diagnostics, Cleveland,
OH, USA), excitation 540 nm, emission 590 nm, in a Spectromax M2 microplate reader (Molecular Devices, Sunnyvale, CA, USA). RNA INTERFERENCE Cells were transfected with small interfering RNA
(siRNA) targeting human Atg7, the human Atg8 homolog LC3B,4 human ERK1 (5′-CAGCUGAGCAAUGACCAUA-3′) and ERK2 (5′-GACACAACACCUCAGCAAU-3′) (Sigma, St. Louis, MO, USA) or a control
non-targeting siRNA pool (Dharmacon, Lafayette, CO, USA) at 2 days before and 3 days after the first dose of MPP+. Efficacy and specificity of knockdown was assessed by western blot,
confirming that siRNA-mediated knockdown of protein expression persisted for more than 10 days after the second dose of siRNA.39 DNA TRANSFECTION Differentiated SH-SY5Y cells transfected
with pCMV6-Neo vector containing HA-tagged human TFAM, which was constructed from TFAM-tGFP plasmid (Origene, Rockville, MD, USA) by replacing the tGFP-tag sequence with an HA-tag coding
sequence (5′-TACCCATACGATGTTCCAGATTACGCTTAA-3′), were treated with chronic MPP+ 48 h after transfection. In some experiments, differentiated SH-SY5Y stably expressing dominant-negative MEK1
(DN-MEK1, S217A) cloned to pBABEpuro eukaryotic expression vector (gift from Dr. CJ Marshall, Cancer Research UK, London, England) were used. TRANSMISSION ELECTRON MICROSCOPY Cells were
fixed in 2.5% glutaraldehyde at 4 °C, processed, and photographed using a JEM 1210 transmission electron microscope (JEOL, Peabody, MA, USA) as previously reported,4 using NIH ImageJ
software to analyze mitochondrial profiles. FLUORESCENCE MICROSCOPY SH-SY5Y cells fixed with 3% paraformaldehyde or methanol were probed with mouse anti-LC3 (1 : 100, Clone 2G6, Nanotools,
San Diego, CA, USA) or mouse anti-human mitochondrial antigen 60KD (clone 113–1; 1 : 100; BioGenex, San Ramon, CA, USA), followed by Cy3-conjugated anti-rabbit antibody (1 : 500; Jackson
Immuno-Research Laboratories, West Grove, PA, USA) or Alexa Fluor 488-conjugated antibody (1 : 500; Molecular Probes, Eugene, OR, USA), and imaged at 541/572 nm and 490/520 nm
(excitation/emission), respectively. MITOCHONDRIAL MEMBRANE POTENTIAL MEASUREMENT Cells were labeled with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazolyl carbocyanine iodide (JC-1,
5 _μ_g/ml; Molecular Probes) for 15 min at 37 °C and rinsed with PBS. The signal intensity was measured by fluorescence well scan photometry (red, ex 535/ em 590 nm; green, ex 485/ em 530
nm) in a Spectromax M2 microplate reader (Molecular Devices), or imaged using an Olympus fluorescence microscope IX-71 (Olympus America Inc., Melville, NY, USA). WESTERN BLOT ANALYSIS AND
DENSITOMETRY Cells were disrupted in 0.1% Triton X-100 with protease/phosphatase inhibitors, electrophoresed through 5–15% gradient polyacrylamide gels and immunoblotted as described
previously,4 using mouse anti-phospho-ERK1/2 (1 : 1000, Cell Signaling, Beverly, MA, USA), rabbit anti-ERK1/2 (1 : 10 000, Millipore, Billerica, MA, USA), mouse anti-p110 mitochondrial
protein (1 : 500; Oncogen, Boston, MA, USA), mouse anti-human mitochondrial antigen 60KD rabbit anti-human tom20 (1 : 10 000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse
anti-pyruvate dehydrogenase E2 subunit (1 : 2000; Molecular Probes), mouse anti-LC3 (1 : 500, clone 5F10, Nanotools), rabbit anti-calnexin (1 : 4000, Calbiochem), rabbit anti-cytochrome p450
reductase (CPR, 1 : 4000, Santa Cruz), rabbit anti-ATG7 (1 : 1000, Rockland, Gilbertsville, PA, USA), Rabbit anti-HA (1 : 1000, Rockland), mouse anti-nuclear-encoded NADH-ubiquinone
oxidoreductase alpha subunit 9 (NDUFA9, 1 : 1000, Abcam, Cambridge, MA, USA), MitoProfile Total OXPHOS antibody cocktail (1 : 1000, MitoSciences, Eugene, Oregon, USA), rabbit
anti-mitochondrial transcription factor A (TFAM, 1 : 10 000, provided by Dr. C Cameron), rabbit anti-nuclear respiratory factor 1 (NRF-1, 1 : 5000, Abcam), rabbit anti-peroxisome
proliferator-activated receptor gamma coactivator 1-alpha (PGC1_α_, 1 : 500, Santa Cruz), mouse anti-_β_-actin (1 : 10 000, Sigma), and rabbit anti-glyceraldehyde-3-phosphate dehydrogenase
(GAPDH, 1 : 10 000, Abcam). Densitometry was performed using the electrophoresis documentation and analysis system 120 (Kodak, Rochester, NY, USA). ERK1/2 ACTIVITY ASSAY _In situ_ ERK1/2
activity was assessed using the PathDetect Elk1 trans-Reporting System (Stratagene, La Jolla, CA, USA) as previous reported.2 This system measures ERK1/2-dependent transactivation of Elk1 in
cells, which drives expression of luciferase. Endogenous ERK1/2 was isolated from treated cells using the MAPK Immunoprecipitation Kinase Assay Kit (Upstate Biotechnology, NY, USA) for _in
vitro_ activity assay using myelin basic protein as substrate. MITOCHONDRIAL RESPIRATION ASSAY Oxygen consumption rates (OCR) in living cells were analyzed at baseline and following the
sequential addition of 1 _μ_M oligomycin, 300 nM FCCP, 120 mM 2-deoxyglucose and 1 _μ_M rotenone (final concentrations) using the XF24 Extracellular Flux Analyzer (Seahorse Bioscience, North
Billerica, MA, USA). Steady-state cellular ATP concentrations were measured using the ATPLite-M luminescence assay (PerkinElmer; Waltham, MA, USA) and a Synergy 2 Multi-Mode Microplate
Reader (BioTek, Winooski, VT, USA) in a black 96-well microplate in glucose-free DMEM with galactose; under these conditions cells rely on OXPHOS for ATP generation.16 [35S]-METHONINE
LABELING OF MITOCHONDRIAL TRANSLATION PRODUCTS _IN VIVO_ Mitochondrial protein synthesis was assayed by [35S]-methionine pulse labeling experiments.40 Cells were washed and incubated in
methionine-free and cysteine-free DMEM for 30 min, and then incubated for 5 min at 37 °C in the same medium containing 100 _μ_g/ml of the eukaryotic translation inhibitor emetine. After the
addition of 0.2 mCi (1175 Ci/mmol) [35S]-methionine, the cells were incubated for 2 h. Lysates (25 _μ_g protein) were resolved on SDS-PAGE gels, and quantified by phosphorimaging. STATISTICS
All graphed data represent mean +/− S.E.M. from replicate experiments. Data were analyzed by one-way analysis of variance with post-hoc Fisher’s least significant difference, two-way ANOVA
or chi-square test as appropriate. Values of _P_<0.05 were considered significant. ABBREVIATIONS * Atg7: Autophagy-related protein 7 * AVs: autophagic vacuoles * DAT: dopamine transporter
* ERK: extracellular signal-regulated protein kinase * FCCP: carbonylcyanide-p-trifluoromethoxyphenylhydrazone * 6-OHDA: 6-hydroxydopamine * LC3: microtubule-associated protein 1 light
