Play all audios:
TO THE EDITOR: Although recently confirmed in an activation-induced cytidine deaminase (AID) reporter mouse model,1 AID expression and immunoglobulin A (IgA) class switch recombination (CSR)
in the intestinal lamina propria (LP) remain a matter of debate.2 In an article published in the last issue of _Mucosal Immunology_, Barone _et al._3 revisited AID expression in the human
intestine. Barone _et al._ observed AID expression in intestinal follicles but not LP.3 In addition, Barone _et al._3 detected a proliferation-inducing ligand (APRIL) and its receptors TACI
and BCMA in both intestinal follicles and LP.3 As previously published data show that human APRIL elicits AID expression and IgA CSR in the absence of T-cell help to B cells by CD40
ligand,4, 5 Barone _et al._ conclude that APRIL and its receptors are part of a molecular mechanism that promotes IgA CSR in intestinal follicles by both T-cell-dependent (TD) and
T-cell-independent (TI) pathways.3 Given the additional involvement of APRIL in plasma cell survival,6 Barone _et al._ further suggest that APRIL promotes plasma cell survival in the LP.3
Although plausible these conclusions are not demonstrated by the data. Indeed, Barone _et al._3 solely document intestinal expression of APRIL, but provide neither functional nor molecular
evidence of APRIL involvement in follicular IgA CSR or LP plasma cell survival. Furthermore, Barone _et al._3 provide neither functional nor molecular evidence to demonstrate the involvement
of APRIL, TACI, and BCMA in TD or TI routes of B-cell activation within intestinal follicles. We also question the approach used by Barone _et al._3 to study the expression of AID, a
hallmark of ongoing CSR,5 in the human LP. Barone _et al._3 stained intestinal tissues for AID and APRIL through immunohistochemistry (IHC) and compared the results of these stainings with
those obtained by our group through immunofluorescence analysis (IFA).5 This comparison is inaccurate, because IHC and IFA are fundamentally different methodologies that deal with
paraffin-embedded and frozen tissues, respectively. Different tissue processing modalities can affect sensitivity. Indeed, the sensitivity of IHC can be attenuated by antigen retrieval
procedures, which modify the antigenic structure of the protein under study due to its exposure to high temperatures. Limited sensitivity explains the detection by Barone _et al._3 of AID in
follicular B cells, but not in LP B cells or tonsillar subepithelial B cells. A sensitivity issue may also explain the finding of APRIL in crypt but not lumen-facing epithelial cells.3
Although we agree with the former observation,5 the lack of APRIL in lumen-facing epithelial cells differs not only from our results,5 but also from the results of Shang _et al._7 and data
available in the Swedish Human Proteome resource program (http://www.proteinatlas.org/show_image.php?image_id=1635790). Because of these reasons and the glaring lack of supporting functional
data, the lack of APRIL expression by lumen-facing epithelial cells should not be used as an argument to question the role of bacterial Toll-like receptor ligands in APRIL release by
intestinal epithelial cells.5 In this regard, Barone _et al._5 fail to recognize that the flagellin receptor Toll-like receptor-5 promotes APRIL secretion rather than APRIL expression by
epithelial cells.3 Antigen retrieval procedures can affect not only the sensitivity, but also the specificity of IHC. Indeed, Barone _et al._3 detected AID in CD68+ LP macrophages, and
attribute this finding to the poor specificity of an EK2-5G9 antibody to AID (anti-AID) used in some of our studies.5 We reject this claim, because we used IFA instead of IHC to visualize
AID. IFA specifically detected AID in LP-activated B cells, plasmablasts, and plasma cells co-expressing IgA, BSAP (Pax5), IRF4, Blimp-1 and/or CD138,5 but not CD68 (B. He and A. Cerutti,
unpublished data). Importantly, we validated and further extended our IFA-based results by means of fluorescence _in situ_ hybridization (FISH) in both CD40-sufficient and CD40-deficient
