Play all audios:
ABSTRACT For most neurodegenerative diseases the precise duration of an individual cell’s death is unknown, which is an obstacle when counteractive measures are being considered. To address
this, we used the _rd1_ mouse model for retinal neurodegeneration, characterized by phosphodiesterase-6 (PDE6) dysfunction and photoreceptor death triggered by high cyclic
guanosine-mono-phosphate (cGMP) levels. Using cellular data on cGMP accumulation, cell death, and survival, we created mathematical models to simulate the temporal development of the
degeneration. We validated model predictions using organotypic retinal explant cultures derived from wild-type animals and exposed to the selective PDE6 inhibitor zaprinast. Together,
photoreceptor data and modeling for the first time delineated three major cell death phases in a complex neuronal tissue: (1) initiation, taking up to 36 h, (2) execution, lasting another 40
h, and finally (3) clearance, lasting about 7 h. Surprisingly, photoreceptor neurodegeneration was noticeably slower than necrosis or apoptosis, suggesting a different mechanism of death
for these neurons. SIMILAR CONTENT BEING VIEWED BY OTHERS THE TEMPORAL PROGRESSION OF RETINAL DEGENERATION AND EARLY-STAGE IDEBENONE TREATMENT IN THE PDE6BRD1/RD1 MOUSE MODEL OF RETINAL
DYSTROPHY Article Open access 23 January 2024 PATHOGENIC MECHANISMS CONTRIBUTING TO THE VULNERABILITY OF AGING HUMAN PHOTORECEPTOR CELLS Article 02 June 2021 HBEGF-TNF INDUCE A COMPLEX OUTER
RETINAL PATHOLOGY WITH PHOTORECEPTOR CELL EXTRUSION IN HUMAN ORGANOIDS Article Open access 19 October 2022 MAIN Neurodegenerative diseases are an increasing health concern in the aging
population, but despite massive research two fundamental questions remain: (1) what are the mechanisms of cell death governing neurodegenerative diseases? The seminal work of Kerr _et al._1
and the introduction of the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method2 rapidly pinpointed apoptosis as the causative cell death pathway, although the
relevance of alternative degeneration mechanisms is becoming increasingly evident.3 (2) How long is the actual cell death process? Although sounding simple, this question has never been
answered satisfactorily,4 primarily because it is difficult to test experimentally.5 The questions are obviously connected, not the least since different cell death pathways run on different
timescales. For instance, necrosis is seen as a rapid, chaotic, and unordered destruction of the cell taking between a few minutes to 1–2 h to complete,3 whereas apoptosis refers to a
comparatively slow, program driven, and orderly cellular disintegration that may take 6–18 h to complete.6, 7 Knowledge on the time course of cell death will define the window-of-opportunity
and may thus strongly influence future therapeutic strategies. To follow cell death, from the first symptoms to the disappearance of a cell, requires sophisticated long-term, live-tissue
imaging technology.5 Yet, previous _in vivo_ imaging experiments8 could not determine the precise time frame for cell death, mainly because markers for the beginning of cellular
deterioration were lacking, and most knowledge on cell death duration hence comes from dissociated cell cultures.9 The use of intact neuronal tissues for _ex vivo_ analyses presents an
alternative and such studies have focused on the late phases of cell death, identified by pyknosis or DNA fragmentation (DAPI or TUNEL staining, respectively) to resolve the time a dying
cell takes to completely disappear. This ‘clearance time’ was suggested to range from 1 to 5 h in different models for neurodegeneration.7, 10 However, as pathological alterations in DNA and
nuclear structure are detectable only toward the end of the cell death process, the clearance time does not indicate how much time any affected cell has spent going from the initiation to
the very end. We set out to study the duration of neuronal cell death, using the _rd1_ mouse, a homologous animal model for retinitis pigmentosa (inherited retinal degeneration, RD) with an
early, rapid loss of photoreceptors, the light-sensitive neurons of the retina. The _rd1_ mutation leads to loss-of-activity in rod photoreceptor cyclic guanosine-mono-phosphate (cGMP)
phosphodiesterase-6 (PDE6)11 and an accumulation of cGMP, triggering cell death.12, 13 The mechanisms behind hereditary photoreceptor neurodegeneration as such are unsettled and have been
suggested to involve apoptosis,14 necrosis,15 as well as non-apoptotic cell death.16 Neuronal degeneration models – including the _rd1_ mouse – often exhibit a constant rate of cell death,
resembling the exponential decay of radioactive elements.17, 18 We built on this knowledge and used markers characteristic for different cell death stages to create a mathematical model,
which for the first time allowed estimating the temporal duration of photoreceptor neurodegeneration _in vivo_. The model predictions were validated using organotypic retinal _in vitro_
culture, demonstrating that the photoreceptor cell death mechanism was considerably slower than both necrosis and apoptosis. RESULTS ACCUMULATION OF CGMP AND PHOTORECEPTOR CELL DEATH IN THE
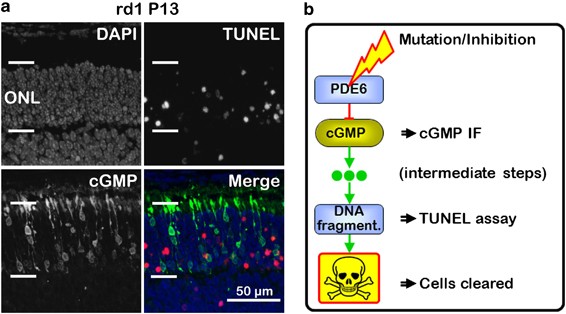
_RD1_ RETINA cGMP accumulation found in _rd1_ photoreceptors is seen as the first sign of impending cellular degeneration.13 Cell death is easily detected using the TUNEL method, which
detects both necrotic and apoptotic cells.2, 19 A variety of different TUNEL-positive phenotypes were observed: some cells stained only in perinuclear areas, in others the entire nucleus was
strongly positive, and yet others showed a very condensed, pyknotic, TUNEL-positive nucleus, all probably relating to different phases of cell death (Figure 1a, Supplementary Figure 1, 2).