chain 3 (Atg8) * MEK1/2: mitogen-activated protein (MAP) kinase kinase 1/2 * MPP+: 1-methyl-4-phenylpyridinium * NDUFA9: NADH-ubiquinone oxidoreductase alpha subunit 9 * NRF-1: nuclear
respiratory factor 1 * OCR: oxygen consumption rate * OXPHOS: oxidative phosphorylation * PDH: Pyruvate dehydrogenase * PGC-1_α_: Peroxisome proliferator-activated receptor gamma coactivator
1-alpha * RA: retinoic acid * RC: reserve capacity or spare respiratory capacity of mitochondria * ROS: reactive oxygen species * siRNA: small interfering RNA * siCtrl: non-targeting
control siRNA * TFAM: mitochondrial transcription factor A * U0126: 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene * VMAT2: vesicular monoamine transporter 2 REFERENCES * Schon
EA, Przedborski S . Mitochondria: the next (neurode)generation. _Neuron_ 2011; 70: 1033–1053. Article CAS Google Scholar * Dagda RK, Zhu J, Kulich SM, Chu CT . Mitochondrially localized
ERK2 regulates mitophagy and autophagic cell stress: implications for Parkinson's disease. _Autophagy_ 2008; 4: 770–782. Article CAS Google Scholar * Cheng HC, Kim SR, Oo TF, Kareva
T, Yarygina O, Rzhetskaya M _et al_. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. _J Neurosci_ 2011; 31: 2125–2135. Article CAS Google
Scholar * Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT . Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced
cell death. _Am J Pathol_ 2007; 170: 75–86. Article CAS Google Scholar * Plowey ED, Cherra SJ, Liu YJ, Chu CT . Role of autophagy in G2019S-LRRK2-associated neurite shortening in
differentiated SH-SY5Y cells. _J Neurochem_ 2008; 105: 1048–1056. Article CAS Google Scholar * Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D . Impaired degradation of mutant
alpha-synuclein by chaperone-mediated autophagy. _Science_ 2004; 305: 1292–1295. Article CAS Google Scholar * Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L . Abberant
alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. _PLoS ONE_ 2009; 4: e5515. Article Google Scholar * Zhu JH, Guo F, Shelburne J,
Watkins S, Chu CT . Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. _Brain Pathol_ 2003; 13: 473–481. Article CAS Google Scholar *
Higashi S, Moore DJ, Minegishi M, Kasanuki K, Fujishiro H, Kabuta T _et al_. Localization of MAP1-LC3 in vulnerable neurons and Lewy bodies in brains of patients with dementia with Lewy
bodies. _J Neuropathol Exp Neurol_ 2011; 70: 264–280. Article CAS Google Scholar * DeFord SM, Wilson MS, Rice AC, Clausen T, Rice LK, Barabnova A _et al_. Repeated mild brain injuries
result in cognitive impairment in B6C3F1 mice. _J Neurotrauma_ 2002; 19: 427–438. Article Google Scholar * Chinta SJ, Andersen JK . Reversible inhibition of mitochondrial complex I
activity following chronic dopaminergic glutathione depletion _in vitro_: implications for Parkinson's disease. _Free Radic Biol Med_ 2006; 41: 1442–1448. Article CAS Google Scholar
* Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M _et al_. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and
alpha-synuclein. _Proc Natl Acad Sci USA_ 2005; 102: 3413–3418. Article CAS Google Scholar * Mizushima N, Yoshimori T . How to interpret LC3 immunoblotting. _Autophagy_ 2007; 3: 542–545.
Article CAS Google Scholar * Pattingre S, Bauvy C, Codogno P . Amino acids interfere with the ERK1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human
colon cancer HT-29 cells. _J Biol Chem_ 2003; 278: 16667–16674. Article CAS Google Scholar * Choi WS, Palmiter RD, Xia Z . Loss of mitochondrial complex I activity potentiates dopamine
neuron death induced by microtubule dysfunction in a Parkinson's disease model. _J Cell Biol_ 2011; 192: 873–882. Article CAS Google Scholar * Rossignol R, Gilkerson R, Aggeler R,
Yamagata K, Remington SJ, Capaldi RA . Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. _Cancer Res_ 2004; 64: 985–993. Article CAS Google Scholar
* Yadava N, Nicholls DG . Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with
rotenone. _J Neurosci_ 2007; 27: 7310–7317. Article CAS Google Scholar * Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR . Peroxisome proliferator-activated receptor
gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. _J Biol Chem_ 2010; 285: 21590–21599. Article CAS
Google Scholar * Chang JS, Huypens P, Zhang Y, Black C, Kralli A, Gettys TW . Regulation of NT-PGC-1alpha subcellular localization and function by protein kinase A-dependent modulation of
nuclear export by CRM1. _J Biol Chem_ 2010; 285: 18039–18050. Article CAS Google Scholar * Flynn JM, Choi SW, Day NU, Gerencser AA, Hubbard A, Melov S . Impaired spare respiratory
capacity in cortical synaptosomes from Sod2 null mice. _Free Radic Biol Med_ 2011; 50: 866–873. Article CAS Google Scholar * Monick MM, Powers LS, Barrett CW, Hinde S, Ashare A,
Groskreutz DJ _et al_. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. _J Immunol_ 2008; 180: 7485–7496. Article CAS Google Scholar * Nowak
G, Clifton GL, Godwin ML, Bakajsova D . Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. _Am J Physiol Renal Physiol_ 2006; 291:
F840–F855. Article CAS Google Scholar * Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG . Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and
necrosis induced by hydrogen peroxide in renal proximal tubular cells. _J Pharmacol Exp Ther_ 2008; 325: 732–740. Article CAS Google Scholar * Sucher R, Gehwolf P, Kaier T, Hermann M,
Maglione M, Oberhuber R _et al_. Intracellular signaling pathways control mitochondrial events associated with the development of ischemia/ reperfusion-associated damage. _Transpl Int_ 2009;
22: 922–930. Article CAS Google Scholar * Zhang CL, Wu LJ, Zuo HJ, Tashiro S, Onodera S, Ikejima T . Cytochrome c release from oridonin-treated apoptotic A375-S2 cells is dependent on
p53 and extracellular signal-regulated kinase activation. _J Pharmacol Sci_ 2004; 96: 155–163. Article CAS Google Scholar * Ebisuya M, Kondoh K, Nishida E . The duration, magnitude and
compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. _J Cell Sci_ 2005; 118 (Pt 14): 2997–3002. Article CAS Google Scholar * Marko AJ, Miller
RA, Kelman A, Frauwirth KA . Induction of glucose metabolism in stimulated T lymphocytes is regulated by mitogen-activated protein kinase signaling. _PLoS ONE_ 2010; 5: e15425. Article
Google Scholar * Scarpulla RC . Transcriptional paradigms in mammalian mitochondrial biogenesis and function. _Physiol Rev_ 2008; 88: 611–638. Article CAS Google Scholar * Ekstrand MI,
Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E _et al_. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. _Proc Natl Acad Sci USA_ 2007; 104:
1325–1330. Article CAS Google Scholar * Iyer S, Thomas RR, Portell FR, Dunham LD, Quigley CK, Bennett JP . Recombinant mitochondrial transcription factor A with N-terminal mitochondrial
transduction domain increases respiration and mitochondrial gene expression. _Mitochondrion_ 2009; 9: 196–203. Article CAS Google Scholar * Sato M, Shegogue D, Hatamochi A, Yamazaki S,
Trojanowska M . Lysophosphatidic acid inhibits TGF-beta-mediated stimulation of type I collagen mRNA stability via an ERK-dependent pathway in dermal fibroblasts. _Matrix Biol_ 2004; 23:
353–361. Article CAS Google Scholar * Zhai B, Yang H, Mancini A, He Q, Antoniou J, Di Battista JA . Leukotriene BBLT receptor signaling regulates the level and stability of
cyclooxygenase-2 (COX-2) mRNA through restricted activation of Ras/Raf/ERK/p42 AUF1 pathway. _J Biol Chem_ 2010; 285: 23568–23580. Article CAS Google Scholar * Piantadosi CA, Suliman HB .
Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. _J Biol Chem_ 2006; 281: 324–333. Article CAS Google Scholar * Chen CX, Huang SY,
Zhang L, Liu YJ . Synaptophysin enhances the neuroprotection of VMAT2 in MPP+-induced toxicity in MN9D cells. _Neurobiol Dis_ 2005; 19: 419–426. Article Google Scholar * Pifl C, Giros B,
Caron MG . Dopamine transporter expression confers cytotoxicity to low doses of the parkinsonism-inducing neurotoxin 1-methyl-4-phenylpyridinium. _J Neurosci_ 1993; 13: 4246–4253. Article
CAS Google Scholar * Nixon RA . Autophagy in neurodegenerative disease: friend, foe or turncoat? _Trends Neurosci_ 2006; 29: 528–535. Article CAS Google Scholar * Chakrabarti L, Eng J,
Ivanov N, Garden GA, La Spada AR . Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death. _Mol Brain_ 2009; 2: 24. Article Google
Scholar * Simpson PB, Bacha JI, Palfreyman EL, Woollacott AJ, McKernan RM, Kerby J . Retinoic acid evoked-differentiation of neuroblastoma cells predominates over growth factor stimulation:
an automated image capture and quantitation approach to neuritogenesis. _Anal Biochem_ 2001; 298: 163–169. Article CAS Google Scholar * Bartlett DW, Davis ME . Insights into the kinetics
of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. _Nucleic Acids Res_ 2006; 34: 322–333. Article CAS Google Scholar * Yang Y, Cimen H, Han MJ, Shi
T, Deng JH, Koc H _et al_. NAD+-dependent deacetylase SIRT3 regulates mitochondrial protein synthesis by deacetylation of the ribosomal protein MRPL10. _J Biol Chem_ 2010; 285: 7417–7429.
Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr. C Cameron (Pennsylvania State University) for TFAM antibodies, CJ Marshall, (Cancer Research UK, London,
England) for DN-MEK1, and SJ Cherra III for his assistance with PGC-1_α_ image analysis. This work was supported by funding from the National Institutes of Health (AG026389 and NS065789) and
PA CURE (BVH). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pathology, Division of Neuropathology, University of Pittsburgh School of Medicine, Pittsburgh, 15213, PA, USA J H
Zhu, A M Gusdon & C T Chu * Department of Biochemistry and Molecular Biology, Pennsylvania State University, University Park, 16802, PA, USA H Cimen * The Department of Pharmacology and
Chemical Biology, University of Pittsburgh Cancer Institute, University of Pittsburgh School of Medicine, Pittsburgh, 15213, PA, USA B Van Houten * Department of Biochemistry &
Microbiology, Marshall University, Huntington, 25701, WV, USA E Koc * The McGowan Institute for Regenerative Medicine, University of Pittsburgh School of Medicine, Pittsburgh, 15213, PA, USA
C T Chu * The Center for Neuroscience, University of Pittsburgh, Pittsburgh, 15213, PA, USA C T Chu Authors * J H Zhu View author publications You can also search for this author inPubMed
Google Scholar * A M Gusdon View author publications You can also search for this author inPubMed Google Scholar * H Cimen View author publications You can also search for this author
inPubMed Google Scholar * B Van Houten View author publications You can also search for this author inPubMed Google Scholar * E Koc View author publications You can also search for this
author inPubMed Google Scholar * C T Chu View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to C T Chu. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by P Salomoni Supplementary Information accompanies the paper on Cell Death and
Disease website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (PDF 688 KB) RIGHTS AND PERMISSIONS This work is licensed under the Creative Commons Attribution-NonCommercial-No
Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Zhu, J., Gusdon, A., Cimen, H. _et al._ Impaired mitochondrial biogenesis contributes to depletion of functional mitochondria in chronic MPP+ toxicity: dual roles for ERK1/2. _Cell Death
Dis_ 3, e312 (2012). https://doi.org/10.1038/cddis.2012.46 Download citation * Received: 13 October 2011 * Revised: 12 March 2012 * Accepted: 30 March 2012 * Published: 24 May 2012 * Issue
Date: May 2012 * DOI: https://doi.org/10.1038/cddis.2012.46 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Parkinson’s disease *
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine * autophagy * mitochondrial biogenesis * mitogen-activated protein kinases * mitochondrial transcription factor A