individuals,5 whereas Barone _et al._3 did not. Moreover, the specificity of EK2-5G9 for both follicular and extrafollicular B cells, including tonsillar subepithelial B cells, is consistent
not only with results by Cattoretti _et al._,8 but also with additional data from our group generated with anti-AID antibodies different from EK2-5G9 (B. He and A. Cerutti, unpublished
data). Barone _et al._3 back up their claim that there is no AID in the LP by comparing the presence of AID transcripts in microdissected tonsillar germinal centers and LP tissue through
quantitative PCR. The sequence of the primers used to amplify AID mRNA is not disclosed. This issue aside, we contend that in this specific setting quantitative PCR is misleading, because
based on the false assumption that germinal centers and LP have comparable B-cell densities and AID expression levels. Instead, germinal centers have a much higher B-cell density than the
LP, which consists of a mixed population of B cells and non-B cells. Furthermore, the vast majority of germinal center B cells express AID, whereas only a fraction of LP B cells does.5, 9
Moreover, individual germinal center B cells are likely to express more AID than individual LP B cells. Thus, the observation that germinal centers contain more AID RNA than the LP is
completely expected and should not allow one to conclude that the LP lacks AID. Similarly, naive B cells exposed to CD40 ligand and interleukin-4 contain negligible AID RNA compared with
germinal center B cells, because only a fraction of naive B cells induce AID and undergo CSR upon _in vitro_ stimulation (B. He and A. Cerutti, unpublished data). A more appropriate method
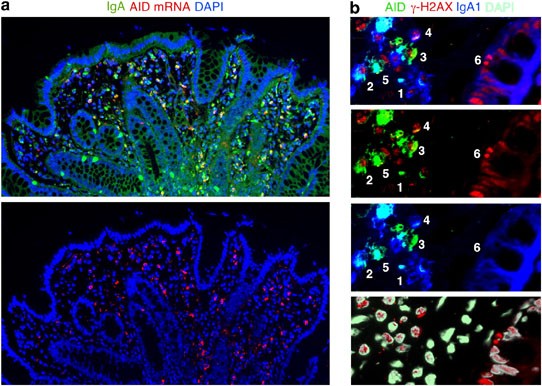
to detect AID in scattered LP B cells is FISH (Figure 1a). Alternatively, one can PCR amplify AID mRNA from purified LP B cells.6 At any rate, even the data provided by Barone _et al._3 seem
to be consistent with the presence of AID in the LP, because CD20+ B-cell-enriched contain more AID mRNA than CD20+ B-cell-poor LP samples.3 This difference would be visually more evident
in a graphic lacking germinal center data. An additional problem relates to the inaccurate discussion of the literature on the inductive function of the LP. For example, Barone _et al._3 do
not discuss recent studies showing LP AID expression and both TD and T1 LP IgA CSR events in an AID-reporter mouse model.1 In addition, Barone _et al._3 do not mention the fact that APRIL
triggers IgA1 CSR in addition to IgA2 CSR,5 which may explain the presence of APRIL not only in IgA2-rich areas such as the colon LP,5 but also in IgA1-rich lymphoid areas such as mucosal
follicles.3, 5, 10 Finally, Barone _et al._3 do not discuss earlier evidence showing that APRIL cooperates with antigen, cytokines, and microbial molecular patterns to induce not only direct
IgM-to-IgA1 CSR, but also sequential IgA1-to-IgA2 CSR and B-cell proliferation.5, 11, 12, 13, 14 This evidence is consistent with the local presence of clonally expanded B cells in the LP15
and with the expression of growth-associated nuclear proteins such as Ki-67 (http://www.proteinatlas.org/show_image.php?image_id=282924) and γ-H2AX (Figure 1b) in some LP B cells. Of note,
the histone γ-H2AX targets AID-induced double-strand switch DNA breaks in B cells transiting through the G1 phase of the cell cycle.16 In summary, we contend that Barone _et al._3 provide
neither functional nor molecular evidence to demonstrate that APRIL signals IgA CSR in B cells from intestinal follicles through TD and TD pathways involving TACI and BCMA. We also question
the conclusion that the human intestinal LP lacks AID, because the experimental approaches used by Barone _et al._3 neither allow an accurate detection of LP AID nor can be compared with
approaches successfully used in earlier works.1, 5, 17, 18 DISCLOSURE The authors declared no conflict of interest. REFERENCES * Crouch, E.E. _et al_. Regulation of AID expression in the
immune response. _J. Exp. Med_. 204, 1145–1156 (2007). Article Google Scholar * Cerutti, A. Location, location, location: B-cell differentiation in the gut lamina propria. _Mucosal.
Immunol_. 1, 8–10 (2008). Article Google Scholar * Barone, F., Patel, P., Sanderson, J. & Spencer, J. Gut-associated lymphoid tissue contains the molecular machinery to support
T-cell-dependent and T-cell-independent class switch recombination. _Mucosal. Immunol_. 2, 495–503 (2009). Article Google Scholar * Litinskiy, M.B. _et al_. DCs induce CD40-independent
immunoglobulin class switching through BLyS and APRIL. _Nat. Immunol_. 3, 822–829 (2002). Article Google Scholar * He, B. _et al_. Intestinal bacteria trigger T cell-independent
immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. _Immunity_ 26, 812–826 (2007). Article Google Scholar * O'Connor, B.P. _et al_. BCMA
is essential for the survival of long-lived bone marrow plasma cells. _J. Exp. Med_. 199, 91–98 (2004). Article Google Scholar * Shang, L. _et al_. Toll-like receptor signaling in small
intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. _Gastroenterology_ 135, 529–538 (2008). Article Google Scholar * Cattoretti, G. _et al_. Nuclear and
cytoplasmic AID in extrafollicular and germinal center B cells. _Blood_ 107, 3967–3975 (2006). Article Google Scholar * Xu, W. _et al_. Epithelial cells trigger frontline immunoglobulin
class switching through a pathway regulated by the inhibitor SLPI. _Nat. Immunol_. 8, 294–303 (2007). Article Google Scholar * Chiu, A. _et al_. Hodgkin lymphoma cells express TACI and
BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. _Blood_ 109, 729–739 (2007). Article Google Scholar * Gupta, M. _et al_. A
proliferation-inducing ligand mediates follicular lymphoma B-cell proliferation and cyclin D1 expression through phosphatidylinositol 3-kinase-regulated mammalian target of rapamycin
activation. _Blood_ 113, 5206–5216 (2009). Article Google Scholar * He, B. _et al_. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. _J. Immunol_. 172,
3268–3279 (2004). Article Google Scholar * Cerutti, A. The regulation of IgA class switching. _Nat. Rev. Immunol_. 8, 421–434 (2008). Article Google Scholar * Ozcan, E. _et al_.
Transmembrane activator, calcium modulator, and cyclophilin ligand interactor drives plasma cell differentiation in LPS-activated B cells. _J. Allergy Clin. Immunol_. 123, 1277–1286.e5
(2009). Article Google Scholar * Yuvaraj, S. _et al_. Evidence for local expansion of IgA plasma cell precursors in human Ileum. _J. Immunol_. PMID 19786537 (2009). * Petersen, S. _et al_.
AID is required to initiate Nbs1/γ-H2AX focus formation and mutations at sites of class switching. _Nature_ 414, 660–665 (2001). Article Google Scholar * Tsuji, M. _et al_. Requirement
for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. _Immunity_ 29, 261–271 (2008). Article Google Scholar *
Fagarasan, S., Kinoshita, K., Muramatsu, M., Ikuta, K. & Honjo, T. _In situ_ class switching and differentiation to IgA-producing cells in the gut lamina propria. _Nature_ 413, 639–643
(2001). Article Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pathology and Laboratory Medicine of Weill Medical College of Cornell
University and Weill Graduate School of Medical Sciences of Cornell University, New York, New York, USA B He, W Xu & A Cerutti Authors * B He View author publications You can also search
for this author inPubMed Google Scholar * W Xu View author publications You can also search for this author inPubMed Google Scholar * A Cerutti View author publications You can also search
for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to A Cerutti. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE He, B., Xu, W. & Cerutti, A. Comment on “Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent
class switch recombination”. _Mucosal Immunol_ 3, 92–94 (2010). https://doi.org/10.1038/mi.2009.125 Download citation * Published: 16 December 2009 * Issue Date: January 2010 * DOI:
https://doi.org/10.1038/mi.2009.125 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative