Play all audios:
ABSTRACT Cilia and flagella are highly conserved organelles that have diverse roles in cell motility and sensing extracellular signals. Motility defects in cilia and flagella often result in
primary ciliary dyskinesia. However, the mechanisms underlying cilia formation and function, and in particular the cytoplasmic assembly of dyneins that power ciliary motility, are only
poorly understood. Here we report a new gene, _kintoun_ (_ktu_), involved in this cytoplasmic process. This gene was first identified in a medaka mutant, and found to be mutated in primary
ciliary dyskinesia patients from two affected families as well as in the _pf13_ mutant of _Chlamydomonas_. In the absence of Ktu/PF13, both outer and inner dynein arms are missing or
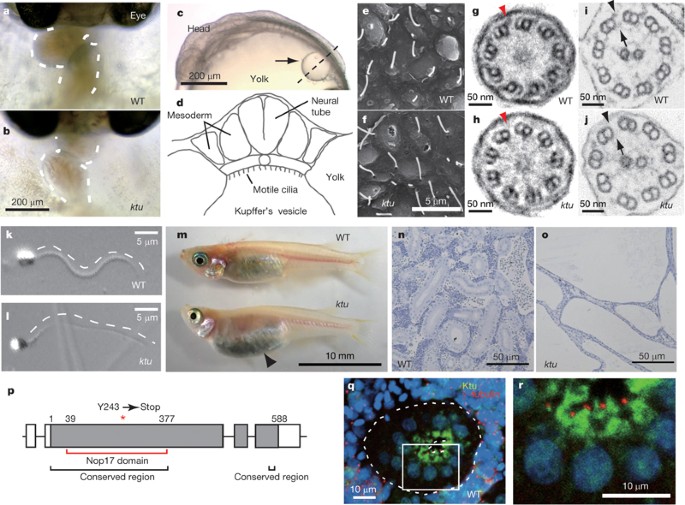
defective in the axoneme, leading to a loss of motility. Biochemical and immunohistochemical studies show that Ktu/PF13 is one of the long-sought proteins involved in pre-assembly of dynein
arm complexes in the cytoplasm before intraflagellar transport loads them for the ciliary compartment. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CORE N-DRC COMPONENTS PLAY A CRUCIAL ROLE
IN EMBRYONIC DEVELOPMENT AND POSTNATAL ORGAN DEVELOPMENT Article Open access 15 March 2025 AXONEMAL STRUCTURES REVEAL MECHANOREGULATORY AND DISEASE MECHANISMS Article Open access 31 May
2023 THE INNER JUNCTION PROTEIN CFAP20 FUNCTIONS IN MOTILE AND NON-MOTILE CILIA AND IS CRITICAL FOR VISION Article Open access 03 November 2022 ACCESSION CODES PRIMARY ACCESSIONS
GENBANK/EMBL/DDBJ * AB455237 * AB455535 * AB455811 * FJ158843 * FJ160770 DATA DEPOSITS The accession numbers are: medaka _ktu_, AB455535; human _KTU_, FJ158843; mouse _ktu_, AB455811;
_Chlamydomonas_ _PF13_ cDNA, AB455237; and _Chlamydomonas_ _PF13_ genome, FJ160770. REFERENCES * Okada, Y. et al. Mechanism of nodal flow: A conserved symmetry breaking event in left-right
axis determination. _Cell_ 121, 633–644 (2005) CAS PubMed Google Scholar * Fliegauf, M., Benzing, T. & Omran, H. When cilia go bad: cilia defects and ciliopathies. _Nature Rev. Mol.
Cell Biol._ 8, 880–893 (2007) CAS Google Scholar * Wilson, P. D. Polycystic kidney disease. _N. Engl. J. Med._ 350, 151–164 (2004) CAS PubMed Google Scholar * Zariwala, M. A., Knowles,
M. R. & Omran, H. Genetic defects in ciliary structure and function. _Annu. Rev. Physiol._ 69, 423–450 (2007) CAS PubMed Google Scholar * Olbrich, H. et al. Mutations in _DNAH5_ cause
primary ciliary dyskinesia and randomization of left-right asymmetry. _Nature Genet._ 30, 143–144 (2002) CAS PubMed Google Scholar * Pennarun, G. et al. Loss-of-function mutations in a
human gene related to _Chlamydomonas reinhardtii_ dynein IC78 result in primary ciliary dyskinesia. _Am. J. Hum. Genet._ 65, 1508–1519 (1999) CAS PubMed PubMed Central Google Scholar *
Bartoloni, L. et al. Mutations in the _DNAH11_ (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. _Proc. Natl
Acad. Sci. USA_ 99, 10282–10286 (2002) ADS CAS PubMed PubMed Central Google Scholar * Budny, B. et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and
ciliary dysfunction is allelic to oral-facial-digital type I syndrome. _Hum. Genet._ 120, 171–178 (2006) CAS PubMed Google Scholar * van Dorp, D. B., Wright, A. F., Carothers, A. D.
& Bleeker-Wagemakers, E. M. A family with RP3 type of X-linked retinitis pigmentosa: an association with ciliary abnormalities. _Hum. Genet._ 88, 331–334 (1992) CAS PubMed Google
Scholar * Duriez, B. et al. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. _Proc. Natl Acad. Sci. USA_
104, 3336–3341 (2007) ADS CAS PubMed PubMed Central Google Scholar * Wittbrodt, J., Shima, A. & Schartl, M. Medaka–a model organism from the far East. _Nature Rev. Genet._ 3, 53–64
(2002) CAS PubMed Google Scholar * Kasahara, M. et al. The medaka draft genome and insights into vertebrate genome evolution. _Nature_ 447, 714–719 (2007) ADS CAS PubMed Google Scholar
* Furutani-Seiki, M. et al. A systematic genome-wide screen for mutations affecting organogenesis in Medaka, Oryzias latipes. _Mech. Dev._ 121, 647–658 (2004) CAS PubMed Google Scholar
* Yokoi, H. et al. Mutant analyses reveal different functions of _fgfr1_ in medaka and zebrafish despite conserved ligand–receptor relationships. _Dev. Biol._ 304, 326–337 (2007) CAS PubMed
Google Scholar * Hojo, M. et al. Right-elevated expression of charon is regulated by fluid flow in medaka Kupffer’s vesicle. _Dev. Growth Differ._ 49, 395–405 (2007) ADS CAS PubMed
Google Scholar * Huang, B., Piperno, G. & Luck, D. J. Paralyzed flagella mutants of _Chlamydomonas reinhardtii_ defective for axonemal doublet microtubule arms. _J. Biol. Chem._ 254,
3091–3099 (1979) CAS PubMed Google Scholar * Fowkes, M. E. & Mitchell, D. R. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. _Mol. Biol. Cell_
9, 2337–2347 (1998) CAS PubMed PubMed Central Google Scholar * Essner, J. J. et al. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right
development of the brain, heart and gut. _Development_ 132, 1247–1260 (2005) CAS PubMed Google Scholar * Kramer-Zucker, A. G. et al. Cilia-driven fluid flow in the zebrafish pronephros,
brain and Kupffer’s vesicle is required for normal organogenesis. _Development_ 132, 1907–1921 (2005) CAS PubMed Google Scholar * Gonzales, F. A., Zanchin, N. I., Luz, J. S. &
Oliveira, C. C. Characterization of _Saccharomyces cerevisiae_ Nop17p, a novel Nop58p-interacting protein that is involved in Pre-rRNA processing. _J. Mol. Biol._ 346, 437–455 (2005) CAS
PubMed Google Scholar * Zhao, R. et al. Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP complex activity that regulates snoRNA accumulation. _J. Cell Biol._ 180, 563–578
(2008) CAS PubMed PubMed Central Google Scholar * Boulon, S. et al. The Hsp90 chaperone controls the biogenesis of L7Ae RNPs through conserved machinery. _J. Cell Biol._ 180, 579–595
(2008) CAS PubMed PubMed Central Google Scholar * Mochizuki, E. et al. Fish mesonephric model of polycystic kidney disease in medaka (_Oryzias latipes_) pc mutant. _Kidney Int._ 68,
23–34 (2005) PubMed Google Scholar * Fliegauf, M. et al. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. _Am. J. Respir. Crit. Care
Med._ 171, 1343–1349 (2005) PubMed PubMed Central Google Scholar * Kamiya, R. Functional diversity of axonemal dyneins as studied in _Chlamydomonas_ mutants. _Int. Rev. Cytol._ 219,
115–155 (2002) CAS PubMed Google Scholar * LeDizet, M. & Piperno, G. The light chain p28 associates with a subset of inner dynein arm heavy chains in _Chlamydomonas_ axonemes. _Mol.
Biol. Cell_ 6, 697–711 (1995) CAS PubMed PubMed Central Google Scholar * Hornef, N. et al. _DNAH5_ mutations are a common cause of primary ciliary dyskinesia with outer dynein arm
defects. _Am. J. Respir. Crit. Care Med._ 174, 120–126 (2006) CAS PubMed PubMed Central Google Scholar * Tam, L. W. & Lefebvre, P. A. Cloning of flagellar genes in _Chlamydomonas
reinhardtii_ by DNA insertional mutagenesis. _Genetics_ 135, 375–384 (1993) CAS PubMed PubMed Central Google Scholar * Yamamoto, R., Yanagisawa, H. A., Yagi, T. & Kamiya, R. A novel
subunit of axonemal dynein conserved among lower and higher eukaryotes. _FEBS Lett._ 580, 6357–6360 (2006) CAS PubMed Google Scholar * Ahmed, N. T. & Mitchell, D. R. ODA16p, a
_Chlamydomonas_ flagellar protein needed for dynein assembly. _Mol. Biol. Cell_ 16, 5004–5012 (2005) CAS PubMed PubMed Central Google Scholar * Merchant, S. S. et al. The _Chlamydomonas_
genome reveals the evolution of key animal and plant functions. _Science_ 318, 245–250 (2007) ADS CAS PubMed PubMed Central Google Scholar * Ahmed, T. N. et al. ODA16 aids axonemal
outer row dynein assembly through an interaction with the intraflagellar transport machinery. _J. Cell Biol._ 183, 313–322 (2008) CAS PubMed PubMed Central Google Scholar * Young, J. C.,
Barral, J. M. & Ulrich Hartl, F. More than folding: localized functions of cytosolic chaperones. _Trends Biochem. Sci._ 28, 541–547 (2003) CAS PubMed Google Scholar * Mitchell, B. F.
et al. ATP production in _Chlamydomonas reinhardtii_ flagella by glycolytic enzymes. _Mol. Biol. Cell_ 16, 4509–4518 (2005) CAS PubMed PubMed Central Google Scholar * Hirokawa, N. &
Takemura, R. Molecular motors and mechanisms of directional transport in neurons. _Nature Rev. Neurosci._ 6, 201–214 (2005) CAS Google Scholar * Rosenbaum, J. L. & Witman, G. B.
Intraflagellar transport. _Nature Rev. Mol. Cell Biol._ 3, 813–825 (2002) CAS Google Scholar * Hagiwara, H., Shibasaki, S. & Ohwada, N. Abnormal cilia in human uterine tube epithelium.
_J. Clin. Electron Microsc._ 23, 493–503 (1990) Google Scholar * Kamiya, R. Mutations at twelve independent loci result in absence of outer dynein arms in _Chylamydomonas reinhardtii_ .
_J. Cell Biol._ 107, 2253–2258 (1988) CAS PubMed Google Scholar * Mitchell, D. R. & Sale, W. S. Characterization of a _Chlamydomonas_ insertional mutant that disrupts flagellar
central pair microtubule-associated structures. _J. Cell Biol._ 144, 293–304 (1999) CAS PubMed PubMed Central Google Scholar * Mastronarde, D. N. et al. Arrangement of inner dynein arms
in wild-type and mutant flagella of _Chlamydomonas_ . _J. Cell Biol._ 118, 1145–1162 (1992) CAS PubMed Google Scholar * Hou, Y. et al. Functional analysis of an individual IFT protein:
IFT46 is required for transport of outer dynein arms into flagella. _J. Cell Biol._ 176, 653–665 (2007) CAS PubMed PubMed Central Google Scholar * Mitchell, D. R. & Rosenbaum, J. L.
Protein–protein interactions in the 18S ATPase of _Chlamydomonas_ outer dynein arms. _Cell Motil. Cytoskeleton_ 6, 510–520 (1986) CAS PubMed Google Scholar * King, S. M., Otter, T. &
Witman, G. B. Characterization of monoclonal antibodies against _Chlamydomonas_ flagellar dyneins by high-resolution protein blotting. _Proc. Natl Acad. Sci. USA_ 82, 4717–4721 (1985) ADS
CAS PubMed PubMed Central Google Scholar * DiBella, L. M. et al. Differential light chain assembly influences outer arm dynein motor function. _Mol. Biol. Cell_ 16, 5661–5674 (2005) CAS
PubMed PubMed Central Google Scholar * Yang, P. & Sale, W. S. The Mr 140,000 intermediate chain of _Chlamydomonas_ flagellar inner arm dynein is a WD-repeat protein implicated in
dynein arm anchoring. _Mol. Biol. Cell_ 9, 3335–3349 (1998) CAS PubMed PubMed Central Google Scholar * Yagi, T. et al. An axonemal dynein particularly important for flagellar movement at
high viscosity. Implications from a new _Chlamydomonas_ mutant deficient in the dynein heavy chain gene DHC9. _J. Biol. Chem._ 280, 41412–41420 (2005) CAS PubMed Google Scholar * Chen,
X., Kindle, K. L. & Stern, D. B. The initiation codon determines the efficiency but not the site of translation initiation in _Chlamydomonas_ chloroplasts. _Plant Cell_ 7, 1295–1305
(1995) CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank C. Lo and D. Morris-Rosendahl for critical reading of this manuscript. We are grateful to M.
Sugimoto, A. Ito-Igarashi, K. Nakaguchi, S. Minami, Y. H. Park, Y. Mochizuki, Y. Ozawa, K. Ohki, T. Obata, A. Heer and C. Reinhardt for excellent fish care and/or experimental assistance.
We also thank A. Shimada and D. Nihei for their help in medaka experiments, J. Freshour and M. Nakatsugawa for help with _Chlamydomonas_, and S. King, H. Qin, W. Sale and D. Stern for
antibodies. Our mutant screening was carried out mainly at the National Institute of Genetics (NIG), supported by NIG Cooperative Research Program (2002–2006). This work was supported in
part by Grants-in-Aid for Scientific Research Priority Area Genome Science and Scientific Research (A and B), Global COE Program (Integrative Life Science Based on the Study of Biosignaling
Mechanisms) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, Yamada Science Foundation, and a Bio-Design Project of the Ministry of Agriculture,
Forestry and Fisheries of Japan. D.K. was a research fellow supported by the 21th century COE program of the University of Tokyo, MEXT, Japan. This work was supported by grants to H.Omran
from the ‘Deutsche Forschungsgemeinschaft’ DFG Om 6/4, GRK1104, BIOSS and the SFB592, and to D.R.M. from the NIH, GM44228. We would like to acknowledge the sequencing activities by K. Borzym
and the Seq-Team at MPI-MG, which was supported by the German Ministry of Science and Education (BMBF) by grant NGFN-2:01GR0414-PDN-S02T17 to R.R. We are grateful for the support by the
‘Primare Ciliaere Dyskinesie and Kartagener Syndrom e.V.’. AUTHOR CONTRIBUTIONS Research planning and supervision was by H.Omran, D.R.M. and H.T.; medaka genetics and phenotypic analyses by
D.K., T.T. and H.T.; biochemical experiments using mouse testis was by T.T., S.K. and Y.W.; high-speed video microscopy of medaka Kupffer’s vesicle cilia was by C.H., H.M., H.K., D.K. and
A.M.; electron microscopy of medaka cilia/flagella was by H.H. and R.K.; experiments on human PCD were by H. Omran, H. Olbrich, N.T.L., M.F., H.Z., H.S. and R.R.; _Chlamydomonas_ experiments
were by D.R.M., Q.Z., G.L., E.O., T.Y. and R.K.; and manuscript writing was by H.Omran, D.R.M. and H.T. AUTHOR INFORMATION Author notes * Daisuke Kobayashi, Qi Zhang & Toshiki Yagi
Present address: Present addresses: Department of Anatomy and Developmental Biology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto 602-8566, Japan
(D.K.); Structural Biology, Graduate School of Science, Kyoto University, Kyoto 606-8502, Japan (T.Y.); Department of Neurobiology, Schering Plough Research Institute, Kenilworth, New Jersey
07033, USA (Q.Z.)., * Heymut Omran, Daisuke Kobayashi, Heike Olbrich and Tatsuya Tsukahara: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of
Pediatrics and Adolescent Medicine, University Hospital Freiburg Mathildenstraße 1, D-79106 Freiburg, Germany Heymut Omran, Heike Olbrich, Niki T. Loges & Manfred Fliegauf * Department
of Biological Sciences, Graduate School of Science, University of Tokyo, Tokyo 113-0033, Japan Daisuke Kobayashi, Toshiki Yagi, Sumito Koshida, Ritsu Kamiya & Hiroyuki Takeda * Institute
of Molecular and Cellular Biosciences, and Graduate Program in Biophysics and Biochemistry, Graduate School of Science, University of Tokyo, Tokyo 113-0032, Japan Tatsuya Tsukahara &
Yoshinori Watanabe * Department of Anatomy and Cell Biology, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan, Haruo Hagiwara * Department of Cell and
Developmental Biology, SUNY Upstate Medical University, Syracuse, New York 13210-1605, USA, Qi Zhang, Gerard Leblond & David R. Mitchell * Department of Molecular, Cellular and
Developmental Biology, University of Colorado, Boulder, Colorado 80309-0347, USA, Eileen O’Toole * Laboratory for Cell Function Dynamics, Advanced Technology Development Group, Brain Science
Institute, RIKEN, Wako, Saitama 351-0198, Japan , Chikako Hara, Hideaki Mizuno, Hiroyuki Kawano & Atsushi Miyawaki * Department of Tumor Virology, German Cancer Research Center, D-69120
Heidelberg, Germany Hanswalter Zentgraf * Klinik für Kinder und Jugendliche, Klinikum Nürnberg Süd, Breslauer Straße 201, 90471 Nürnberg, Germany , Horst Seithe * Max-Planck-Institut für
molekulare Genetik, D-14195 Berlin, Germany Richard Reinhardt Authors * Heymut Omran View author publications You can also search for this author inPubMed Google Scholar * Daisuke Kobayashi
View author publications You can also search for this author inPubMed Google Scholar * Heike Olbrich View author publications You can also search for this author inPubMed Google Scholar *
Tatsuya Tsukahara View author publications You can also search for this author inPubMed Google Scholar * Niki T. Loges View author publications You can also search for this author inPubMed
Google Scholar * Haruo Hagiwara View author publications You can also search for this author inPubMed Google Scholar * Qi Zhang View author publications You can also search for this author
inPubMed Google Scholar * Gerard Leblond View author publications You can also search for this author inPubMed Google Scholar * Eileen O’Toole View author publications You can also search
for this author inPubMed Google Scholar * Chikako Hara View author publications You can also search for this author inPubMed Google Scholar * Hideaki Mizuno View author publications You can
also search for this author inPubMed Google Scholar * Hiroyuki Kawano View author publications You can also search for this author inPubMed Google Scholar * Manfred Fliegauf View author
publications You can also search for this author inPubMed Google Scholar * Toshiki Yagi View author publications You can also search for this author inPubMed Google Scholar * Sumito Koshida
View author publications You can also search for this author inPubMed Google Scholar * Atsushi Miyawaki View author publications You can also search for this author inPubMed Google Scholar *
Hanswalter Zentgraf View author publications You can also search for this author inPubMed Google Scholar * Horst Seithe View author publications You can also search for this author inPubMed
Google Scholar * Richard Reinhardt View author publications You can also search for this author inPubMed Google Scholar * Yoshinori Watanabe View author publications You can also search for
this author inPubMed Google Scholar * Ritsu Kamiya View author publications You can also search for this author inPubMed Google Scholar * David R. Mitchell View author publications You can
also search for this author inPubMed Google Scholar * Hiroyuki Takeda View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS
Correspondence to Heymut Omran, David R. Mitchell or Hiroyuki Takeda. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains Supplementary Tables S1-S5, Supplementary Figures
S1-S7 with legends, and legends for Supplementary movies S1-S10. (PDF 6645 kb) SUPPLEMENTARY MOVIE 1 Movie S1. Dorsal view of cilia in wild-type Kupffer's vesicle. The wild-type motile
cilia rotate on the KV epithelial cells. (MOV 1845 kb) SUPPLEMENTARY MOVIE 2 Movie S2. Dorsal view of cilia in ktu mutant Kupffer's vesicle. The cilia rotation is completely blocked.
(MOV 1539 kb) SUPPLEMENTARY MOVIE 3 Movie S3. Flagellar waveform of wild-type sperm. The wild-type flagellar bending beautifully propagate to the tip of the sperm tail. (MOV 1718 kb)
SUPPLEMENTARY MOVIE 4 Movie S4. Flagellar waveform of ktu mutant sperm. The mutant sperm looks paralyzed and the waveform of flagellar beating is affected. The flagellar bending does not
propagate to the tip of the sperm tail. (MOV 2096 kb) SUPPLEMENTARY MOVIE 5 Movie S5. Motility of cilia in respiratory cells from control patients. (AVI 420 kb) SUPPLEMENTARY MOVIE 6 Movie
S6. Motility of cilia in respiratory cells from PCD patient OP146II1. (AVI 244 kb) SUPPLEMENTARY MOVIE 7 Movie S7. Motility of cilia in respiratory cells from PCD patient OP146II3. (AVI 311
kb) SUPPLEMENTARY MOVIE 8 Movie S8. Motility of cilia in respiratory cells from PCD patient OP234II1. (AVI 417 kb) SUPPLEMENTARY MOVIE 9 Movie S9. Motility of sperm flagella from control
patients. (AVI 951 kb) SUPPLEMENTARY MOVIE 10 Movie S10. Motility of sperm flagella from PCD patient OP146II3. (AVI 542 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR
FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Omran, H., Kobayashi, D., Olbrich, H. _et
al._ Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. _Nature_ 456, 611–616 (2008). https://doi.org/10.1038/nature07471 Download citation * Received: 19 May 2008 *
Accepted: 25 September 2008 * Published: 01 December 2008 * Issue Date: 04 December 2008 * DOI: https://doi.org/10.1038/nature07471 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative