Play all audios:
ABSTRACT A cDNA molecule encoding a major part of the human Norepinephrine transporter(hNET) was synthesized by means of Polymerase Chain Reaction(PCR) technique and used as a probe for
selecting the human genomic NET gene. A positive clone harbouring the whole gene was obtained from a human lymphocyte genomic library through utilizing the “genomic walking” technique. The
clone, designated as phNET, harbours a DNA fragment of about 59 kb in length inserted into BamH I site in cosmid pWE15. The genomic clone contains 14 exons encoding all amino acid residues
in the protein. A single exon encodes a distinct transmembrane domain, except for transmembrane domain 10 and 11, which are encoded by part of two exons respectively, and exon 12, which
encodes part of domain 11 and all of domain 12. These results imply that there is a close relationship between exon splicing of a gene and structural domains of the protein, as is the case
for the human γ-aminobutyric acid transporter(hGAT) and a number of other membrane proteins. SIMILAR CONTENT BEING VIEWED BY OTHERS SUBSTRATE BINDING AND INHIBITION MECHANISM OF
NOREPINEPHRINE TRANSPORTER Article 14 August 2024 LABEL-FREE HIGH-THROUGHPUT SCREENING ASSAY FOR THE IDENTIFICATION OF NOREPINEPHRINE TRANSPORTER (NET/SLC6A2) INHIBITORS Article Open access
10 June 2021 PHOSPHATIDYLINOSITOL 4,5-BISPHOSPHATE (PIP2) FACILITATES NOREPINEPHRINE TRANSPORTER DIMERIZATION AND MODULATES SUBSTRATE EFFLUX Article Open access 17 November 2022 INTRODUCTION
Norepinephrine(NE) is a major catecholamine neurotransmitter in the peripheral and central nervous systems1. In noradrenergic neurons, synaptic transmisslon includes three steps: release of
NE into the synaptic cleft, interaction with a postsynaptic receptor, and subsequent removal of NE from the cleft into the presynaptic terminals or surrounding glial cells. This uptake
process is carried out via a sodium-dependent NE transporter(NET). The NET is also apparently the initial site of action for therapeutic antidepressants and drugs such as cocaine and the
amphetamines2, 3. Over the past five years, the cDNAs for many of the known neurotransmitter transporters have been elucidated using PCR, homology screening and expression cloning
techniques3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. According to their dependence on Na+/K+ or Na+/Cl−, these transporters can
be divided into two families. Family I, with 6, 8 or 10 transmembrane domains, includes transporters for glutamate / aspartate4, glutamate5, certain neutral amino acids6, 7 and excitatory
amino-acid carrier 18. Family II, based on the homology and other characteristics of the transport systems, can be further divided into three subfamilies: i) transporters for γ-aminobutyric
acid(GABA) 9, 10, 11, 12, 13, taurine14, 15 and choline16 (enzymaticly dissolved from acetylcholine), but betaine transporter17 and creatine transporter18 can also be ineluded here, although
betaine is an osmolyte and creatine is a component involved in energy exchange; ii) transporters for glycine19, 20, 21 and proline22; and iii) transporters for norepinephrine3, dopamine23,
24, 25, 26 and serotonin[27-291. All members belonging to family II have 12 constant transmembrane domains. It is noteworthy that transporters are expressed from prokaryote ie. E.coli.31 to
eukaryote ie. yeast32, Drosophila29 and mammalian systems, and that the expressions of transporters during development in one species appear to be tightly programmed13. Since
neurotransmitter transporters are involved in diseases of the nervous sys- tem, drug addiction and synaptic plasticity33, 34, 35, they had been studied pharmacologically and biochemically