Interestingly, although high cGMP triggers TUNEL-positive _rd1_ cell death,12 cGMP did not co-label with TUNEL in photoreceptor cells (Figure 1a). Hence, cGMP and TUNEL labeled two distinct
degeneration stages, separated in time by a transition period. Seen from a mechanistic point of view, PDE6 dysfunction caused a temporary rise in cGMP, followed by (yet unidentified)
intermediate processes in a transition stage, before the cells turned TUNEL positive to be finally cleared away (Figure 1b). Our methodology thus provided an opportunity to study three
different and temporally unique events during an individual photoreceptor cell’s death. Cellular photoreceptor cGMP accumulation (Figure 2a) was an extremely rare event in wild-type (_wt_)
retina, with only few positive cells observed per retinal cross-section, in particular at early post-natal (P) days. In contrast, the number of cGMP-positive photoreceptors was significantly
elevated in the outer nuclear layer (ONL) of the _rd1_ retina already from P8. The early post-natal mouse retina displays a measurable amount of developmental photoreceptor cell death,20
seen also here by the TUNEL assay in _wt_ specimens (Supplementary Figure 1A). Although _rd1_ photoreceptor cell death was numerically higher at P9 when compared with _wt_, statistically
significant differences were found only from P11 onward (Figure 2a). The cGMP-dependent cell death under study was negative for caspase activity (Supplementary Figure 2) – indicating a
non-apoptotic cell death mechanism – and topologically independent (i.e., no clumping of dying cells), suggesting cell-autonomous, non-necrotic processes. The delay between the significant
rise of cGMP at P8, and TUNEL at P11, indicated that photoreceptor death execution could take as long as 2 to 3 days. The percentage of cGMP-positive ONL cells in the _rd1_ retina peaked at
P13, coinciding with the peak of cell death (Figure 2a), after which both cGMP and TUNEL-positive cells declined, but cells with high cGMP remained more numerous than TUNEL-positive cells.
As the cellular life-time of a marker determines its detection probability, these results proposed that cGMP positivity lasted longer than TUNEL positivity. The amount of surviving
photoreceptor rows showed a minor decrease in _wt_ retina (because of developmental processes), whereas in _rd1_ retina photoreceptor numbers strongly declined from P13 onward (Figure 2b).
These data served as an index of the clearance of cells. MODELING PHOTORECEPTOR CELL DEATH KINETICS Based on the _in vivo_ data on cGMP accumulation, TUNEL positivity, and survival of
photoreceptors, we constructed a mathematical model for the temporal progression of neurodegeneration in the _rd1_ retina (Figure 2b). We reasoned that in addition to three phases defined by
cGMP, TUNEL, and clearance of cells, at least two additional transition states must exist: (1) a first one relating to unknown molecular events that, while cGMP negative, eventually cause
unphysiologically high cGMP. (2) A second one in between cGMP and TUNEL positivity, because there was no apparent colocalization between these two markers (Figure 1a). The model also
included an initial stage in which the cells were healthy. Thus, in total six different stages were considered, going from healthy (H) to transition-1 (Tr1) to high cGMP (cGMP⊕) to
transition-2 (Tr2) to TUNEL-positive (TU⊕) to dead (D). These stages were represented by a set of differential equations (1, 2, 3, 4, 5, 6), to allow calculating the average life-times for
each stage as the inverse of the respective decay constant _k_. The values for these equation constants representing the five cell death stages are given in Table 1, together with
approximate average life-times and parameter errors. The decay constant _k_3 of cGMP⊕ was considerably larger than the decay of the upstream stage Tr1 (_k_2), hence the average life time of
cGMP⊕ was governed by _k_2, rather than _k_3. The model reproduces the progression of cell death as evidenced by cGMP accumulation (sum squared error (SQE)=0.82), TUNEL assay (SQE=0.79), and
loss of photoreceptor rows (SQE=0.98). Altogether, the average time for an individual cell to die was predicted to take 83.8±9.4 h (Table 1). As the loss of photoreceptor cells (Figure 2c)
did not follow a strictly exponential decay, we tested an alternative mathematical approach in which _k_1 was defined by an asymmetric, generalized logistic function rather than a step
function. This may reflect photoreceptor biology in the sense that toward the end of differentiation, the risk for a photoreceptor to die increases dramatically, to then remain constant once
full differentiation is reached. Indeed, this approach allowed for a better model fit to cell loss in the initial stage of the degeneration, but in later stages the model fit was not
significantly improved (Supplementary Figure 3). Another, independent estimate of how long the cell death process took, was obtained by plotting data on cGMP accumulation (Figure 2a)
together with the derived photoreceptor survival data (Figure 2c) and fitting Gauss curves to these data sets (Figure 2d, see Table 2 for parameters). The distance between the peaks of the
two curves was approximately 30.2±4.9 h and this was interpreted as the average duration of a cell’s transit from displaying high levels of cGMP until complete disappearance. PROGRESSION OF
ZAPRINAST–CGMP-INDUCED CELL DEATH To test and validate the model predictions, we used organotypic retinal explant cultures derived from _wt_ animals, exposed to the selective PDE6 inhibitor
zaprinast.20 Zaprinast raises intracellular cGMP levels and induces _wt_ photoreceptor degeneration similar to what is seen in _rd1_ retina.12, 21 The effects of zaprinast on cGMP levels and
TUNEL positivity were investigated at time points ranging from 8 h to 10 d. A significant rise in cGMP-positive cells was detected after 36 h of zaprinast treatment and at all later time
points assessed (Figure 3a). Retinal explantation is a traumatic event and the cultures therefore displayed elevated rates of cell death (TUNEL assay) even under control conditions (Figure
3a). This can be regarded as basal level of cell death. Zaprinast caused a significant rise in cell death, but only after 72 h of treatment. As a result of the culture situation, the number
of photoreceptor rows in the ONL _in vitro_ is decreasing more strongly than it would _in vivo,_ in healthy _wt_ retina (Figure 3b). Yet, zaprinast significantly exacerbated this cell loss
from 6 d of treatment onward. The delay of almost 36 h between the zaprinast induced rise of cGMP and the rise of cell death another 36 h later corresponded to both the _in vivo_ findings
and the results of mathematical modeling (Figure 4). PRESERVATION OF CONE PHOTORECEPTORS In inherited RD in humans, primary rod degeneration is often followed by a secondary,
mutation-independent loss of cone photoreceptors. The _rd1_ mouse also suffers from such a secondary loss of cones. Zaprinast inhibits both rod and cone PDE6 with similar specificity.22 To
test how zaprinast treatment affected rod and cone photoreceptors, we performed immunostaining for the cone marker glycogen phosphorylase (GP)23 on retinal cultures after 10 d of zaprinast
treatment (i.e., P20). Owing to a somewhat slower retinal development, P20 _in vitro_ corresponds to P18 _in vivo_. At P18 in _wt_ retina _in vivo_, approximately 5% of ONL cells stained
positively for GP. In relative terms cone numbers were far higher in _rd1_ retina because of rod loss at this time point _in vivo_ (Supplementary Figure 4A, B; quantified in E). Higher
numbers of cones were also found in zaprinast-treated _versus_ -untreated _in vitro_ retina (Supplementary Figure 4C, D; quantified in E). Even if the relative effect was much smaller than
_in vivo_, this suggested that zaprinast-induced degeneration of ONL cells affected mostly rods, particularly when absolute numbers of cones at P18 were considered (Supplementary Figure 4F).
DISCUSSION Here, we show for the first time that inherited neuronal cell death in the retina – despite a rapid progression of overall tissue degeneration – is a surprisingly slow process at
the level of the individual cell. This affords interesting insights into the underlying mechanism, since a total duration of cell death of approximately 80 h is incompatible with the
execution of conventionally assumed necrotic or apoptotic cell death. CGMP IN PHOTORECEPTOR CELL DEATH High levels of cGMP are known to cause photoreceptor degeneration.13 In the _rd1_
retina accumulation of cGMP is caused by PDE6 dysfunction,11 and this situation can be replicated by pharmacological inhibition of PDE6.13, 21 The _rd1_ mouse seems particularly well suited
for studies into the temporal characteristics of cell death, because cGMP accumulation provides a clear label for cell death induction, an event otherwise difficult to assess. cGMP has two
prototypic targets: cGMP-dependent protein kinase G (PKG)12 and cyclic nucleotide gated (CNG) channels.24 In photoreceptors, cGMP levels are controlled by a physiologic feedback mechanism
via activation of CNG channels, resulting in Ca2+ influx, which in turn inhibits guanylyl cyclase (GC) and cGMP production.25, 26 Why this control mechanism fails to keep cGMP in check after
prolonged elevation of cGMP is unknown, but may be linked to cGMP-dependent activation of PKG12 and its effects on gene regulation.27 Indeed, extensive gene expression changes were seen in
_rd1_ retina already at P11.28 At the same time, excessively high cGMP-levels may precipitate _rd1_ cell death via over-activation of CNG channels and subsequent influx of Ca2+ ions.29, 30
CELL DEATH MECHANISMS A variety of different mechanisms have been connected with neuronal cell death,3, 14 but necrosis and apoptosis are possibly the best well known and often seen as
conceptual counterparts. While necrosis usually is very rapid, taking no more than a few hours to complete,3 apoptosis is somewhat slower, requiring 6–18 h typically.6, 7 Characteristic
features of necrosis not seen in apoptosis are activation of the immune system and an inflammation, as well as necrotic clumping of dying cells. Photoreceptor cell death during primary RD is
topologically independent and generally there is no evidence for inflammation,16 although an upregulation of innate immunity31 and secondary infiltration of microglial cells into the ONL
has been reported.32 Both _rd1_ and _wt_ retina show small numbers of cells with activated caspase-3 – a key feature of apoptosis and likely related to early post-natal developmental cell
death.20 However, _rd1_ mutation-induced cell death is caspase-independent and devoid of classical apoptotic features.16 It is tempting to speculate that instead of necrosis or apoptosis, we
may be facing an alternative cell death mechanism,3 possibly involving metabolic activities of PKG,12 histone deacetylase (HDAC),33 poly-ADP-ribose polymerase (PARP),28 and calpains.34 Our
data indicate that this form of cell death is considerably more time consuming than both necrosis and apoptosis. This is in line with findings in caspase-deficient neurons suggesting that
caspase-independent cell death may be slower than caspase-mediated apoptosis.35 An alternative explanation for the exceptionally slow progression observed here, could be that – maybe in the
initial phases – the execution of cell death is counteracted by endogenous survival mechanisms.36 THE KINETICS OF PHOTORECEPTOR DEATH Rod photoreceptors in the mouse are born over a 16-d
period ranging from 7 d before to 9 d after birth,37 and one could hypothesize that the lifespan of _rd1_ photoreceptors might be constant and predetermined by their respective date of
birth. On the other hand, _rd1_ photoreceptor degeneration at the tissue level is governed by a constant rate of cell death and first-order kinetics,17 with one cell’s death entirely
independent from another cell’s death. This is very similar to what is observed in the exponential decay of radioactive elements and hence, the lifespan of an individual photoreceptor cell
will be random and governed by probabilistic and stochastic effects.38 In this model, the average life-span of a cell population will be governed by the severity of the (genetic) insult.18
The kinetics of cell death presented here are generally compatible with the ‘one-hit-model’.17 We introduced several novel parameters to this model, including different cell death stages and
a variable risk for the initial stage. Indeed, we found that an individual _rd1_ photoreceptor’s risk to die may increase during early post-natal differentiation and reach a constant value
only once the fully differentiated state is reached. This would result in an apparent wave of cell death at the time of differentiation and may serve to explain the center to periphery
progression of the _rd1_ degeneration, which follows the pattern of retinal development. ZAPRINAST TREATMENT: SIMULATION OF AN INHERITED DISEASE? Studies of inherited RDs can be helped by
disease simulation on different genetic backgrounds or in different species. Pioneering works by Lolley _et al._13 used the general PDE inhibitor 3-isobutyl-1-methylxanthine to
pharmacologically induce selective, cGMP-dependent photoreceptor degeneration in _Xenopus_ embryos. In mammalian systems, zaprinast is a highly selective PDE6 inhibitor,22 which – as used
here (100 _μ_M) – causes cGMP accumulation and exclusive photoreceptor death.12, 21 Interestingly, and very similar to the _in vivo_ characteristics of the _rd1_ retina, zaprinast affected
sub-populations of photoreceptor cells at different times, which also _in vitro_ might be connected to ongoing development of photoreceptors,37 superseded by stochastic effects.38
Unexpectedly, the dramatic rise of photoreceptor cGMP levels was not an immediate effect of zaprinast treatment. Although zaprinast increases Ca2+-levels cGMP dependently in mouse
photoreceptors within minutes of application,39 a catastrophic rise of cellular cGMP to levels detectable with immunostaining appeared only after 36 h and beyond. These non-linear kinetics
of cGMP accumulation suggest that feedback control mechanisms, such as the CNG-Ca2+-GC loop,25 prevent the rise of cGMP for approximately 1.5 d, before a changing metabolism causes cGMP
levels to go unchecked. Contrary to what might be expected from its inhibitory capacity on both rod and cone PDE6 isoforms,20 zaprinast did not seem to affect cones. This may point at an
increased resistance of cones to higher cGMP levels40 but could also be due to their later differentiation.41 Anyhow, within the time frame of our experiments, _in vitro_ application of
zaprinast on wt retina faithfully reproduced the selective rod photoreceptor loss seen in _rd1_ retina _in vivo_. This approach could prove very useful to study retinal neurodegenerative
mechanisms, for instance on non-degenerating knock-out rodent models21 or on large, non-rodent animal models. THE SLOW DEATH OF PHOTORECEPTORS: THREE PHASES IN CELL DEATH Our different
experimental approaches delineate three major phases in the progression of cell death and give estimates on their duration (Figure 4). * 1 _The initiation phase_: this phase of cell death is
inherently difficult to study because of our lack of understanding of relevant metabolic processes and suitable markers. Nevertheless, both _in vivo_ and _in vitro_ data suggested that
after inhibition or genetic inactivation of PDE6, cGMP levels rise but are maintained within physiological limits by feedback control mechanisms.25 Possibly, the cell is not yet committed to
die at this stage, although toward its end the feedback control is shut down and cGMP rises beyond physiological limits. PKG is involved in the degeneration12 and has a 100-fold higher
sensitivity to cGMP compared with CNG channels.42 PKG could therefore have a preeminent role during this phase, which our data suggest takes about 36 h. * 2 _The execution phase_: once the
cellular metabolism has switched to allow for a catastrophic rise of cGMP, the cell likely becomes committed to die and enters the death execution phase. This phase may be characterized by
an over-activation of HDAC33 and subsequently PARP,28 resulting in chromatin changes and rearrangements. In addition, high cGMP likely acts on CNG channels,30 to cause excessive Ca2+
influx29 and calpain activation.34 The execution phase may last between 36 and 48 h. Similarly, chick embryo neuronal precursor cells became committed to die approximately 2 d before clear
signs of impending cell death were observed.6 * 3 _The clearance phase_: once the cell has reached the final stages of cell death, extensive DNA fragmentation sets in, as evidenced by the
TUNEL assay. It is worth mentioning that the TUNEL assay generally labels dying cells, including in necrosis19 and apoptosis.2 Our model suggests that it may take about 4 h until a maximal
degree of DNA fragmentation is reached, and from then on the nucleus becomes more and more condensed and pyknotic until the cellular debris is completely removed, a stage that may take
another 3 h. This is in agreement with studies on clearance of TUNEL-positive cells in rat and mouse neocortex, estimated to last between 1 and 4 h,10 and clearance of pyknotic ganglion
cells during retinal development within 1 h.43 Even with cautious extrapolation to the human situation, where RD caused by homologous mutations may take several decades to complete, the
observed delays suggest a window-of-opportunity sufficiently large for therapeutic interventions in patients. They also confirm previous observations that photoreceptor metabolism may suffer
significantly before evident cell loss leads to first clinical symptoms.44 With respect to potential neuroprotective treatments, targeting of metabolic processes36 during initiation or
early execution phases may be particularly promising. CONCLUSION Our study for the first time provides consistent estimates on the duration of neuronal cell death in a hereditary
neurodegenerative disease. With a period of about 80 h – from initiation, to cGMP accumulation, to TUNEL-positive reaction, to clearance – the time an individual cell needs to die is
remarkably long and points toward execution of non-necrotic, non-apoptotic, and relatively slow cell death mechanisms. This has clear relevance for the development of potential therapies,
for instance in acute neurodegenerative disorders such as stroke or spinal-cord injury, where the time frame for cell death will directly determine the window-of-opportunity and potential
therapeutic options. Similarly, for chronic and inherited forms of neurodegeneration, knowledge on the temporal progression of cell death will provide insights into the underlying
degenerative mechanisms and again define possible treatment approaches. The mathematical model presented here may be extended to also include other processes causally involved in cell death,
such as enzymatic activities of PKG, HDAC, PARP, and calpain. Combined with the use of transgenic biosensors for cGMP and Ca2+,39 this could allow precise delineation of the temporal
progression and interdependence of different metabolic processes causing cell death. Furthermore, the current experimental approach and type of mathematical modeling may be used to study
cell death in general, provided at least two temporally distinct degeneration markers can be identified. MATERIALS AND METHODS ANIMALS C3H _rd1_/_rd1_ (_rd1_) and control C3H _wt_ mice were
housed under standard white cyclic lighting, had free access to food and water, and were used irrespective of gender. All procedures were performed in accordance with the local ethics
committee at Tübingen University (§4 registration from 23 January 2008), and the ARVO statement for the use of animals in ophthalmic and visual research. All efforts were made to minimize
the number of animals used and their suffering. Day of birth was considered as post-natal day (P) 0. ORGANOTYPIC RETINAL EXPLANT CULTURE Retinae from P5 _rd1_ and _wt_ animals were used to
generate retinal explants as described before.21, 33 Explanted retinae were cultured on Millicell HA culture dish filter inserts (Millipore, Carrigtwohill, Cork, Ireland; PIHA03050) with the
retinal pigment epithelium facing the membrane. Inserts were put into six-well culture plates and incubated in R16 nutrient medium with supplements at 37 °C. Every second day the full
volume of nutrient medium, 1.5 ml per well, was replaced with fresh medium. After 5 d _in vitro_ (i.e., P10), the two retinal explants obtained from one animal were split into two groups,
one was exposed to 100 _μ_M zaprinast in DMSO (treatment group), one was exposed to DMSO only (control group; 0.3% DMSO). The culture period was ended by immediate fixation in 4%
paraformaldehyde in PBS at post-treatment time points ranging from 8 h to 10 d. IMMUNOSTAINING AND TUNEL ASSAY Retinal cryosections obtained either from _in vivo_ animals or following _in
vitro_ explant culture, were dried for 30–60 min at 37 °C. Subsequently, the tissue was rehydrated in PBS, and pre-incubated for 1 h at room temperature in blocking solution, containing 10%
normal serum, and 0.1% or 0.3% Triton in PBS (PBST). Immunostaining was performed overnight at 4 °C, using primary antibodies against cGMP (provided by Harry Steinbusch, Maastricht
University, The Netherlands), Cngb1 (provided by Stylianos Michalakis, Ludwig Maximilian University of Munich, Germany), and GP (provided by Brigitte Pfeiffer-Gugliemi, University of
Tübingen, Germany) diluted 1 : 500 in blocking solution. The tissue was rinsed with PBST, and incubated for 1 h with a corresponding, Alexafluor-488 conjugated, secondary antibody (1 : 200–1
: 750, Life technologies, Darmstadt, Germany), diluted in PBST. Sections were rinsed in PBS, and mounted in Vectashield with DAPI for nuclear counterstaining (Vector Laboratories Inc.,
Burlingame, CA, USA). The TUNEL assay was performed on using an _in situ_ cell death detection kit conjugated with tetra-methyl-rhodamine or fluorescein isothiocyanate (Roche Diagnostics,
Mannheim, Germany). For controls terminal deoxynucleotidyl transferase enzyme was either omitted from the labeling solution (negative control), or sections were pre-treated for 30 min with
DNAse I (Roche, 3 U/ml) in 50 mM Tris-HCl, pH 7.5, 1 mg/ml BSA to induce DNA strand breaks (positive control). Although negative control gave no staining, positive control stained all nuclei
in all layers of the retina.28 MICROSCOPY, CELL COUNTING, AND STATISTICAL ANALYSIS Morphological observations and routine light microscopy were performed on a Zeiss Imager Z1 Apotome
Microscope (Zeiss, Oberkochen, Germany), equipped with a Zeiss Axiocam digital camera. Images were captured using Zeiss Axiovision 4.8 software; image overlays and contrast enhancement were
done using Adobe Photoshop CS5. Images shown in figures are representative for least three different animals for each genotype/treatment. Percentages of TUNEL-positive cells were assessed in
a blinded fashion as reported previously.33, 34 The mean value for photoreceptor rows in the ONL was determined using DAPI staining on six different locations in close proximity to the
optic nerve (_in vivo_) or in central areas of the retinal explant (_in vitro_). To generate the data set for dead cells and to account for early post-natal developmental cell death, the
_wt_ photoreceptor row count was subtracted from the respective _rd1_ values. Values are mean±S.E.M. Statistical significance was tested using one-way ANOVA with Bonferroni correction and
GraphPad Prism 5 software (San Diego, CA, USA), significance levels were: *_P_<0.05, **_P_<0.01, and ***_P_<0.001. MATHEMATICAL MODELS For modeling cell death progression and
estimating its duration, irreversible first-order kinetics were assumed.17 The model consisted of six stages, termed ‘healthy’(H), ‘transition state-1’ (Tr1), ‘cGMP-positive’ (cGMP⊕),
‘transition state-2’ (Tr2), ‘TUNEL-positive’ (TU⊕) and ‘dead’ (D). The changes in the six stages are represented by an ordinary differential equation system: _k_1, _k_2, _k_3, _k_4, and _k_5
stand for the decay constants of the respective equation and cell death stage (see additional considerations in results). The average life-time _τ_x of a given cell death stage _x_ will be
the inverse of the respective decay constant _k_x. This model was implemented in Matlab 2010a (Mathworks, Natick, MA, USA). The system of differential equations was solved using ‘ode45’
algorithm (explicit Runge–Kutta based). The SQE was calculated using equation (8) where _y_ represents the measured values of healthy, cGMP-positive, TUNEL-positive and dead cells stages,
_y_p represents predicted values of these variables, whereas _n_ stands for the total number of observations. For the SQE calculation, we summed the predicted values for the healthy the Tr1
state, because these two states were indistinguishable experimentally. To fit the parameters, we used the Nelder–Mead Simplex Algorithm (‘fminsearch’) to minimize SQE, and to match the model
as closely as possible to the observed cell death processes. Initially, decay constants and starting time _t_0 of the system of differential equations were part of the optimization and
subsequent bootstrapping. From the probability density function of possible start times, we choose the time with the highest probability below 10 d post-natal, which was 9.84 d. For
biological reasons, solutions beyond 10 d were not considered. The final parameters were then optimized with a fixed starting time _t_0 of 9.84 d. To model the continuous decay of the
healthy stage, an extension of the above described model was tested, which used a generalized logistic function equation (9) instead of a constant _k_1 (Supplementary Figure 4). The
simulation of this model and estimation of the generalized logistic functiońs parameters were performed using COPASI – software45 and the simulated annealing algorithm for parameter
estimation. The decay constants k2–k5 were left unchanged. An alternative, empirical approach is given in a second model, which provides an estimate of the ’life-time’ of the sick (_S_)
state of a cell (i.e., the cell death execution phase). The ‘life-time’ of the sick cells Δ_t_ was assumed to be the time _t_2 (peak of cell death) minus the time _t_1 (peak of cGMP-positive
cells). To estimate the time point _t_1 with the maximum number of cells that are in the sick state _S_(_t_), a Gauss function equation (10) was fitted to the normalized cGMP⊕ data peak,
where _f_1 is a parameter for peak height and _σ_1 represents peak width. The maximal increase in dead cells was determined in the same way by fitting a Gauss function equation (11) to the
derivative of the dead cell data. Δ_t_ equation (12) gives the average life-time of sick cells. The second model was realized in Microsoft Excel 2010 (Microsoft, Unterschleissheim, Germany)
using the ‘Solver’ functionality with genetic algorithms and SQE to fit the functions to the measured data. ABBREVIATIONS * cGMP: cyclic guanosine-mono-phosphate * d: days * DPN: days
post-natal * GP: glycogen phosphorylase * HDAC: histone deacetylase * ONL: outer nuclear layer * PDE6: phosphodiesterase-6 * PARP: poly-ADP-ribose-polymerase * PKG: protein kinase G * RD:
retinal degeneration * SQE: sum squared error * TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling * wt: wild-type REFERENCES * Kerr JF, Wyllie AH, Currie AR . Apoptosis: a
basic biological phenomenon with wide-ranging implications in tissue kinetics. _Br J Cancer_ 1972; 26: 239–257. Article CAS PubMed PubMed Central Google Scholar * Gavrieli Y, Sherman Y,
Ben-Sasson SA . Identification of programmed cell death _in situ_ via specific labeling of nuclear DNA fragmentation. _J Cell Biol_ 1992; 119: 493–501. Article CAS PubMed Google Scholar
* Zong WX, Thompson CB . Necrotic death as a cell fate. _Genes Dev_ 2006; 20: 1–15. Article CAS PubMed Google Scholar * Henson PM, Hume DA . Apoptotic cell removal in development and
tissue homeostasis. _Trends Immunol_ 2006; 27: 244–250. Article CAS PubMed Google Scholar * Skommer J, Darzynkiewicz Z, Wlodkowic D . Cell death goes LIVE: technological advances in
real-time tracking of cell death. _Cell Cycle_ 2010; 9: 2330–2341. Article CAS PubMed Google Scholar * Oppenheim RW . Cell death during development of the nervous system. _Annu Rev
Neurosci_ 1991; 14: 453–501. Article CAS PubMed Google Scholar * Wong RO, Hughes A . Role of cell death in the topogenesis of neuronal distributions in the developing cat retinal
ganglion cell layer. _J Comp Neurol_ 1987; 262: 496–511. Article CAS PubMed Google Scholar * Cordeiro MF, Guo L, Coxon KM, Duggan J, Nizari S, Normando EM _et al_. Imaging multiple
phases of neurodegeneration: a novel approach to assessing cell death _in vivo_. _Cell Death Dis_ 2010; 1: e3. Article CAS PubMed PubMed Central Google Scholar * Alborzinia H, Can S,
Holenya P, Scholl C, Lederer E, Kitanovic I _et al_. Real-time monitoring of cisplatin-induced cell death. _PLoS ONE_ 2011; 6: e19714. Article CAS PubMed PubMed Central Google Scholar *
Gohlke JM, Griffith WC, Faustman EM . The role of cell death during neocortical neurogenesis and synaptogenesis: implications from a computational model for the rat and mouse. _Brain Res
Dev Brain Res_ 2004; 151: 43–54. Article CAS PubMed Google Scholar * Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB . Retinal degeneration in the rd mouse is caused by a
defect in the beta subunit of rod cGMP-phosphodiesterase. _Nature_ 1990; 347: 677–680. Article CAS PubMed Google Scholar * Paquet-Durand F, Hauck SM, van Veen T, Ueffing M, Ekström P .
PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. _J Neurochem_ 2009; 108: 796–810. Article CAS PubMed Google Scholar * Lolley RN, Farber DB, Rayborn ME,
Hollyfield JG . Cyclic GMP accumulation causes degeneration of photoreceptor cells: simulation of an inherited disease. _Science_ 1977; 196: 664–666. Article CAS PubMed Google Scholar *
Chang GQ, Hao Y, Wong F . Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. _Neuron_ 1993; 11: 595–605. Article CAS PubMed Google Scholar *
Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM _et al_. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for
inhibition of apoptosis. _Proc Natl Acad Sci USA_ 2010; 107: 21695–21700. Article CAS PubMed PubMed Central Google Scholar * Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, Romero
FJ, van Veen T, Zrenner E _et al_. Photoreceptor cell death mechanisms in inherited retinal degeneration. _Mol Neurobiol_ 2008; 38: 253–269. Article CAS PubMed Google Scholar * Clarke G,
Collins RA, Leavitt BR, Andrews DF, Hayden MR, Lumsden CJ _et al_. A one-hit model of cell death in inherited neuronal degenerations. _Nature_ 2000; 406: 195–199. Article CAS PubMed
Google Scholar * Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS . Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. _Nat Rev Genet_ 2010; 11:
273–284. Article CAS PubMed Google Scholar * Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R . _In situ_ detection of fragmented DNA (TUNEL assay)
fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. _Hepatology_ 1995; 21: 1465–1468. CAS PubMed Google Scholar * Young RW . Cell death during
differentiation of the retina in the mouse. _J Comp Neurol_ 1984; 229: 362–373. Article CAS PubMed Google Scholar * Sahaboglu A, Tanimoto N, Kaur J, Sancho-Pelluz J, Huber G, Fahl E _et
al_. PARP1 gene knock-out increases resistance to retinal degeneration without affecting retinal function. _PLoS ONE_ 2010; 5: e15495. Article PubMed PubMed Central Google Scholar *
Zhang X, Feng Q, Cote RH . Efficacy and selectivity of phosphodiesterase-targeted drugs in inhibiting photoreceptor phosphodiesterase (PDE6) in retinal photoreceptors. _Invest Ophthalmol Vis
Sci_ 2005; 46: 3060–3066. Article PubMed Google Scholar * Nihira M, Anderson K, Gorin FA, Burns MS . Primate rod and cone photoreceptors may differ in glucose accessibility. _Invest
Ophthalmol Vis Sci_ 1995; 36: 1259–1270. CAS PubMed Google Scholar * Huttl S, Michalakis S, Seeliger M, Luo DG, Acar N, Geiger H _et al_. Impaired channel targeting and retinal
degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. _J Neurosci_ 2005; 25: 130–138. Article PubMed PubMed Central Google Scholar * Olshevskaya EV, Ermilov AN,
Dizhoor AM . Factors that affect regulation of cGMP synthesis in vertebrate photoreceptors and their genetic link to human retinal degeneration. _Mol Cell Biochem_ 2002; 230: 139–147.
Article CAS PubMed Google Scholar * Michalakis S, Mühlfriedel R, Tanimoto N, Krishnamoorthy V, Koch S, Fischer MD _et al_. Restoration of cone vision in the CNGA3(-/-) mouse model of
congenital complete lack of cone photoreceptor function. _Mol Ther_ 2010; 18: 2057–2063. Article CAS PubMed PubMed Central Google Scholar * Fiscus RR . Involvement of cyclic GMP and
protein kinase G in the regulation of apoptosis and survival in neural cells. _Neurosignals_ 2002; 11: 175–190. Article CAS PubMed Google Scholar * Paquet-Durand F, Silva J, Talukdar T,
Johnson LE, Azadi S, van Veen T _et al_. Excessive activation of poly(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. _J
Neurosci_ 2007; 27: 10311–10319. Article CAS PubMed PubMed Central Google Scholar * Fox DA, Poblenz AT, He L . Calcium overload triggers rod photoreceptor apoptotic cell death in
chemical-induced and inherited retinal degenerations. _Ann N Y Acad Sci_ 1999; 893: 282–285. Article CAS PubMed Google Scholar * Paquet-Durand F, Beck S, Michalakis S, Goldmann T, Huber
G, Mühlfriedel R _et al_. A key role for cyclic nucleotide gated (CNG) channels in cGMP-related retinitis pigmentosa. _Hum Mol Genet_ 2011; 20: 941–947. Article CAS PubMed Google Scholar
* Azadi S, Paquet-Durand F, Medstrand P, van Veen T, Ekström PA . Up-regulation and increased phosphorylation of protein kinase C (PKC) delta, mu and theta in the degenerating rd1 mouse
retina. _Mol Cell Neurosci_ 2006; 31: 759–773. Article CAS PubMed Google Scholar * Murakami Y, Matsumoto H, Roh M, Suzuki J, Hisatomi T, Ikeda Y _et al_. Receptor interacting protein
kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. _Proc Natl Acad Sci USA_ 2012; 109: 14598–14603. Article CAS PubMed PubMed Central Google
Scholar * Sancho-Pelluz J, Alavi MV, Sahaboglu A, Kustermann S, Farinelli P, Azadi S _et al_. Excessive HDAC activation is critical for neurodegeneration in the rd1 mouse. _Cell Death
Disease_ 2010; 1: 1–9. Article Google Scholar * Paquet-Durand F, Sanges D, McCall J, Silva J, van Veen T, Marigo V _et al_. Photoreceptor rescue and toxicity induced by different calpain
inhibitors. _J Neurochem_ 2010; 115: 930–940. Article CAS PubMed Google Scholar * Oppenheim RW, Flavell RA, Vinsant S, Prevette D, Kuan CY, Rakic P . Programmed cell death of developing
mammalian neurons after genetic deletion of caspases. _J Neurosci_ 2001; 21: 4752–4760. Article CAS PubMed PubMed Central Google Scholar * Trifunovic D, Sahaboglu A, Kaur J, Mencl S,
Zrenner E, Ueffing M _et al_. Neuroprotective strategies for the treatment of inherited photoreceptor degeneration. _Curr Mol Med_ 2012; 12: 598–612. Article CAS PubMed Google Scholar *
Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D . Cell fate determination in the vertebrate retina. _Proc Natl Acad Sci USA_ 1996; 93: 589–595. Article CAS PubMed PubMed Central
Google Scholar * Skommer J, Raychaudhuri S, Wlodkowic D . Timing is everything: stochastic origins of cell-to-cell variability in cancer cell death. _Front Biosci_ 2011; 16: 307–314.
Article CAS Google Scholar * Wei T, Schubert T, Paquet-Durand F, Tanimoto N, Chang L, Koeppen K _et al_. Generation and functional characterization of a transgenic mouse expressing a Ca2+
biosensor in cone photoreceptors. _J Neurosci_ 2012; 32: 6994–6981. Google Scholar * Johnson JE, Perkins GA, Giddabasappa A, Chaney S, Xiao W, White AD _et al_. Spatiotemporal regulation
of ATP and Ca2+ dynamics in vertebrate rod and cone ribbon synapses. _Mol Vis_ 2007; 13: 887–919. CAS PubMed Central PubMed Google Scholar * Szél Á, van Veen T, Röhlich P . Retinal cone
differentiation. _Nature_ 1994; 370: 336. Article PubMed Google Scholar * Lincoln TM, Cornwell TL . Intracellular cyclic GMP receptor proteins. _FASEB J_ 1993; 7: 328–338. Article CAS
PubMed Google Scholar * Cellerino A, Galli-Resta L, Colombaioni L . The dynamics of neuronal death: a time-lapse study in the retina. _J Neurosci_ 2000; 20: RC92. Article CAS PubMed
PubMed Central Google Scholar * Acosta ML, Shin YS, Ready S, Fletcher EL, Christie DL, Kalloniatis M _et al_. Retinal metabolic state of the proline-23-histidine rat model of retinitis
pigmentosa. _Am J Physiol Cell Physiol_ 2010; 298: C764–C774. Article CAS PubMed Google Scholar * Hoops S, Sahle S, Gauges R, Lee C, Pahle J, Simus N _et al_. COPASI- A COmplex PAthway
SImulator. _Bioinformatics_ 2006; 22: 3067–3074. Article CAS PubMed Google Scholar * Lindner O, Hitzmann B . Experimental design for optimal parameter estimation of an enzyme kinetic
process based on the analysis of the Fisher information matrix. _J Theor Biol_ 2006; 238: 111–123. Article Google Scholar Download references ACKNOWLEDGEMENTS We thank T Euler, B
Arango-Gonzalez and D Trifunović for helpful comments and discussions. This work was supported by grants from DFG (Pa1751/4-1, 1-1), and Open Access Publishing Fund of Tuebingen University,
EU (DRUGSFORD: HEALTH-F2-2012-304963), CIN (PP2009-20), and Kerstan Foundation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute for Ophthalmic Research, University of Tübingen,
Tübingen, Germany A Sahaboglu, J Dietter, S Bernhard-Kurz, M Ueffing & F Paquet-Durand * Institute of Food Science and Biotechnology, University of Stuttgart Hohenheim, Stuttgart,
Germany O Paquet-Durand & B Hitzmann * Skin Clinic, University of Tübingen, Tübingen, Germany K Dengler * Department of Clinical Sciences, Lund, University of Lund, Lund, Sweden P AR
Ekström Authors * A Sahaboglu View author publications You can also search for this author inPubMed Google Scholar * O Paquet-Durand View author publications You can also search for this
author inPubMed Google Scholar * J Dietter View author publications You can also search for this author inPubMed Google Scholar * K Dengler View author publications You can also search for
this author inPubMed Google Scholar * S Bernhard-Kurz View author publications You can also search for this author inPubMed Google Scholar * P AR Ekström View author publications You can
also search for this author inPubMed Google Scholar * B Hitzmann View author publications You can also search for this author inPubMed Google Scholar * M Ueffing View author publications You
can also search for this author inPubMed Google Scholar * F Paquet-Durand View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to F Paquet-Durand. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by A Verkhratsky Supplementary
Information accompanies the paper on Cell Death and Disease website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 (JPG 390 KB) SUPPLEMENTARY FIGURE 2 (JPG 247 KB) SUPPLEMENTARY FIGURE 3
(JPG 1905 KB) SUPPLEMENTARY FIGURE 4 (JPG 110 KB) SUPPLEMENTARY INFORMATION (DOC 2633 KB) RIGHTS AND PERMISSIONS This work is licensed under the Creative Commons Attribution-NonCommercial-No
Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Sahaboglu, A., Paquet-Durand, O., Dietter, J. _et al._ Retinitis pigmentosa: rapid neurodegeneration is governed by slow cell death mechanisms. _Cell Death Dis_ 4, e488 (2013).
https://doi.org/10.1038/cddis.2013.12 Download citation * Received: 09 November 2012 * Revised: 21 December 2012 * Accepted: 03 January 2013 * Published: 07 February 2013 * Issue Date:
February 2013 * DOI: https://doi.org/10.1038/cddis.2013.12 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * TUNEL * apoptosis * necrosis *
retina * cGMP