Play all audios:
It has been suggested that proangiotensin-12 (proang-12), a novel angiotensin peptide recently discovered in rat tissues, may function as a component of the tissue renin-angiotensin system
(RAS). To investigate the role of proang-12 in the production of angiotensin II (Ang II), we measured its plasma and tissue concentrations in Wistar–Kyoto (WKY) and spontaneously
hypertensive (SHR) rats, with and without RAS inhibition. The 15-week-old male WKY and SHR rats were left untreated or were treated for 7 days with 30 mg kg−1 per day losartan, an
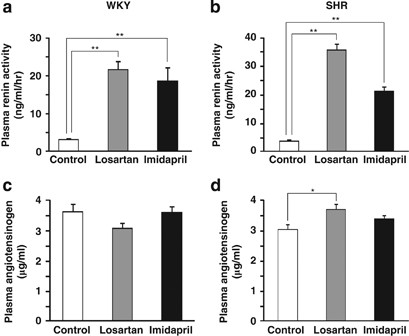
angiotensin receptor blocker, or with 20 mg kg−1 per day imidapril, an angiotensin-converting enzyme (ACE) inhibitor. Both treatments increased renin activity and the concentrations of
angiotensin I (Ang I) and Ang II in the plasma of WKY and SHR rats, but neither affected plasma proang-12 levels. In contrast to the comparatively low level of proang-12 seen in plasma,
cardiac and renal levels of proang-12 were higher than those of Ang I and Ang II. In addition, despite activation of the RAS in the systemic circulation, tissue concentrations of proang-12
were significantly reduced following treatment with losartan or imidapril. Similar reductions were also observed in the tissue concentrations of Ang II in both strains, without a reduction
in Ang I. These results suggest that tissue concentrations of proang-12 and Ang II are regulated independently of the systemic RAS in WKY and SHR rats, which is consistent with the notion
that proang-12 is a component of only the tissue RAS.
The renin-angiotensin system (RAS) has a crucial role in the regulation of blood pressure and fluid balance. It is well established that renin secreted from the kidneys cleaves
angiotensinogen circulating in the blood to produce angiotensin I (Ang I), which is in turn cleaved by angiotensin-converting enzyme (ACE) to produce angiotensin II (Ang II), a potent
pressor peptide mediating the major actions of the circulating (systemic) RAS.1, 2, 3 By contrast, much less is known about the tissue RAS, and many questions about the angiotensin
processing cascade and the role of the tissue RAS in regulating blood pressure and fluid balance remain unanswered.4, 5, 6, 7
Proangiotensin-12 (proang-12) is a 12-amino acid, C-terminal extended form of Ang I, which we recently isolated from rat small intestine.8 In vitro, proang-12 constricts aortic strips and,
when intravenously infused into rats, raises blood pressure. The vasoconstrictor and pressor effects of proang-12 are abolished by ACE inhibitors and angiotensin receptor blockers, which
suggests ACE is involved in the conversion of proang-12 to Ang II.8, 9, 10 However, Prosser et al.11, 12 reported that chymase inhibition attenuates proang-12-induced cardiac damage caused
by ischemia-reperfusion in rat hearts ex vivo, as well as proang-12-induced constriction of isolated rat arteries. This suggests that chymase is also involved in the conversion of proang-12
to Ang II. Moreover, Ahmad et al.13 demonstrated that ACE, neprilysin and chymase are all involved in the metabolism of proang-12 in neonatal cardiac myocytes. Taken together, these findings
suggest proang-12 may be metabolized or converted to Ang II by several enzymes. In addition, proang-12 is also metabolized to two other angiotensin-related peptides, Ang(1–7)14 or
Ang(1–9).15
In various tissues, including the heart and kidneys, the concentration of proang-12 is much higher than those of Ang I and Ang II.8, 9, 16 By contrast, the concentration of proang-12 in
plasma is lower than that of either Ang I or Ang II. This suggests that proang-12 may be a component of only the tissue RAS in rats. To investigate the role of systemic renin in the
production of proang-12, we previously measured tissue proang-12 concentrations in rats subjected to bilateral nephrectomy17 or fed a low-salt diet.18 Both of these experiments showed that
tissue proang-12 is regulated in a manner that is independent of the plasma renin activity.
Therefore, our aim in the present study was to clarify the role of proang-12 in the production of Ang II in tissue and blood. To accomplish this, we assessed the plasma and tissue
concentrations of Ang II, Ang I and proang-12 in Wistar–Kyoto (WKY) and spontaneously hypertensive (SHR) rats, with and without RAS inhibitor.
The 15-week-old male WKY and SHR rats were purchased from Charles River Laboratories (Kanagawa, Japan). Losartan and imidapril were kindly provided by Merck (Whitehouse Station, NJ, USA) and
Mitsubishi Tanabe Pharma Corporation (Osaka, Japan), respectively.
The rats were maintained under a 12-h light/12-h dark cycle and specific pathogen-free conditions, and were fed a normal diet. Before experimentation, the rats were randomly divided into
three groups (n=6–8 in each group): the control group was left untreated; the losartan group received 30 mg kg−1 losartan in their drinking water daily for 7 days; and the imidapril group
received 20 mg kg−1 imidapril daily over the same period. We selected doses of both agents that reportedly suppress the systemic RAS to a substantial degree.19, 20 Blood pressures were
measured using the tail-cuff method (model BP-98A; Softron, Tokyo, Japan), before and after treatment. At the end of the treatment period the rats were decapitated, and blood samples were
collected into tubes containing 10 mg ml−1 EDTA and 500 KIU ml−1 aprotinin, and were then immediately centrifuged for 10 min (3000 g and 4 °C) to obtain the plasma.
The present study was performed in accordance with the Animal Welfare Act and with the approval of the University of Miyazaki Institutional Animal Care and Use Committee (2008-501-2).
Samples were prepared for RIA as described previously.21, 22 After decapitating the rats, tissues of interest were carefully resected and boiled for 10 min in 10 volumes of distilled H2O.
Acetic acid was then added to the samples to a final concentration of 1.0 mol l−1, and the samples were homogenized using a Polytron mixer and then centrifuged for 20 min (12 000 r.p.m. at 4
°C). Finally, the plasma and tissue samples were separately applied to a Sep-Pak C18 cartridge and eluted with 60% acetonitrile in 0.1% trifluoroacetic acid. The eluted samples were
lyophilized and stored at −20 °C until used for RIA.
To specifically detect proang-12 in tissues and plasma, we developed an RIA using antiserum raised against the C-terminal portion of the peptide. The details of this RIA, including its
cross-reactivity with other angiotensin peptides and comparison with a high-performance liquid chromatographic analysis of immunoreactive proang-12, are provided elsewhere.8 The Ang I
concentrations in tissues and plasma were determined using a specific RIA developed using an antibody raised against the C-terminus of Ang I (Miles). Ang II concentrations were measured
using a RIA developed against Ang II antiserum (Cortex Biochem., San Leandro, USA).8 The Ang I RIA cross-reacted with proang-12 at a level of 1.6%; there was no cross-reaction with Ang II.
The Ang II RIA showed no cross-reactivity with either proang-12 or Ang I. The angiotensinogen concentrations in plasma were determined using an ELISA purchased from Immuno-Biological
Laboratories (Gunma, Japan).21, 22 Plasma renin activity was determined using a GammaCoat Plasma Renin Activity kit from Kyowa Medex (Tokyo, Japan). Estimates of renin activity were based on
the plasma concentrations of generated Ang I following incubation at 37 °C.
Data are presented as means±s.e. Comparisons of data from multiple groups were made using ANOVA followed by the Tukey–Kramer test. Values of P