Play all audios:
ABSTRACT Common fragile sites have long been identified by cytogeneticists as chromosomal regions prone to breakage upon replication stress1. They are increasingly recognized to be
preferential targets for oncogene-induced DNA damage in pre-neoplastic lesions2 and hotspots for chromosomal rearrangements in various cancers3. Common fragile site instability was
attributed to the fact that they contain sequences prone to form secondary structures that may impair replication fork movement, possibly leading to fork collapse resulting in DNA breaks4.
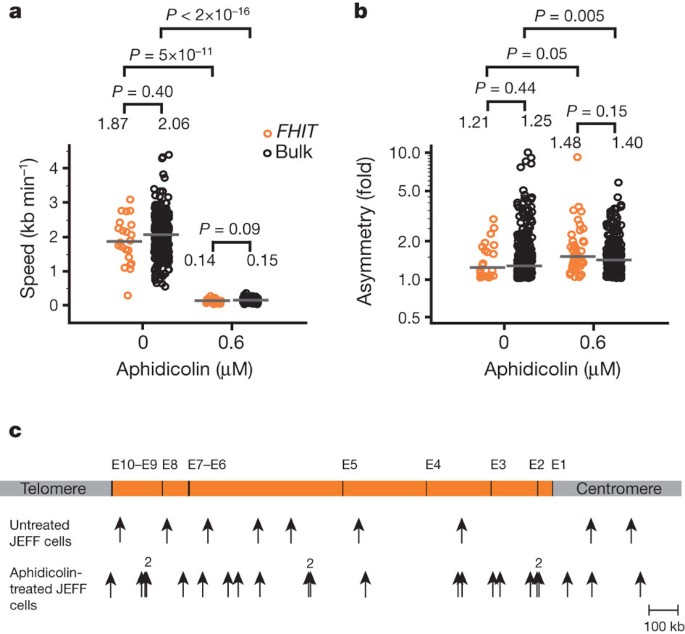
Here we show, in contrast to this view, that the fragility of _FRA3B_—the most active common fragile site in human lymphocytes—does not rely on fork slowing or stalling but on a paucity of
initiation events. Indeed, in lymphoblastoid cells, but not in fibroblasts, initiation events are excluded from a _FRA3B_ core extending approximately 700 kilobases, which forces forks
coming from flanking regions to cover long distances in order to complete replication. We also show that origins of the flanking regions fire in mid-S phase, leaving the site incompletely
replicated upon fork slowing. Notably, _FRA3B_ instability is specific to cells showing this particular initiation pattern. The fact that both origin setting5,6 and replication timing are
highly plastic7,8 in mammalian cells explains the tissue specificity of common fragile site instability we observed. Thus, we propose that common fragile sites correspond to the latest
initiation-poor regions to complete replication in a given cell type. For historical reasons, common fragile sites have been essentially mapped in lymphocytes1. Therefore, common fragile
site contribution to chromosomal rearrangements in tumours should be reassessed after mapping fragile sites in the cell type from which each tumour originates. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print
issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS 3D GENOME ORGANIZATION CONTRIBUTES TO GENOME INSTABILITY AT FRAGILE SITES Article Open access 17 July 2020 MISTIMED ORIGIN LICENSING AND ACTIVATION STABILIZE COMMON
FRAGILE SITES UNDER TIGHT DNA-REPLICATION CHECKPOINT ACTIVATION Article 06 April 2023 REPLICATION STRESS INDUCES POLQ-MEDIATED STRUCTURAL VARIANT FORMATION THROUGHOUT COMMON FRAGILE SITES
AFTER ENTRY INTO MITOSIS Article Open access 06 November 2024 REFERENCES * Sutherland, G. R. & Richards, R. I. The molecular basis of fragile sites in human chromosomes. _Curr. Opin.
Genet. Dev._ 5, 323–327 (1995) Article CAS Google Scholar * Negrini, S., Gorgoulis, V. G. & Halazonetis, T. D. Genomic instability—an evolving hallmark of cancer. _Nature Rev. Mol.
Cell Biol._ 11, 220–228 (2010) Article CAS Google Scholar * Bignell, G. R. et al. Signatures of mutation and selection in the cancer genome. _Nature_ 463, 893–898 (2010) Article ADS CAS
Google Scholar * Schwartz, M., Zlotorynski, E. & Kerem, B. The molecular basis of common and rare fragile sites. _Cancer Lett._ 232, 13–26 (2006) Article CAS Google Scholar *
Grégoire, D., Brodolin, K. & Méchali, M. HoxB domain induction silences DNA replication origins in the locus and specifies a single origin at its boundary. _EMBO Rep._ 7, 812–816 (2006)
PubMed PubMed Central Google Scholar * Dazy, S., Gandrillon, O., Hyrien, O. & Prioleau, M. N. Broadening of DNA replication origin usage during metazoan cell differentiation. _EMBO
Rep._ 7, 806–811 (2006) CAS PubMed PubMed Central Google Scholar * Hansen, R. S. et al. Sequencing newly replicated DNA reveals widespread plasticity in human replication timing. _Proc.
Natl Acad. Sci. USA_ 107, 139–144 (2010) Article ADS CAS Google Scholar * Hiratani, I. et al. Global reorganization of replication domains during embryonic stem cell differentiation.
_PLoS Biol._ 6, e245 (2008) Article Google Scholar * Tourriere, H. & Pasero, P. Maintenance of fork integrity at damaged DNA and natural pause sites. _DNA Repair_ 6, 900–913 (2007)
Article CAS Google Scholar * Branzei, D. & Foiani, M. Maintaining genome stability at the replication fork. _Nature Rev. Mol. Cell Biol._ 11, 208–219 (2010) Article CAS Google
Scholar * Durkin, S. G. & Glover, T. W. Chromosome fragile sites. _Annu. Rev. Genet._ 41, 169–192 (2007) Article CAS Google Scholar * Helmrich, A., Stout-Weider, K., Hermann, K.,
Schrock, E. & Heiden, T. Common fragile sites are conserved features of human and mouse chromosomes and relate to large active genes. _Genome Res._ 16, 1222–1230 (2006) Article CAS
Google Scholar * Tsantoulis, P. K. et al. Oncogene-induced replication stress preferentially targets common fragile sites in preneoplastic lesions. A genome-wide study. _Oncogene_ 27,
3256–3264 (2008) Article CAS Google Scholar * Palumbo, E., Matricardi, L., Tosoni, E., Bensimon, A. & Russo, A. Replication dynamics at common fragile site _FRA6E_ . _Chromosoma_
(2010) * Huebner, K. & Croce, C. M. Cancer and the _FRA3B_/_FHIT_ fragile locus: it’s a HIT. _Br. J. Cancer_ 88, 1501–1506 (2003) Article CAS Google Scholar * Pichiorri, F. et al.
Molecular parameters of genome instability: roles of fragile genes at common fragile sites. _J. Cell. Biochem._ 104, 1525–1533 (2008) Article CAS Google Scholar * Lebofsky, R., Heilig,
R., Sonnleitner, M., Weissenbach, J. & Bensimon, A. DNA replication origin interference increases the spacing between initiation events in human cells. _Mol. Biol. Cell_ 17, 5337–5345
(2006) Article CAS Google Scholar * Cha, R. S. & Kleckner, N. ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. _Science_ 297, 602–606 (2002)
Article ADS CAS Google Scholar * Rothstein, R., Michel, B. & Gangloff, S. Replication fork pausing and recombination or “gimme a break”. _Genes Dev._ 14, 1–10 (2000) CAS PubMed
Google Scholar * Farkash-Amar, S. et al. Global organization of replication time zones of the mouse genome. _Genome Res._ 18, 1562–1570 (2008) Article CAS Google Scholar * Anglana, M.,
Apiou, F., Bensimon, A. & Debatisse, M. Dynamics of DNA replication in mammalian somatic cells: nucleotide pool modulates origin choice and interorigin spacing. _Cell_ 114, 385–394
(2003) Article CAS Google Scholar * Courbet, S. et al. Replication fork movement sets chromatin loop size and origin choice in mammalian cells. _Nature_ 455, 557–560 (2008) Article ADS
CAS Google Scholar * Bielinsky, A. K. Replication origins: why do we need so many? _Cell Cycle_ 2, 307–309 (2003) Article CAS Google Scholar * El Achkar, E., Gerbault-Seureau, M.,
Muleris, M., Dutrillaux, B. & Debatisse, M. Premature condensation induces breaks at the interface of early and late replicating chromosome bands bearing common fragile sites. _Proc.
