Play all audios:
ABSTRACT Fragile X syndrome, an X-linked dominant disorder with reduced penetrance, is associated with intellectual and emotional disabilities ranging from learning problems to mental
retardation, and mood instability to autism. It is most often caused by the transcriptional silencing of the _FMR1_ gene, due to an expansion of a CGG repeat found in the 5′-untranslated
region. The _FMR1_ gene product, FMRP, is a selective RNA-binding protein that negatively regulates local protein synthesis in neuronal dendrites. In its absence, the transcripts normally
regulated by FMRP are over translated. The resulting over abundance of certain proteins results in reduced synaptic strength due to AMPA receptor trafficking abnormalities that lead, at
least in part, to the fragile X phenotype. SIMILAR CONTENT BEING VIEWED BY OTHERS THE MOLECULAR BIOLOGY OF FMRP: NEW INSIGHTS INTO FRAGILE X SYNDROME Article 19 February 2021 ELEVATED
_FMR1_-MRNA AND LOWERED FMRP – A DOUBLE-HIT MECHANISM FOR PSYCHIATRIC FEATURES IN MEN WITH _FMR1_ PREMUTATIONS Article Open access 23 June 2020 CHANNELOPATHIES IN FRAGILE X SYNDROME Article
07 April 2021 IN BRIEF * Fragile X syndrome is a common inherited form of mental retardation that can be associated with features of autism. * The physical features of fragile X syndrome are
subtle and may not be obvious. * The vast majority of cases of fragile X syndrome are caused by the expansion to over 200 copies of a CGG repeat in the 5′-untranslated region of _FMR1_ that
shuts off transcription of the gene. * Genetic testing for this repeat expansion is diagnostic for this syndrome, and testing is appropriate in all children with developmental delay, mental
retardation or autism. * Fragile X syndrome is inherited from individuals, usually females, who typically carry an unstable premutation allele of the CGG-repeat tract in _FMR1_. *
Premutation carriers are themselves at risk of premature ovarian failure and the fragile X-associated tremor/ataxia syndrome. INTRODUCTION Fragile X syndrome (FXS) is the most common
inherited cause of mental retardation with approximately 1 in 4000 males affected.1 In the vast majority of cases, this X-linked disorder is caused by expansions of a CGG repeat in the
5′-untranslated (UTR) region of the _FMR1_ gene that arises due to the meiotic instability of certain alleles of this repeat tract. FXS causing alleles, or full mutations, contain 200 or
more copies of the repeat that are hypermethylated and transcriptionally silenced. The unstable alleles that give rise to full mutations are called premutations and are associated with
phenotypes distinct from FXS. The mutational mechanism, combined with the location of this gene on the X chromosome, leads to remarkable inheritance patterns in which the relevant alleles
are passed from intellectually normal men through their unaffected daughters and then to affected sons.2 FXS may be suspected in both sexes, and includes a variable clinical phenotype.
Individuals with FXS may present with anything from learning problems and a normal IQ to severe mental retardation and autistic behaviors. Physical features have been described but are often
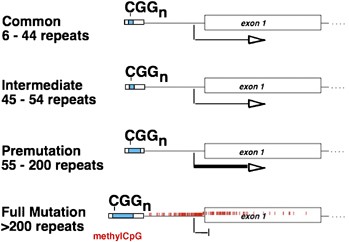
nonspecific. Thus, diagnosis is made based upon the detection of alterations to _FMR1_ (Figure 1). CLINICAL OVERVIEW The physical characteristics of FXS are fairly subtle, and the first
clinical indication is often delayed developmental milestones, such as mild motor delays and/or language delays.3 Autistic-like behaviors such as hand flapping, poor eye contact, and hand
biting may be noted. The average IQ in adult men with the completely methylated full mutation is approximately 40.4 Less-affected males typically have incomplete methylation, resulting in an
incomplete activation of _FMR1_, and they may even have an IQ in the borderline or low normal range. In general, for FXS, cognitive deficits include problems with working and short-term
memory, executive function, and mathematic and visuospatial abilities.5 Because the disorder is X-linked, females are generally much more mildly affected than males, particularly in terms of
cognitive functioning, but they tend to have higher risk for emotional problems compared to the general population.6 Females with the full mutation usually have normal or borderline IQ, and
most will have associated learning disabilities and/or emotional problems.7 Individuals with FXS usually do not have significant medical issues. Recurrent otitis media and recurrent
sinusitis are common during childhood.8 Joint laxity with hyperextensibility finger joints and pes planus (flat feet) may be present and usually improve with age.9 Gastroesophageal reflux
disease occurs in a third of young infants with FXS, and may present with irritability or recurrent emesis.9 Seizures and EEG findings consistent with epilepsy are another common feature of
FXS during childhood, with an incidence between 13 and 18% in boys and 5% in girls.10 The majority of individuals with the premutation have normal intelligence, but males are prone to have
attentional problems, executive dysfunction, social deficits, and obsessive-compulsive behavior.11 Approximately 20% of women who carry an _FMR1_ premutation have premature ovarian failure
(POF), which is the premature cessation of menses before the age of 40.12 A subgroup of men with a premutation develop neurologic deficits beyond age 50.13 Although many of these individuals
would have been given a diagnosis of parkinsonism in the past, a distinct tremor/ataxia syndrome due to premutations in _FMR1_ has been recently recognized. The fragile X-associated
tremor/ataxia syndrome (FXTAS) causes intentional tremors, balance problems, frequent falls, neuropathy, autonomic dysfunction, cognitive decline, and dementia, which may progressively
worsen over time.14 BEHAVIORAL ASPECTS The behavioral phenotype may be helpful in suggesting the diagnosis of FXS. Autistic-like features are common in individuals with FXS and include hand
flapping, hand biting, gaze avoidance, tactile defensiveness, and hyperarousal to sensory stimuli.15, 16 These features – along with impaired social skills, such as socio-emotional
reciprocity – are expressed with varying degrees in children with FXS and may be indicative of a concurrent diagnosis of autism spectrum disorder or autistic-like behavior.17 Anxiety and
mood disorders, hyperactivity, impulsivity, and aggressive behavior can also be present.18 The emotional and behavioral characteristics in females with FXS are usually variable. Females with
the full mutation are prone to social anxiety, shyness, social avoidance, withdrawal, language deficits, mood lability, and depression.6 Furthermore, females with the premutation have also
been described to have social anxiety.19 PHYSICAL FEATURES Physical features include macroorchidism that is apparent just prior to puberty20 and those related to a connective tissue
dysplasia, which include a long, narrow face, prominent ears, joint hypermobility, and flat feet.21 The facial characteristics (Figure 2) can be subtle and may become more apparent with
increasing age. NEUROANATOMY At autopsy, no gross abnormalities are observed in the brains of individuals with FXS.22 In males with FXTAS, MRI findings include global brain atrophy and white
matter abnormalities, including involvement of the middle cerebellar peduncles.23 A key neurological feature of individuals with FXS is that, in certain areas of the brain, their neurons
have immature and dense dendritic spines.24 The spines are the site at which the majority of excitatory synapses occur, and, although it is not known whether they are a cause or an effect,
similar abnormalities have been associated with other forms of mental retardation.25 It is believed that these differences represent a defect in dendritic spine development and maturation.24
MOLECULAR AND GENETIC BASIS OF THE DISEASE In 1991, the gene responsible for FXS, _FMR1_, was identified.26 Fragile X was the first known example of a trinucleotide repeat disorder (Figure
1). There are four allelic classes for the CGG-repeat tract in the 5′-UTR of _FMR1_. The repeat sizes for each group are not well defined, and this complicates genetic counseling. In the
general population, the repeat tract contains up to 40 repeats, with 30 being the most common (normal or common alleles).27 Next are the intermediate alleles, which range from 41to 54
repeats and which usually are not associated with instability of the repeat tract. Full mutations, which cause FXS, have over 200 copies of the repeat.27, 28 Hypermethylation of this
expanded repeat tract and the upstream CpG island silences _FMR1_ expression.29 Full mutations arise from the allele class known as premutations, which range in size from approximately
55–200 repeats and are meiotically unstable.27, 28 Premutations are unmethylated, transcriptionally active, and produce FMRP, although possibly in lower quantities than normal.30 These
alleles can expand in small amounts to yield a slightly larger premutation allele, or they can expand massively into the full mutation range. Expansion to the full mutation occurs only in
females because the full mutation cannot be maintained during spermatogenesis.31 The expanded repeat may be unstable and exhibits somatic heterogeneity in affected individuals, called
mosaics. Mosaicism may be both in terms of repeat length as well as methylation status (methylation mosaics). Affected males with methylation mosaicism have, on average, higher IQ scores
than those with fully methylated alleles, presumably because they express some FMRP.32 Although _FMR1_ premutation carriers are not affected by FXS, they are at risk for the additional
phenotypes of POF and FXTAS, as mentioned previously. In contrast to the lack of _FMR1_ transcription associated with FXS, the premutation-associated diseases are caused by a toxic RNA
effect caused by an excess of _FMR1_ transcription and/or repeat-containing mRNA.33 With FXTAS, this toxic effect is associated with the presence of intranuclear inclusions in the neurons
and astrocytes of affected brains.33 FRAGILE X MENTAL RETARDATION PROTEIN FMRP binds to specific mRNAs and has an important role in the regulation of protein synthesis at a local level in
the dendrites of neurons. The finding of abnormally long and immature dendritic spines in the brains of people with FXS and the importance of these spines in synaptic transmission, synaptic
plasticity, learning, and memory suggest that understanding the function of FMRP at the synapse could be the key to understanding the development of FXS syndrome. A proposed role for FMRP at
the synapse is that it is a negative regulator of protein synthesis stimulated by group 1 metabotropic glutamate receptor (mGluR) activation.34 FXS, then, is at least partially a result of
exaggerated responses to mGluR stimulation. In particular, one of the primary defects associated with the absence of FMRP appears to be excessive AMPA receptor internalization in response to
mGluR signaling.35 These alterations to AMPA receptor trafficking effect persistent changes in synaptic activity. This disease model is supported by the fact that treatment with the mGluR
antagonist 2-methyl-6-phenylethynyl-pyridine rescues the defect in AMPA receptor trafficking in cultured FMRP-deficient neurons,35 as well as some of the defects associated with the lack of
FMRP in _Drosophila_ and mouse models lacking FMRP function.36, 37 In addition, _Fmr1_-deficient mice that express 50% of the normal level of mGluR5 are rescued for several phenotypes,
including changes to the density of dendritic spines, susceptibility to audiogenic seizures, and altered plasticity in the visual cortex.38 DIAGNOSTIC APPROACHES A checklist of phenotypic
criteria has been established in order to identify individuals with undiagnosed developmental delay who would be appropriate candidates for FXS molecular testing3 (Table 1). These
checklists, of which several had been devised prior to that of Maes, may increase the diagnostic yield slightly, but because children may not have apparent physical features, it is accepted
practice to order fragile X testing in all children with developmental delay, mental retardation, or autism,39 although this may have a diagnostic yield of only approximately 1–2%.40 The
presence in the proband's family of movement disorders, learning disabilities, mental retardation, or primary ovarian insufficiency should increase suspicion of the presence of _FMR1_
mutations in the family.41 A suggested targeted family history questionnaire can be found in Table 2. The testing procedure encompasses two complementary analyses. PCR with primers flanking
the repeat is used to determine the number of CGG repeats in the _FMR1_ 5′-UTR, and a Southern blot of genomic DNA is used to determine the methylation status and to gauge the size of full
mutations, which are often resistant to PCR amplification.18 The combination of Southern blot and PCR for the detection of fragile X-associated mutations has a sensitivity of 99%. The
remaining 1% of mutations include missense mutations and full or partial deletions of _FMR1_.39 There is a deficit of reported missense mutations of _FMR1_ due to the over reliance on
CGG-repeat testing. Thus, in any individual presenting with a clinical suspicion of FXS but with normal CGG-repeat lengths, sequencing of _FMR1_, which also uncovers deletions in males,
should be considered. Fragile X testing of an individual with isolated cognitive impairment should be done in conjunction with cytogenetic evaluation because constitutional chromosomal
abnormalities are as common, if not more common, than FXS in this population.39 The same testing approach can also be used to identify premutation carriers. Prenatal diagnosis for full
mutations can be performed on either chorionic villus or amniocentesis samples and has proven to be highly reliable.43 MANAGEMENT GENETIC COUNSELING If a positive FXS test is discovered, the
proband and family should be referred for genetic counseling and cascade testing of family members at risk of carrying a full mutation or premutation.41 Premutation carriers should be
counseled regarding their risks of passing a full mutation onto their children, and they should also be counseled of their own risks of POF and/or FXTAS. When planning cascade testing in an
affected family, particular attention should be considered for family members with mental retardation, learning disabilities, autism, or social and behavioral disorders; female relatives
with infertility or premature menopause; and those with tremor, ataxia, or other neurological and psychiatric problems. Genetic counseling becomes difficult when _FMR1_ alleles in the ∼45–54
repeat range are identified. This is a ‘gray zone’ because unstable alleles of this size have been reported in families but the expansion is unlikely. Premutations are most often clinically
reported when the repeat tract is 55 repeats or greater, although the smallest repeat known to expand to a full mutation in a single generation was 59 repeats. The American College of
Medical Genetics provides guidelines for fragile X testing at http://www.acmg.net/Pages/ACMG_Activities/stds-2002/fx.htm. TREATMENT AND CARE Current approaches to therapy for FXS are all
symptom based, and few controlled trials have been performed to determine their effectiveness.44 Psychopharmacologic intervention should be combined with other supportive strategies,
including speech therapy, sensory integration occupational therapy, individualized educational plans, and tailored behavioral interventions to maximize functioning. In boys with FXS, the
most frequently used medications are stimulants.44 These medications are targeted to symptoms of hyperactivity, impulsivity, and distractability and can be quite helpful in these areas.44
Despite being the most common medication in FXS, the efficacy of these drugs and their side effects vary for each individual. The response rate to stimulants may be relatively lowered in
adult men with FXS because of their increased anxiety and decreased activity level.44 Some researchers believe that many of the behavioral problems observed in individuals with FXS are
secondary to problems with hyperarousal to sensory stimuli.18 Although it can be difficult to implement, structuring the environment of the affected individual such that they are comfortable
with their surroundings is one approach to alleviating this issue.18 Another approach is the use of _α_2-adrenergic agonists, which are thought to dampen the response to sensory input to
the brain and show good efficacy in treating some of these behaviors in boys with FXS.44 Selective serotonin reuptake inhibitors (SSRIs) are quite commonly used to treat mood disorder,
anxiety, and obsessive-compulsive behaviors associated with FXS. They are effective, particularly in alleviating social anxiety, tantrums, and aggression.44 Individuals treated with SSRIs
should be monitored for restlessness, mood changes, hyperactivity, and disinhibited behavior including aggression.44 Atypical antipsychotics have been used to treat self-injury, aggressive
behaviors, and autism. Parental reports of improvement in mood stabilization, attention, and academic performance have been noted with the atypical antipsychotic aripiprazole, which should
be used at low doses to avoid the agitation that can be induced by higher doses.45 Adverse events associated with antipsychotics include weight gain (although not with aripiprazole),
sedation, nausea, constipation, diabetes, and tardive dyskinesia. In individuals with rapid weight gain, monitoring for diabetes and metabolic syndrome must be considered. As mentioned
previously, the phenotype in FXS is caused by excessive mGluR5 signaling, thus drugs targeting mGluR5 may be an effective treatment for FXS.34 Several mGluR5 anatagonists are presently in
pharmaceutical development for an array of targeted clinical symptoms, such as anxiety disorder, Parkinson's Disease, and substance abuse.46 In addition, a recent placebo-controlled
clinical trial of the AMPA receptor positive modulator (Ampakine) CX 516 indicates no significant improvements in memory, language, attention/executive function, behavior, and cognitive
functioning in subjects with FXS, but suggest improvement in subjects cotreated with antipsychotics compared with those on placebo.47 Similar to other studies, it is possible that CX516 is a
low potency agent and the dosing may have been inadequate for therapeutic effect. Future clinical trials involving mGluR5 antagonists and AMPA receptor signaling may potentially provide a
targeted treatment of FXS in alleviating the core psychiatric and neurologic symptoms. Environmental variables may influence an individual's development of adaptive behaviors, cognitive
abilities, and behavioral symptoms.48 For instance, children with FXS residing in a higher quality home environment displayed fewer autistic behaviors, better adaptive behavior, and higher
IQ level. Some of the characteristics of the home environment that may be a factor in these effects are parenting ability, parental expectations of child behavior, organization of the home,
emotional climate, and enrichments in the home.48 Further studies in this area may lead to the development of nonpharmaceutical therapies that can help improve the outcomes of individuals
with FXS. At present, there are virtually no studies on the effectiveness of specific behavioral interventions in FXS despite our knowledge regarding their distinct behavioral phenotypes.
CONCLUSIONS Although current therapies for FXS are aimed at symptom management, it is hoped that future molecular therapies, whether they are aimed at mGluR5, the AMPA receptor, or other
molecular targets, will be directed at preventing the development of some of the symptoms of FXS. This may be a challenge because neurological evidence of FXS on a cellular level can be seen
very early postnatally.49 Early diagnosis will be key to making these therapies more effective, and there are efforts to implement newborn or infant screening for FXS. In a survey of
parents of children with FXS during the 1990s, a significant delay was found between the time they first became concerned about the child's development (median age: 12 months) and the
time they received a diagnosis of FXS (median age: 26 months).50 Thus, clinicians should address parental concern and consider the diagnosis of FXS in any infant or toddler with
developmental delays. REFERENCES * Crawford DC, Acuna JM, Sherman SL : FMR1 and the fragile X syndrome: human genome epidemiology review. _Genet Med_ 2001; 3: 359–371. Article CAS Google
Scholar * Sherman SL, Jacobs PA, Morton NE _et al_: Further segregation analysis of the fragile X syndrome with special reference to transmitting males. _Hum Genet_ 1985; 69: 289–299.
Article CAS Google Scholar * Maes B, Fryns JP, Ghesquiere P, Borghgraef M : Phenotypic checklist to screen for fragile X syndrome in people with mental retardation. _Ment Retard_ 2000;
38: 207–215. Article CAS Google Scholar * Merenstein SA, Sobesky WE, Taylor AK, Riddle JE, Tran HX, Hagerman RJ : Molecular-clinical correlations in males with an expanded FMR1 mutation.
_Am J Med Genet_ 1996; 64: 388–394. Article CAS Google Scholar * Kemper MB, Hagerman RJ, Altshul-Stark D : Cognitive profiles of boys with the fragile X syndrome. _Am J Med Genet_ 1988;
30: 191–200. Article CAS Google Scholar * Freund LS, Reiss AL, Abrams MT : Psychiatric disorders associated with fragile X in the young female. _Pediatrics_ 1993; 91: 321–329. CAS PubMed
Google Scholar * de Vries BB, Wiegers AM, Smits AP _et al_: Mental status of females with an FMR1 gene full mutation. _Am J Hum Genet_ 1996; 58: 1025–1032. CAS PubMed PubMed Central
Google Scholar * Hagerman RJ, Altshul-Stark D, McBogg P : Recurrent otitis media in the fragile X syndrome. _Am J Dis Child (1960)_ 1987; 141: 184–187. CAS Google Scholar * Hagerman RJ :
Medical follow-up and pharmacotherapy; in Hagerman RJ, Hagerman PJ (eds):: _Fragile X Syndrome: Diagnosis, Treatment and Research_, 3rd edn. Baltimore: The Johns Hopkins University Press,
2002, pp 287–338. Google Scholar * Musumeci SA, Hagerman RJ, Ferri R _et al_: Epilepsy and EEG findings in males with fragile X syndrome. _Epilepsia_ 1999; 40: 1092–1099. Article CAS
Google Scholar * Farzin F, Perry H, Hessl D _et al_: Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. _J Dev Behav Pediatr_
2006; 27: S137–S144. Article Google Scholar * Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D _et al_: Fragile X premutation is a significant risk factor for premature ovarian failure: the
International Collaborative POF in Fragile X study – preliminary data. _Am J Med Genet_ 1999; 83: 322–325. Article CAS Google Scholar * Jacquemont S, Hagerman RJ, Leehey MA _et al_:
Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. _JAMA_ 2004; 291: 460–469. Article CAS Google Scholar * Willemsen R, Mientjes E, Oostra
BA : FXTAS: a progressive neurologic syndrome associated with Fragile X premutation. _Curr Neurol Neurosci Rep_ 2005; 5: 405–410. Article CAS Google Scholar * Hagerman RJ, Jackson III AW,
Levitas A, Rimland B, Braden M : An analysis of autism in fifty males with the fragile X syndrome. _Am J Med Genet_ 1986; 23: 359–374. Article CAS Google Scholar * Brown WT, Jenkins EC,
Cohen IL _et al_: Fragile X and autism: a multicenter survey. _Am J Med Genet_ 1986; 23: 341–352. Article CAS Google Scholar * Kaufmann WE, Cortell R, Kau AS _et al_: Autism spectrum
disorder in fragile X syndrome: communication, social interaction, and specific behaviors. _Am J Med Genet_ 2004; 129: 225–234. Article Google Scholar * Tsiouris JA, Brown WT :
Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. _CNS Drugs_ 2004; 18: 687–703. Article CAS Google Scholar * Franke P, Leboyer M, Gansicke M _et al_:
Genotype – phenotype relationship in female carriers of the premutation and full mutation of FMR-1. _Psychiatry Res_ 1998; 80: 113–127. Article CAS Google Scholar * Lachiewicz AM, Dawson
DV : Do young boys with fragile X syndrome have macroorchidism? _Pediatrics_ 1994; 93: 992–995. CAS PubMed Google Scholar * Hagerman RJ, Van Housen K, Smith AC, McGavran L : Consideration
of connective tissue dysfunction in the fragile X syndrome. _Am J Med Genet_ 1984; 17: 111–121. Article CAS Google Scholar * Hinton VJ, Brown WT, Wisniewski K, Rudelli RD : Analysis of
neocortex in three males with the fragile X syndrome. _Am J Med Genet_ 1991; 41: 289–294. Article CAS Google Scholar * Jacquemont S, Hagerman RJ, Leehey M _et al_: Fragile X premutation
tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. _Am J Hum Genet_ 2003; 72: 869–878. Article CAS Google Scholar * Irwin SA, Patel B, Idupulapati M _et al_:
Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. _Am J Med Genet_ 2001; 98: 161–167. Article CAS
Google Scholar * Kaufmann WE, Moser HW : Dendritic anomalies in disorders associated with mental retardation. _Cereb Cortex_ 2000; 10: 981–991. Article CAS Google Scholar * Verkerk AJ,
Pieretti M, Sutcliffe JS _et al_: Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome.
_Cell_ 1991; 65: 905–914. Article CAS Google Scholar * Snow K, Doud LK, Hagerman R, Pergolizzi RG, Erster SH, Thibodeau SN : Analysis of a CGG sequence at the FMR-1 locus in fragile X
families and in the general population. _Am J Hum Genet_ 1993; 53: 1217–1228. CAS PubMed PubMed Central Google Scholar * Fu YH, Kuhl DP, Pizzuti A _et al_: Variation of the CGG repeat at
the fragile X site results in genetic instability: resolution of the Sherman paradox. _Cell_ 1991; 67: 1047–1058. Article CAS Google Scholar * Sutcliffe JS, Nelson DL, Zhang F _et al_:
DNA methylation represses FMR-1 transcription in fragile X syndrome. _Hum Mol Genet_ 1992; 1: 397–400. Article CAS Google Scholar * Kenneson A, Zhang F, Hagedorn CH, Warren ST : Reduced
FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. _Hum Mol Genet_ 2001; 10: 1449–1454. Article CAS
Google Scholar * Malter HE, Iber JC, Willemsen R _et al_: Characterization of the full fragile X syndrome mutation in fetal gametes. _Nat Genet_ 1997; 15: 165–169. Article CAS Google
Scholar * McConkie-Rosell A, Lachiewicz AM, Spiridigliozzi GA _et al_: Evidence that methylation of the FMR-I locus is responsible for variable phenotypic expression of the fragile X
syndrome. _Am J Hum Genet_ 1993; 53: 800–809. CAS PubMed PubMed Central Google Scholar * Hagerman PJ, Hagerman RJ : The fragile-X premutation: a maturing perspective. _Am J Hum Genet_
2004; 74: 805–816. Article CAS Google Scholar * Bear MF, Huber KM, Warren ST : The mGluR theory of fragile X mental retardation. _Trends Neurosci_ 2004; 27: 370–377. Article CAS Google
Scholar * Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST : Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of
AMPA receptors. _Proc Natl Acad Sci USA_ 2007; 104: 15537–15542. Article CAS Google Scholar * McBride SM, Choi CH, Wang Y _et al_: Pharmacological rescue of synaptic plasticity, courtship
behavior, and mushroom body defects in a _Drosophila_ model of fragile X syndrome. _Neuron_ 2005; 45: 753–764. Article CAS Google Scholar * Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP :
Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. _Neuropharmacology_ 2005; 49: 1053–1066. Article CAS Google Scholar * Dolen G, Osterweil
E, Rao BS _et al_: Correction of fragile X syndrome in mice. _Neuron_ 2007; 56: 955–962. Article CAS Google Scholar * Sherman S, Pletcher BA, Driscoll DA : Fragile X syndrome: diagnostic
and carrier testing. _Genet Med_ 2005; 7: 584–587. Article Google Scholar * Rauch A, Hoyer J, Guth S _et al_: Diagnostic yield of various genetic approaches in patients with unexplained
developmental delay or mental retardation. _Am J Med Genet_ 2006; 140: 2063–2074. Article Google Scholar * McConkie-Rosell A, Abrams L, Finucane B _et al_: Recommendations from
multi-disciplinary focus groups on cascade testing and genetic counseling for fragile X-associated disorders. _J Genet Couns_ 2007; 16: 593–606. Article Google Scholar * McConkie-Rosell A,
Finucane B, Cronister A, Abrams L, Bennett RL, Pettersen BJ : Genetic counseling for fragile x syndrome: updated recommendations of the national society of genetic counselors. _J Genet
Couns_ 2005; 14: 249–270. Article Google Scholar * Brown WT, Houck Jr GE, Jeziorowska A _et al_: Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test.
_JAMA_ 1993; 270: 1569–1575. Article CAS Google Scholar * Berry-Kravis E, Potanos K : Psychopharmacology in fragile X syndrome – present and future. _Ment Retard Dev Disabil Res Rev_
2004; 10: 42–48. Article Google Scholar * Hagerman RJ : Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. _J Dev Behav Pediatr_ 2006; 27: 63–74. Article Google
Scholar * Slassi A, Isaac M, Edwards L _et al_: Recent advances in non-competitive mGlu5 receptor antagonists and their potential therapeutic applications. _Curr Top Med Chem_ 2005; 5:
897–911. Article CAS Google Scholar * Berry-Kravis E, Krause SE, Block SS _et al_: Effect of CX516, an AMPA-modulating compound, on cognition and behavior in fragile X syndrome: a
controlled trial. _J Child Adolesc Psychopharmacol_ 2006; 16: 525–540. Article Google Scholar * Glaser B, Hessl D, Dyer-Friedman J _et al_: Biological and environmental contributions to
adaptive behavior in fragile X syndrome. _Am J Med Genet_ 2003; 117: 21–29. Article Google Scholar * Nimchinsky EA, Oberlander AM, Svoboda K : Abnormal development of dendritic spines in
FMR1 knock-out mice. _J Neurosci_ 2001; 21: 5139–5146. Article CAS Google Scholar * Bailey DB, Skinner D, Sparkman K, Moore CA, Olney RS, Crawford DC : Delayed diagnosis of fragile X
syndrome – United States, 1990–1999. _MMWR Morb Mortal Wkly Rep_ 2002; 51: 740–742. Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Human
Genetics, Emory University School of Medicine, Atlanta, GA, USA Kathryn B Garber, Jeannie Visootsak & Stephen T Warren Authors * Kathryn B Garber View author publications You can also
search for this author inPubMed Google Scholar * Jeannie Visootsak View author publications You can also search for this author inPubMed Google Scholar * Stephen T Warren View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Stephen T Warren. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Garber, K., Visootsak, J. & Warren, S. Fragile X syndrome. _Eur J Hum Genet_ 16, 666–672 (2008). https://doi.org/10.1038/ejhg.2008.61 Download citation *
Received: 06 December 2007 * Revised: 08 February 2008 * Accepted: 20 February 2008 * Published: 09 April 2008 * Issue Date: June 2008 * DOI: https://doi.org/10.1038/ejhg.2008.61 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Fragile X syndrome * FMR1 * permutation * full mutation * autism