for a long time before. In contrast, studies on the genomic structures of these transporters have been much more limited36, 37. To learn the structure, function and regulation of
neurotransmitter transporters in the nervous system, we cloned and analyzed genomic NET gene. The result of this study indicated the unique characters of the biogenic amine
transporters' subfamily. MATERIAL AND METHODS 1. MOLECULAR CLONING OF THE HUMAN NET GENE To clone and analyze NET gene, a human brain stem cDNA library cloned in Lambda
ZAPII(Stratagene) was amplified and its DNA molecules were extracted according to published procedures. A pair of primers were designed for PCR amplification. The sense primer was
corresponding to bases 1111-1137 of NET eDNA molecule with the sequence 5′GAACACAAGGTCAACATTGAGGATGTG 3′ , the antisense primer was corresponding to bases 1911-1892 with the sequence
5′CGGAAGCTTGTGACCTGGACATTGGCATGG 3′ , HinclI and HindIII were underlined. The amplification protocol consisted of a 1 min denaturation at 94°C, a 1.5 min annealing at 55°C, and a 2 min
extension at 70°C for 30 cycles on DNA Amplifier. A 801 bp DNA fragment was generated by PCR. The 801 bp fragment was cloned into pTZ19u between SmaI and HindIII. The recombinant plasmid was
called pNET1. Identification was done by sequencing using the method of Guo and Wu38. Using pNET1 insert as a probe to isolate human NET gene from a human genomic library cloned in Cosmid
pWE15 (Stratagene), one positive clone harbouring 29.7 kb fragment was obtained and mapped. Far 5′terminal of the insert was used as a probe for chromosome walking. Together this technique
was proceeded three times. Then the clone named as phNET contains the entire open reading frame(ORF) of NET. 2. STRUCTURAL ANALYZING OF THE HUMAN NET GENE Standard protocols were used for
restriction mapping, Southern hybridization, subclone and sequencing39. RESULTS AND DISCUSSION A 801 bp fragment corresponding to 1111-1911 of NET cDNA molecular was obtained from a human
brain stem cDNA libray (where hNET is highly expressed) by using PCR technique. After cloning and identification, it was used as a probe to isolate the human NET gene. One positive clone
harbouring a 29.7 kb fragment was obtained and mapped. Southern hybridization with γ-32P-dATP labelled oligonucleotides corresponding to 5′terminal in NET cDNA showed that the clone contains
only part of the gene. Far 5′terminal of the insert was used as a probe for chromosome walking. Altogether this technique was proceeded for three times, giving 11 kb, 9.5 kb and 8.6 kb
extensions towards 5′direction of the gene respectively. Then the clone named as phNET with the entire open reading frame(ORF) of NET was obtained. Fourteen exons encoding all amino acids of
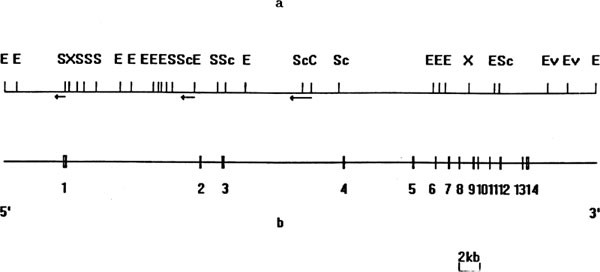
NET were determined on ∼ 59kb genomic DNA fragment by restriction mapping, Southern hybridization, subclone, and sequencing. Exon 1 to exon 6 were determined by restriction sites in NET
cDNA sequence and synthesized oligonucleotides. The human NE transporter gene is much larger than the human GABA transporter gene36. In the NET genomic DNA, intron 1 and 2 exceed 10 kb in
length, and exons are more concentrated on the 3′ terminal (Fig 1). The deduced amino acid sequence of this gene is identical to that deduced from published NET cDNA molecule. The
intron-exon junctions of this gene are shown in Tab 1. The statistics of all splice donor and acceptor sequences is.......g100t100a54a46g70t61.......c93a100g100. The twelve putative
transmembrane regions of the human NET are encoded by exons 1–12. In general, each transmembrane domain is encoded by a single different exon, with the exception of transmembrane domain 10
and 11, which are encoded by parts of exon 10 and 11, and exon 11 and 12, respectively. Furthermore, exon 12 encodes part of domain 11 as well as the entire domain 12. Compared with the
human GABA transporter36, the organization of these two proteins is rather similar. There are, however, three distinct differences: i) while the human GABA transporter gene is about 25 kb,
consists of 16 exons, the human NE transporter is about 46 kb, consists of 14 exons; ii) in the human GABA transporter gene, the largest outside loop is encoded by a separate exon---exon 6
(the translation starting site is in exon 3), while in NE transporter gene, this loop is encoded by 3′ terminal of exon 3 and 5′terminal of exon 4; iii) in the human GABA transporter, most
of the amino acid residues of the cytoplasmic carboxyl terminus are encoded by exon 16, whereas in the human NE transporter, the C terminal in cytoplasm was encoded by three exons: part of
exon 12, and all of exon 13 and 14 (Fig 2). Amino acid residues at domain boundaries are listed in Tab 2. It is noted that glycine or polar amino acid residues always occur there, except for
amino acid residues between exon 11 and 12, which are inside transmembrane domain 11. This is possibly a result of evolution, since glycine, an amino acid without any branches in the
residue, has the greatest flexibility. The human NET gene(NETHG) reported here is the first human catecholamine transporter gene that has been cloned and analyzed. From the relationship
between exons and domains of NETHG, we predict that for this gene, 14 exons encode 13 functional domains in the NET protein. Exon 14 only encodes seven amino acids which may not comprise an
independent domain. Additionally, with the exception of domain 10 and 11, the exon-intron junctions are all located at the border of, or outside, the membrane (Fig 2). The significance of
these observations is not known. Future studies will be focused on the molecular, developmental and pharmacological properties of this protein as well as the regulation of the NET gene. Such
analyses should enhance our understanding of the molecular mechanisms of neurotransmitter transporters, and their relationships with synaptic plasticity, learning and certain neurological
disorders such as drug addiction and depression. REFERENCES * Zygmunt LK, Christopher JP(Eds) . _Neurotransmitters and Drugs_. Third Edition. Chapmanl Hall. 1992. * Axelrod J .
Noradrenaline: fate and control of its biosynthesis. _Science_ 1971; 173:598–606 Article CAS PubMed Google Scholar * Pacholczyk T, Blakely R, Amara S . Expression cloning of a cocaine-
and antidepressant-sensitive human noradrenaline transporter. _Nature_ 1991; 350:350–4. Article CAS PubMed Google Scholar * Stork T, Schulte S, Hofmann K, Stoffel W . Structure,
expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. _Proc Natl Acad Sci USA_ 1992; 89:10955–9. Article Google Scholar * Pines G, Danbolt
NC, Bjoras M, _et al_. Cloning and expression of a rat brain L-glutamate transporter. _Nature_ 1992; 360:464–7. Article CAS PubMed Google Scholar * Arriza JL, Kavanaugh MP, Fairman WA,
_et al_. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. _J Biol Chem_ 1993; 268:15329–32. CAS PubMed
Google Scholar * Shafqat S, Tamarappoo BK, Kilberg M, _et al_. Cloning and expression of a novel Na+-dependent neutral amino acid transporter structurally related to mammalian Na+
/glutamate cotrans- porters. _J Biol Chem_ 1993; 268:15351–5. CAS PubMed Google Scholar * Kanai Y, Hediger MA . Primary structure and functional characterization of a high-affinity
glutamate transporter. _Nature_ 1992; 360:467–71. Article CAS PubMed Google Scholar * Guastella J, Nelson N, Lester H, _et al_. Cloning and expression of a rat brain GABA transporter.
_Science_ 1990; 249:1303–6. Article CAS PubMed Google Scholar * Nelson H, Mandiyan S, Nelson N . Cloning of the human brain GABA transporter. _FEBS Lett_ 1990; 269:181–4. Article CAS
PubMed Google Scholar * Lopez-Corcuera B, Liu QR, Mandiyan S, Nelson H, Nelson N . Expression of a mouse brain cDNA encoding novelγ- aminobutyric acid transporter. _J Biol Chem_ 1992;
267:17491–3. CAS PubMed Google Scholar * Borden LA, Smith KE, Hartig PR, Branchek TA, Weinshank RL . Molecular heterogeneity of the γ-aminobutyric acid(GABA) transporter system, cloning
of two novel high affinity GABA transporter from rat brain. _Neuron_ 1992; 9:337–48. Article Google Scholar * Liu QR, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N . Molecular
characterization of four pharmacologically distinct γ-aminobutyric acid transporters in mouse brain. _J Biol Chem_ 1993; 268:2106–12. CAS PubMed Google Scholar * Smith KE, Borden LA, Wang
CHD, Hartig PR, Branchek TA, Weinshank RL . Cloning and expression of a high affinity taurine transporter from rat brain. _Mol Pharmacol_ 1992; 42:563–9. CAS PubMed Google Scholar * Liu
QR, Lopez-Corcuera B, Nelson H, Madiyan S, Nelson N . Cloning and expression of a cDNA encoding the transporter of taurine and beta-alanine in mouse brain. _Proc Natl Acad Sci USA_ 1992;
89:12145–9. Article CAS PubMed PubMed Central Google Scholar * Mayser W, Schloss P, Betz H . Primary structure and functional expression of a choline trans- porter expressed in the rat
nervous system. _FEBS Lett_ 1992; 305:31–6. Article CAS PubMed Google Scholar * Yamauchi A, Uchida S, Kwon HM, _et al_. Cloning of a Na+ and C1−-dependent betaine trans-porter that is
regulated by hypertonicity. _J Biol Chem_ 1992; 267:649–52. CAS PubMed Google Scholar * Guimbal C, Kilimann MW . A Na+- dependent creatine transporter in rabbit brain, muscle, heart, and
kidney. _J Biol Chem_ 1993; 268:8418–21. CAS PubMed Google Scholar * Guastella J, Brecha N, Weigmann C, Lester HA . Cloning, expression, and localization of a rat brain high-affinity
glycine transporter. _Proc Natl Acad Sci USA_ 1992; 89:7189–93. Article CAS PubMed PubMed Central Google Scholar * Liu QL, Nelson H, Mandiyan S, Lopez-Corcuera B, Nelson N . Cloning and
expression of a glycine transporter from mouse brain. _FEBS Lett_ 1992; 305:110–4. Article CAS PubMed Google Scholar * Borowsky B, Mezey E, Hoffman BJ . Two glycine transporter variants
with distinct localization in the CNS and peripheral tissues are encoded by a common gene. _Neuron_ 1993; 10:851–63. Article CAS PubMed Google Scholar * Fremeau RT, Caron MG, Blakely RD
. Molecular cloning and expression of a high affinity L- proline transporter expressed in putative glutamatergic pathways of rat brain. _Neuron_ 1992; 8:915–26. Article CAS PubMed Google
Scholar * Shimada S, Kitayama S, Lin CL, _et al_. Cloning and expression of a cocaine sensitive dopamine transporter complementary DNA. _Science_ 1991; 254:576–8. CAS PubMed Google
Scholar * Kilty JE, Lorang D, Amara SG . Cloning and expression of a cocaine-sentitive rat dopamine transporter. _Science_ 1991; 254:578–9. CAS PubMed Google Scholar * Giros B, Mestikawy
SE, Bertrand L, Caron MG . Cloning and functional characterization of a cocaine- sensitive dopamine transporter. _FEBS Lett_ 1991; 295:149–54. Article CAS PubMed Google Scholar * Giros
B, Mestickawy SE, Godinot N, _et al_. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. _Mol Pharmacol_ 1992; 42:391–7. Google Scholar
* Hoffman B, Mezey E, Brownstein MJ . Cloning of a serotonin transporter affected by antide-pressants. _Science_ 1991; 254:579–80. Article CAS PubMed Google Scholar * Blakely RD, Berson
HE, Fremeau RT Jr, _et al_. Cloning and expression of a functional serotonin transporter from rat brain. _Nature_ 1991; 354:66–70. Article CAS PubMed Google Scholar * Corey JL, Quick MW,
Davidson N, Lester HA, Guastella J . A cocaine-sensitive drosophila serotonin transporter: cloning, expression, and electrophysiological characterization. _Proc Natl Acad Sci USA_ 1994;
91:1188–92. Article CAS PubMed PubMed Central Google Scholar * Liu QR, Mandiyan S, Lopez-Corcuera B, Nelson H, Nelson N . A rat brain cDNA encoding the neurotransmitter transporter with
an unusual structure. _FEBS Lett_ 1993; 315:114–8. Article Google Scholar * Seol W, Shatkin AJ . Escherichia coli kgtP encodes an alpha-ketoglutarate transporter. _Proc Natl Acad Sci USA_
1991; 88:3802–6. Article CAS PubMed PubMed Central Google Scholar * Szkutnicka K, Tschopp J, Andrews L, Cirillo VP . Sequence and structure of the yeast galactose transporter. _J
Bacteriol_ 1989; 171:4486–93. Article CAS PubMed PubMed Central Google Scholar * Wheal H, Thomson A (Eds). Excitatory Amino Acids and Synaptic Transmission. Academic Press. 1992. *
Huang F, Fei J, Guo LH . Neurotransmitter transporters. _LIFE SCIENCES (China)_ 1994; 6:5–8. Google Scholar * Iversen LL . Uptake processes for biogenic amines. In L.L. Iversen, S.D.
Iversen and S.H. Sny-der(Eds.), _Handbook of psychophaxmacology_. VOL 3. Plenum. New York. 1978: pp.381–442. * Lam DMK, Fei J, Zhang XY, _et al_. Molecular cloning and structure of the
human(GABATHG) GABA transporter gene. _Mol Brain Res_ 1993; 19:227–32. Article CAS PubMed Google Scholar * Liu QR, Mandiyan S, Nelson H, Nelson N . A family of genes encoding
neurotransmitter trans- porters. _Proc Natl Acad Sci USA_ 1992; 89:6639–43. Article CAS PubMed PubMed Central Google Scholar * Guo LH, Wu R . In R. Wu, L. Grossman and K. Moldave (Eds),
_Recombinant DNA Methodology_. Academic Press.1989. pp.73–109. * Sambrook J, Fritsch EF, Maniatis T (Eds). _Molecular Cloning_. Second Edition. Cold Spring Harbor Laboratory Press. 1989.
Download references ACKNOWLEDGEMENTS We thank Drs. Steven King and Ken Beattie for their valuable advice and Ms. Teddy Woodyard for preparation of the manuscript. This work was supported by
research grants from the Retina Research Foundation (Houston) GES Pharmaceuticals Inc. (Houston) and the Croucher Foundation (Hong Kong). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Shanghai Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, 200031, China Lihe Guo, Fang Huang, Jian Fei & Xiaoyong Zhang * Hong Kong Institute of Biotechnology, Shatin,
Hong Kong, NT, Hong Kong Lihua Zhu, Anthony CW Tam & Zengchan Ye * LifeTech Industries Ltd., 100 Hawthorn Road, Conroe, 77301, Texas, USA Dominic Man-Kit Lam Authors * Lihe Guo View
author publications You can also search for this author inPubMed Google Scholar * Lihua Zhu View author publications You can also search for this author inPubMed Google Scholar * Fang Huang
View author publications You can also search for this author inPubMed Google Scholar * Anthony CW Tam View author publications You can also search for this author inPubMed Google Scholar *
Zengchan Ye View author publications You can also search for this author inPubMed Google Scholar * Jian Fei View author publications You can also search for this author inPubMed Google
Scholar * Xiaoyong Zhang View author publications You can also search for this author inPubMed Google Scholar * Dominic Man-Kit Lam View author publications You can also search for this
author inPubMed Google Scholar ADDITIONAL INFORMATION *Dedicated to Professor Yao Zhen's 80th Birthday RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Guo, L., Zhu, L., Huang, F. _et al._ Molecular cloning and structural analysis of human norepinephrine transporter gene(NETHG). _Cell Res_ 5, 93–100 (1995).
https://doi.org/10.1038/cr.1995.9 Download citation * Received: 14 April 1995 * Revised: 09 May 1995 * Accepted: 13 May 1995 * Issue Date: 01 June 1995 * DOI:
https://doi.org/10.1038/cr.1995.9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Human norepinephrine transporter gene * neurotransmitter uptake *
cloning