Natl Acad. Sci. USA_ 102, 18069–18074 (2005) Article ADS CAS Google Scholar * Le Beau, M. M. et al. Replication of a common fragile site, FRA3B, occurs late in S phase and is delayed
further upon induction: implications for the mechanism of fragile site induction. _Hum. Mol. Genet._ 7, 755–761 (1998) Article CAS Google Scholar * Durkin, S. G. et al. Replication stress
induces tumor-like microdeletions in _FHIT_/FRA3B. _Proc. Natl Acad. Sci. USA_ 105, 246–251 (2008) Article ADS CAS Google Scholar * Ryba, T. et al. Evolutionarily conserved replication
timing profiles predict long-range chromatin interactions and distinguish closely related cell types. _Genome Res._ 20, 761–770 (2010) Article CAS Google Scholar * O’Keefe, L. V. &
Richards, R. I. Common chromosomal fragile sites and cancer: focus on FRA16D. _Cancer Lett._ 232, 37–47 (2006) Article Google Scholar * Masai, H., Matsumoto, S., You, Z., Yoshizawa-Sugata,
N. & Oda, M. Eukaryotic chromosome DNA replication: where, when, and how? _Annu. Rev. Biochem._ 79, 89–130 (2010) Article CAS Google Scholar * Michalet, X. et al. Dynamic molecular
combing: stretching the whole human genome for high-resolution studies. _Science_ 277, 1518–1523 (1997) Article CAS Google Scholar * Labit, H. et al. A simple and optimized method of
producing silanized surfaces for FISH and replication mapping on combed DNA fibers. _Biotechniques_ 45, 649–658 (2008) Article CAS Google Scholar * R Development Core Team. _R: A Language
and Environment for Statistical Computing_ (R Foundation for Statistical Computing, 2006) Download references ACKNOWLEDGEMENTS We thank E. Blackburn for critical reading of the manuscript.
We thank Genomic Vision for making the DNA combing technology available to us. We acknowledge the Nikon Imaging Centre at Institut Curie-CNRS. We thank C. Rouzaud for help in combing
experiments. A.L. is supported by a grant from the ARC (Association pour la recherche sur le cancer). The M.D. team is supported by La Ligue Nationale contre le Cancer (LNCC) (Equipe
Labellisée EL2008.LNCC/MD), INCa (Institut National du Cancer) (2009-1-PLBIO-10-IC-1) and the PIC Réplication, Instabilité Chromosomique et Cancer (Institut Curie). AUTHOR INFORMATION Author
notes * Stéphane Koundrioukoff, Anne-Marie Lachagès and Nicolas Vogt: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Institut Curie, Centre de Recherche, 26 rue
d’Ulm, 75248 Paris, France , Anne Letessier, Gaël A. Millot, Stéphane Koundrioukoff, Anne-Marie Lachagès, Nicolas Vogt, Bernard Malfoy, Olivier Brison & Michelle Debatisse * UPMC Univ.
Paris 06, F-75005 Paris, France , Anne Letessier, Gaël A. Millot, Stéphane Koundrioukoff, Anne-Marie Lachagès, Nicolas Vogt, Bernard Malfoy, Olivier Brison & Michelle Debatisse * CNRS
UMR 3244, F-75248 Paris, France , Anne Letessier, Gaël A. Millot, Stéphane Koundrioukoff, Anne-Marie Lachagès, Nicolas Vogt, Bernard Malfoy, Olivier Brison & Michelle Debatisse *
Department of Medicine, Division of Medical Genetics, University of Washington School of Medicine, Seattle, 98195, Washington, USA R. Scott Hansen Authors * Anne Letessier View author
publications You can also search for this author inPubMed Google Scholar * Gaël A. Millot View author publications You can also search for this author inPubMed Google Scholar * Stéphane
Koundrioukoff View author publications You can also search for this author inPubMed Google Scholar * Anne-Marie Lachagès View author publications You can also search for this author inPubMed
Google Scholar * Nicolas Vogt View author publications You can also search for this author inPubMed Google Scholar * R. Scott Hansen View author publications You can also search for this
author inPubMed Google Scholar * Bernard Malfoy View author publications You can also search for this author inPubMed Google Scholar * Olivier Brison View author publications You can also
search for this author inPubMed Google Scholar * Michelle Debatisse View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.L. performed and
analysed combing experiments. G.A.M. performed statistical and Repli-Seq analyses. S.K., A.-M.L. and O.B. performed cytogenetic analyses. N.V. and B.M. designed the Morse code. R.S.H.
contributed to Repli-Seq analysis. A.L., G.A.M., O.B. and M.D. wrote the paper. M.D. planned the project. CORRESPONDING AUTHOR Correspondence to Michelle Debatisse. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION The file contains Supplementary Figures 1-9 with legends and
Supplementary Tables 1-4. (PDF 2012 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Letessier, A., Millot, G., Koundrioukoff, S. _et al._ Cell-type-specific replication initiation programs set fragility of the _FRA3B_ fragile site.
_Nature_ 470, 120–123 (2011). https://doi.org/10.1038/nature09745 Download citation * Received: 24 September 2010 * Accepted: 08 December 2010 * Published: 23 January 2011 * Issue Date: 03
February 2011 * DOI: https://doi.org/10.1038/nature09745 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative