Play all audios:
ABSTRACT The analysis of age-specific genetic effects on human survival over extreme ages is confronted with a deceleration pattern in mortality that deviates from traditional survival
models and sparse genetic data available. As human late life is a distinct phase of life history, exploring the genetic effects on extreme age survival can be of special interest to
evolutionary biology and health science. We introduce a non-parametric survival analysis approach that combines population survival information with individual genotype data in assessing the
genetic effects in cohort-based longitudinal studies. Our approach is characterized by non-parametric analysis of late age survival to capture the observed pattern of mortality deceleration
and frailty modeling to account for individual heterogeneity in unobserved frailty. The method is applied to ApoE genotype data in the Danish 1905 birth cohort to estimate effect of the e4
allele. Our results revealed an age-specific relative risk of the allele that increases nonlinearly with age and non-proportional patterns in hazard of death for carriers and non-carriers of
the allele, suggesting that the e4 mutation preserves its deleterious effect that progressively affect human survival even at extreme ages. SIMILAR CONTENT BEING VIEWED BY OTHERS MENDELIAN
RANDOMIZATION EVIDENCE FOR THE CAUSAL EFFECTS OF SOCIO-ECONOMIC INEQUALITY ON HUMAN LONGEVITY AMONG EUROPEANS Article 29 June 2023 IMPLAUSIBILITY OF RADICAL LIFE EXTENSION IN HUMANS IN THE
TWENTY-FIRST CENTURY Article Open access 07 October 2024 EFFICIENT AND ACCURATE FRAILTY MODEL APPROACH FOR GENOME-WIDE SURVIVAL ASSOCIATION ANALYSIS IN LARGE-SCALE BIOBANKS Article Open
access 16 September 2022 INTRODUCTION The evolutionary theory of aging assumes that the effect of a gene could change over an individual’s life course as genetic mutation functioning at late
ages are subject to weaker selection than early-acting mutations.1 The age-specific genetic effects have been shown to affect fitness traits in animal models.2 In humans, age-specific
effects of genetic variations have been reported to influence body mass index,3 blood pressure4, 5 and survival.6 At late life, the force of natural selection during the reproductive period
stops. In term of survival, mortality deviates significantly from the popular Gompertz model with a reliable attribute characterized by deceleration in age-specific mortality rates.7 The
paradoxical ‘plateaued’ mortality pattern implies that late life is a distinct phase of life history8 for which exploring the genetic effects can be of special interest to evolutionary
biology and health science. The estimation of an age-dependent genetic effect on survival can often be confounded by differential life course exposure to environmental factors or the birth
cohort effect in age-structured populations.9 For that reason, a good choice is to conduct a follow-up or longitudinal study on a birth cohort, which has only been feasible in animal
experiments. In human studies, however, longitudinal analysis on genetic association with human longevity can be done with old-aged birth cohorts, for example, the Danish 1905 birth
cohort,10 to look for genes that affect extreme age survival.9, 11, 12 Although of great interest, estimating genetic effects on late life survival is confronted with the distinct mortality
pattern and sparse genetic data available. In the literature, different theories or models have been proposed to explain the late life-mortality pattern,8 among them the heterogeneity
model,13 which assumes individual heterogeneity in unobserved frailty that follows a gamma distribution. Jacobsen _et al_11 applied a Cox regression model with gamma-distributed frailty to
the Danish 1905 birth cohort data to estimate the age-dependent effect on extreme age survival for the ApoE gene, the only gene whose role on longevity has been consistently demonstrated.14
This paper introduces a demographic heterogeneity model that combines sparse individual genotype data with population survival information to measure age-specific genetic effect on survival
at advanced ages. The method is applied to ApoE genotype data from the Danish 1905 birth cohort10 to illustrate the patterns of the age-specific effect of the e4 allele in affecting extreme
age survival. Results with and without consideration of unobserved frailty will be compared and genotype-specific mortality patterns illustrated. METHODS For a given genetic variation, for
example, a SNP, individuals can be grouped according to their genotypes for a certain allele as non-carriers (0 allele), heterozygous (1 allele) and homozygous (2 alleles) carriers based on
which effect of the allele can be assumed to be additive, dominant or recessive. For simplicity, we divide individuals as carriers and non-carriers of the allele, which is equivalent to a
dominant assumption. In term of survival, the population survival rate in a birth cohort is the weighted mean for allele carriers (≥1 allele) and non-carriers (0 allele),15 Here, s̄(_x_) is
the mean survival rate in the birth cohort at age _x_, _p_ is frequency of carriers of the allele, _s_1(_x_) and _s_o(_x_) are survival rates for carriers and non-carriers of the allele. The
relationship between _s_1(_x_) and _s_0(_x_) reflects relative risk of the allele on survival. In a simple proportional hazard model, the hazards of death corresponding to _s_1(_x_) and
_s_0(_x_) are related as _μ_1(_x_)=_rμ_0(_x_) such that The relationship above is based on the assumption that individuals are homogenous except for their genotypes of the allele. However,
in reality, individuals are heterogeneous in their unobserved factors or frailty, including genetic make-ups, which serves as the basis for existing theories that explain mortality
deceleration at advanced ages, among which is the demographic heterogeneity theory by Vaupel _et al._13 It follows that, when an individual’s unobserved frailty designated as _z_ is
gamma-distributed with mean 1 and variance _σ_2, instead of (2), the relationship between _s_1(_x_) and _s_0(_x_) becomes _s_′(_x_) is a homogenous baseline survival function. Note that the
integration of (3) with (1) combines population survival with genotype frequency and relative risk parameters, which allows assessment of genetic effect on survival. Based on (1), the
proportions of carriers and non-carriers of the allele at any age _x_ can be estimated as and , respectively. When genotype data is available for a random sample from the cohort, a
likelihood function based on binomial distribution can be constructed at each age _x_ as In (4), _n_1(_x_) and _n_0(_x_) are the number of counts for carriers and non-carriers of the allele
at age _x_, _p_ is proportion of carriers in the population, which can be available for specific populations and s̄(_x_ is population survival rate at age _x_ obtainable from population
statistics. With known s̄(_x_ and _p_, (4) can be maximized to estimate the relative risk on survival for carrying the allele. In a longitudinal study on a birth cohort, (4) can be done for
each age or year of follow-up so that age-specific effects can be estimated. The maximum likelihood estimation (MLE) is obtained by introducing a constraint as specified in (1) and
optimization of (4) with numerical gradient and Hessian. Note that our MLE is free from specification of any parametric form for the survival function and is thus a non-parametric approach.
In addition, it combines or makes use of population data in the analysis of genetic effect. Moreover, genotype-specific survival or mortality rates can be calculated at each age to further
illustrate the genetic influence on mortality at advanced ages. Finally, our model allows analysis of sex-specific effect16 by simply replacing the mean cohort survival in (1) with survival
rate for males or females and performing the analysis for each sex separately. However, because most of the survivors at extreme ages are females, insignificant results in males due to small
sample sizes available may not necessarily mean sex-specific effect. In this case, a combined analysis should be preferred. RESULTS We applied our method to the ApoE genotype data collected
on 2662 individuals (584 males and 2078 females) from the Danish 1905 birth cohort10 collected in a longitudinal survey initiated in 1998. All participants were genotyped at age 92–93
years. Individual survival information has been collected with the latest update at the end of 2010 when 10 subjects were still alive with their ages over 104 or 105. For the entire 1905
birth cohort, cohort-specific survival information is available from the Human Mortality Database at http://www.mortality.org/ jointly hosted by University of California, Berkeley, and the
Max Planck Institute for Demographic Research, Rostock, Germany. For the ApoE gene, frequency for the allele of interest, that is, the e4 allele, was estimated to be 0.174 in the Danish
population,17 which corresponds to a carrier frequency of 0.318. As a ‘thrifty’ allele,18 carriers of e4 have been shown to have a higher susceptibility to cardiovascular and Alzheimer’s
diseases, and are associated with higher mortality as compared with non-carriers under the contemporary environmental condition.17, 19 As such, frequency of the allele is expected to
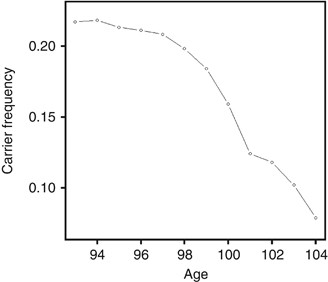
decrease with increasing age in a birth cohort. In our genotype data for the 1905 birth cohort, it is interesting to see that the deceasing pattern continues even at extreme ages starting
with 21.7% at age 93 until 7.8% at age 104, a rapid decrease of about 14% in 11 years (Figure 1). The declining nonlinear pattern in e4 allele frequency that accelerates with age gives a
clear indication of a deleterious effect of the allele on human extreme age survival, which needs to be characterized or measured by proper statistical models. With known population survival
for the entire 1905 birth cohort and frequency of e4 allele in the Danish population, we first fitted the likelihood function in (4) without frailty using genotype-specific survival as
defined in (2). For each age _x_, our procedure estimated an age-specific relative risk on surviving from age _x_ to _x_+1 (Table 1). Our results showed that the estimated risks were all
significantly different from one over all ages with a slight trend of increase at later ages. Figure 2a plots the estimated age-specific relative risks together with their 95% confidence
intervals. The figure clearly displays the increasing risk for the e4 allele in the oldest survivors. The highest risk of 1.23 (_P_=0.026) was obtained at the highest age of 104. We
continued our analysis with frailty modeling by introducing gamma-distributed frailty with mean of 1 and variance of 0.1 (according to our experience in fitting frailty models to oldest-old
mortality). From the estimated relative risks (Table 1), one could see that the frailty model gives higher risk estimates as compared with the no frailty model. In addition to the increased
risk, the age-dependent increase in risk estimates is more clearly seen with frailty modeling, although the overall pattern of increase remained (Figure 2b). Using the relationships in (2)
and (3), age-specific survivals for carriers and non-carriers of the e4 allele can be calculated with the estimated relative risk and baseline survival rate. This allows calculation of
age-specific hazard rate _μ_(_x_) because _μ_(_x_)=−d(ln _s_(_x_))/d_x_ . In Figure 3, we show the non-parametric age-specific hazard functions for the total population starting from age 80
(solid line) and the e4 allele carriers (dashed line) and non-carriers (dash-dotted line) starting from age 93. Although mortality patterns for carriers and non-carriers followed the main
pattern of the whole cohort, carriers had higher whereas non-carriers had lower instant probability of death than that for the mean population, and overall this deviation grew larger at
later ages. Moreover, the population mortality pattern in Figure 3 also exhibits the mortality leveling-off at high ages, suggesting the necessity of frailty modeling. Note that the
calculated patterns of genotype-specific hazards were the same for both frailty and no frailty models as optimization of (4) was done for each age, however, the genetic risk was
underestimated when unobserved heterogeneity in frailty is ignored. Finally, we applied the frailty model to another example for SNP rs2764264 in the FOXO3A gene. The SNP was first reported
to show association with human longevity in a case–control study conducted in the Italian population.20 Recently, the SNP was tested in both case–control samples and the Danish 1905 cohort
with the significant association replicated only in the case–control samples.21 In Figure 4, we show age-specific risks estimated from our frailty modeling (frequency of carriers of minor
allele set to 0.495 according to Soerensen _et al_21). Different from the e4 all of ApoE gene, no risk estimate in Figure 4 reached statistical significance, although there is a slight trend
toward a protective effect similar to that reported in the literature in case–control studies.20, 21 DISCUSSION The cohort study is deemed as the most ideal design for assessing risk
factors that affect human longevity9 and in characterizing their age-specific effects. In humans, longitudinal following up for survival analysis is only feasible in very old cohorts, such
as the Danish 1905 birth cohort. However, at advanced ages, human survival is characterized by mortality deceleration, which challenges conventional survival models.8 We introduced a
non-parametric survival analysis that combines population survival information with individual genotype data in estimating the genetic effects on human longevity. Our method conducts frailty
modeling by introducing the simple gamma frailty model. Our comparison with a model that ignores unobserved heterogeneity showed underestimated genetic effect by the latter, which
emphasizes the importance of frailty modeling in genetic risk assessment at advanced ages. The constraint likelihood for parameter estimation integrates population data with individual
genotype data and allows non-parametric estimation of genetic risk parameters and the baseline survival function to avoid specification of parametric survival models that deviate from the
observed mortality pattern. In addition to parameter estimation, our procedure also calculates non-parametric genotype-specific hazard of death over the observed ages to allow comparison
with population mean death rate (Figure 3). Our likelihood-based procedure is made possible by restricting estimation on each age separately. As an advantage, this allows measurement of
age-specific genetic effect. As shown by Figure 2, the age-specific pattern of the estimated genetic risk deviates clearly from being constant or linear, which contradicts to the
proportional hazard assumption. From the hazard functions for carriers and non-carriers of e4 allele, one can easily see that they are far from proportional. Such a pattern will be missed by
traditional survival analysis, such as the Cox’s proportional hazard model. In Table 2, we compare the different analyses that have been applied to the ApoE genotype data in the 1905
cohort. The early analysis (with high censoring rate of 17%) by Bathum _et al_22 (Table 2) obtained an overall risk for e4 carriers, which was only borderline significant. Jacobsen _et al_11
introduced Aalen’s additive hazards model,23 an extended Cox model, to estimate age-dependent risk assuming additive risks over age intervals. It is interesting that, when applied to the
same updated data set (censoring rate 4%), their analysis also reported the increased effect of the e4 allele on longevity, although their analysis was limited to three age intervals. In
comparison, our combined analysis of population and individual data enabled estimation for each age until the age as high as 104 years such that patterns of the mean genetic effects and
genotype-specific mortality at extreme ages can be examined (Table 2). It can be expected that, with the rapid development in the SNP genotyping and genome sequencing, more genetic data will
be available for association analysis of human extreme age survival for which proper statistical models can contribute. REFERENCES * Zwaan BJ : The evolutionary genetics of ageing and
longevity. _Heredity_ 1999; 82 (Part 6): 589–597. Article Google Scholar * Leips J, Gilligan P, Mackay TF : Quantitative trait loci with age-specific effects on fecundity in _Drosophila
melanogaster_. _Genetics_ 2006; 172: 1595–1605. Article CAS Google Scholar * Atwood LD, Heard-Costa NL, Fox CS, Jaquish CE, Cupples LA : Sex and age specific effects of chromosomal
regions linked to body mass index in the Framingham Study. _BMC Genet_ 2006; 7: 7. Article Google Scholar * Tambs K, Eaves LJ, Moum T _et al_. Age-specific genetic effects for blood
pressure. _Hypertension_ 1993; 22: 789–795. Article CAS Google Scholar * Joubert BR, Diao G, Lin D, North KE, Franceschini N : Longitudinal age-dependent effect on systolic blood
pressure. _BMC Proc_ 2009; 3 (Suppl 7): S87. Article Google Scholar * Tan Q, Bathum L, Christiansen L _et al_. Logistic regression models for polymorphic and antagonistic pleiotropic gene
action on human aging and longevity. _Ann Hum Genet_ 2003; 67 (Part 6): 598–607. Article CAS Google Scholar * Vaupel JW, Carey JR, Christensen K _et al_. Biodemographic trajectories of
longevity, 1998 _Science_ 280: 855–860. Article CAS Google Scholar * Rauser CL, Mueller LD, Rose MR : The evolution of late life. _Ageing Res Rev_ 2006; 5: 14–32. Article Google Scholar
* Tan Q, Kruse TA, Christensen K : Design and analysis in genetic studies of human ageing and longevity. _Ageing Res Rev_ 2006; 5: 371–387. Article CAS Google Scholar * Nybo H, Gaist D,
Jeune B _et al_. The Danish 1905 cohort: a genetic-epidemiological nationwide survey. _J Aging Health_ 2001; 13: 32–46. Article CAS Google Scholar * Jacobsen R, Martinussen T,
Christiansen L _et al_. Increased effect of the ApoE gene on survival at advanced age in healthy and long-lived Danes: two nationwide cohort studies. _Aging Cell_ 2010; 9: 1004–1009. Article
CAS Google Scholar * Sørensen M, Thinggaard M, Nygaard M _et al_. Genetic variation in TERT and TERC and human leukocyte telomere length and longevity: a cross-sectional and longitudinal
analysis. _Aging Cell_ 2012; 11: 223–227. Article Google Scholar * Vaupel JW, Manton KG, Stallard E : The impact of heterogeneity in individual frailty on the dynamics of mortality.
_Demography_ 1979; 16: 439–454. Article CAS Google Scholar * Christensen K, Johnson TE, Vaupel JW : The quest for genetic determinants of human longevity: challenges and insights. _Nat
Rev Genet_ 2006; 7: 436–448. Article CAS Google Scholar * Vaupel JW, Yashin AI : Heterogeneity’s ruses: some surprising effects of selection on population dynamics. _Am Stat_ 1985; 39:
176–185. CAS PubMed Google Scholar * Rosvall L, Rizzuto D, Wang HX, Winblad B, Graff C, Fratiglioni L : APOE-related mortality: effect of dementia, cardiovascular disease and gender.
_Neurobiol Aging_ 2009; 30: 1545–1551. Article CAS Google Scholar * Gerdes LU, Klausen IC, Sihm I, Faergeman O : Apolipoprotein E polymorphism in a Danish population compared to findings
in 45 other study populations around the world. _Genet Epidemiol_ 1992; 9: 155–167. Article CAS Google Scholar * Corbo RM, Scacchi R : Apolipoprotein E (APOE) allele distribution in the
world. Is APOE*4 a ‘thrifty’ allele? _Ann Hum Genet_ 1999; 63 (Part 4): 301–310. Article CAS Google Scholar * Tan Q, Christiansen L, Christensen K, Kruse TA, Bathum L : Apolipoprotein E
genotype frequency patterns in aged Danes as revealed by logistic regression models. _Eur J Epidemiol_ 2004; 19: 651–656. Article Google Scholar * Willcox BJ, Donlon TA, He Q _et al_.
FOXO3A genotype is strongly associated with human longevity. _Proc Natl Acad Sci USA_ 2008; 105: 13987–13992. Article CAS Google Scholar * Soerensen M, Dato S, Christensen K _et al_.
Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. _Aging Cell_ 2010; 9: 1010–1017. Article CAS Google
Scholar * Bathum L, Christiansen L, Jeune B, Vaupel J, McGue M, Christensen K : Apolipoprotein e genotypes: relationship to cognitive functioning, cognitive decline, and survival in
nonagenarians. _J Am Geriatr Soc_ 2006; 54: 654–658. Article Google Scholar * Aalen OO : A linear regression model for the analysis of life times. _Stat Med_ 1989; 8: 907–925. Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS This work was partially supported by the EU Seventh Framework Programme (FP7/2007–2011) under grant agreement no 259679 and NIH/NIA
grant P01 AG08761. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Epidemiology, Institute of Public Health, University of Southern Denmark, Odense C, Denmark Qihua Tan, Rune Jacobsen, Mette
Sørensen, Lene Christiansen & Kaare Christensen * Department of Clinical Genetics, Odense University Hospital, Odense C, Denmark Qihua Tan, Lene Christiansen, Torben A Kruse & Kaare
Christensen * Department of Biochemistry and Pharmacology, Odense University Hospital, Odense C, Denmark Kaare Christensen Authors * Qihua Tan View author publications You can also search
for this author inPubMed Google Scholar * Rune Jacobsen View author publications You can also search for this author inPubMed Google Scholar * Mette Sørensen View author publications You can
also search for this author inPubMed Google Scholar * Lene Christiansen View author publications You can also search for this author inPubMed Google Scholar * Torben A Kruse View author
publications You can also search for this author inPubMed Google Scholar * Kaare Christensen View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to Qihua Tan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Tan, Q., Jacobsen, R., Sørensen, M. _et al._ Analyzing age-specific genetic effects on human extreme age survival in cohort-based longitudinal studies. _Eur J
Hum Genet_ 21, 451–454 (2013). https://doi.org/10.1038/ejhg.2012.182 Download citation * Received: 30 March 2012 * Revised: 21 May 2012 * Accepted: 31 May 2012 * Published: 15 August 2012 *
Issue Date: April 2013 * DOI: https://doi.org/10.1038/ejhg.2012.182 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * genetic effect * extreme age
survival * cohort design * population data * frailty modeling