Play all audios:
ABSTRACT All attempts at treating strokes by pharmacologically reducing the human brain’s vulnerability to ischaemia have failed, leaving stroke as a leading cause of death, disability and
massive socioeconomic loss worldwide1. Over decades, research has failed to translate over 1,000 experimental treatments from discovery in cells and rodents to use in humans2,3,4, a
scientific crisis that gave rise to the prevailing belief that pharmacological neuroprotection is not feasible or practicable in higher-order brains. To provide a strategy for advancing
stroke therapy, we used higher-order gyrencephalic non-human primates, which bear genetic, anatomical and behavioural similarities to humans5,6 and tested neuroprotection by PSD-95
inhibitors—promising compounds that uncouple postsynaptic density protein PSD-95 from neurotoxic signalling pathways7,8,9,10. Here we show that stroke damage can be prevented in non-human
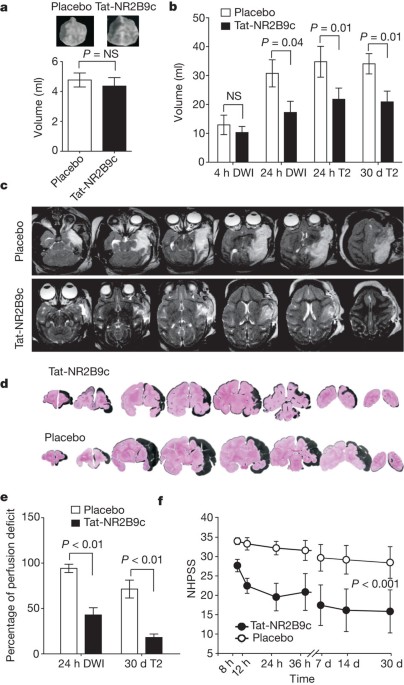
primates in which a PSD-95 inhibitor is administered after stroke onset in clinically relevant situations. This treatment reduced infarct volumes as gauged by magnetic resonance imaging and
histology, preserved the capacity of ischaemic cells to maintain gene transcription in genome-wide screens of ischaemic brain tissue, and significantly preserved neurological function in
neurobehavioural assays. The degree of tissue neuroprotection by magnetic resonance imaging corresponded strongly to the preservation of neurological function, supporting the intuitive but
unproven dictum that integrity of brain tissue can reflect functional outcome. Our findings establish that tissue neuroprotection and improved functional outcome after stroke is
unequivocally achievable in gyrencephalic non-human primates treated with PSD-95 inhibitors. Efforts must ensue to translate these findings to humans. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online
access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
A CLINICALLY RELEVANT MODEL OF FOCAL EMBOLIC CEREBRAL ISCHEMIA BY THROMBUS AND THROMBOLYSIS IN RHESUS MONKEYS Article 27 June 2022 THE NEUROPROTECTIVE MECHANISM OF LITHIUM AFTER ISCHAEMIC
STROKE Article Open access 03 February 2022 ABERRANT NEURONAL EXCITATION PROMOTES NEUROINFLAMMATION IN THE PRIMARY MOTOR CORTEX OF ISCHEMIC STROKE MICE Article 12 March 2025 ACCESSION CODES
PRIMARY ACCESSIONS GENE EXPRESSION OMNIBUS * GSE35589 DATA DEPOSITS Microarray data are deposited in National Center for Biotechnology Information (NCBI) Gene Expression Omnibus under
accession number GSE35589. REFERENCES * Roger, V. L. et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. _Circulation_ 123, e18–e209 (2011)
Article Google Scholar * Anon The bitterest pill. _Nature_ 444, 532–533 (2006) Article Google Scholar * O’Collins, V. E. et al. 1,026 experimental treatments in acute stroke. _Ann.
Neurol._ 59, 467–477 (2006) Article Google Scholar * Sacchetti, M. L. Is it time to definitely abandon neuroprotection in acute ischemic stroke? _Stroke_ 39, 1659–1660 (2008) Article
Google Scholar * Courtine, G. et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? _Nature Med._ 13, 561–566 (2007) Article
CAS Google Scholar * Enard, D., Depaulis, F. & Roest Crollius, H. Human and non-human primate genomes share hotspots of positive selection. _PLoS Genet._ 6, e1000840 (2010) Article
Google Scholar * Aarts, M. et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. _Science_ 298, 846–850 (2002) Article ADS CAS Google Scholar
* Sattler, R. et al. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. _Science_ 284, 1845–1848 (1999) Article CAS Google Scholar * Soriano,
F. X. et al. Specific targeting of pro-death NMDA receptor signals with differing reliance on the NR2B PDZ ligand. _J. Neurosci._ 28, 10696–10710 (2008) Article CAS Google Scholar * Sun,
H. S. et al. Effectiveness of PSD-95 inhibitors in permanent and transient focal ischemia in the rat. _Stroke_ 39, 2544–2553 (2008) Article CAS Google Scholar * Roitberg, B. et al.
Chronic ischemic stroke model in cynomolgus monkeys: behavioral, neuroimaging and anatomical study. _Neurol. Res._ 25, 68–78 (2003) Article Google Scholar * Fisher, M. The ischemic
penumbra: identification, evolution and treatment concepts. _Cerebrovasc. Dis._ 17 (suppl. 1). 1–6 (2004) Article Google Scholar * Bardutzky, J. et al. Characterizing tissue fate after
transient cerebral ischemia of varying duration using quantitative diffusion and perfusion imaging. _Stroke_ 38, 1336–1344 (2007) Article Google Scholar * Fisher, M. et al. Update of the
stroke therapy academic industry roundtable preclinical recommendations. _Stroke_ 40, 2244–2250 (2009) Article Google Scholar * Committee, S. Recommendations for standards regarding
preclinical neuroprotective and restorative drug development. _Stroke_ 30, 2752–2758 (1999) Article Google Scholar * Kornau, H. C., Schenker, L. T., Kennedy, M. B. & Seeburg, P. H.
Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. _Science_ 269, 1737–1740 (1995) Article ADS CAS Google Scholar * Cui, H. et al. PDZ protein
interactions underlying NMDA-receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. _J. Neurosci._ 27, 9901–9915 (2007) Article CAS Google Scholar * Bratane, B. T. et
al. Neuroprotection by freezing ischemic penumbra evolution without cerebral blood flow augmentation with a postsynaptic density-95 protein inhibitor. _Stroke_ 42, 3265–3270 (2011) Article
CAS Google Scholar * Marshall, J. W. & Ridley, R. M. Assessment of cognitive and motor deficits in a marmoset model of stroke. _ILAR J._ 44, 153–160 (2003) Article CAS Google
Scholar * Reagan-Shaw, S., Nihal, M. & Ahmad, N. Dose translation from animal to human studies revisited. _FASEB J._ 22, 659–661 (2008) Article CAS Google Scholar * Brott, T. et al.
Measurements of acute cerebral infarction: a clinical examination scale. _Stroke_ 20, 864–870 (1989) Article CAS Google Scholar * Johnston, K. C. et al. Validation of an acute ischemic
stroke model: does diffusion-weighted imaging lesion volume offer a clinically significant improvement in prediction of outcome? _Stroke_ 38, 1820–1825 (2007) Article Google Scholar *
Hand, P. J. et al. MR diffusion-weighted imaging and outcome prediction after ischemic stroke. _Neurology_ 66, 1159–1163 (2006) Article CAS Google Scholar * Yanagihara, T. Experimental
stroke in gerbils: effect on translation and transcription. _Brain Res._ 158, 435–444 (1978) Article CAS Google Scholar * Hacke, W. et al. Association of outcome with early stroke
treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. _Lancet_ 363, 768–774 (2004) Article Google Scholar * Hacke, W. et al. Thrombolysis with alteplase 3 to 4.5
hours after acute ischemic stroke. _N. Engl. J. Med._ 359, 1317–1329 (2008) Article CAS Google Scholar * Sanossian, N. et al. Simultaneous ring voice-over-Internet phone system enables
rapid physician elicitation of explicit informed consent in prehospital stroke treatment trials. _Cerebrovasc. Dis._ 28, 539–544 (2009) Article Google Scholar * The National Institute of
Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke
Study Group. _N. Engl. J. Med._ 333, 1581–1587 (1995) Article Google Scholar * Savitz, S. I. & Fisher, M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT
trials. _Ann. Neurol._ 61, 396–402 (2007) Article CAS Google Scholar * Savitz, S. I. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous
testing of neuroprotective agents in animal models of stroke. _Exp. Neurol._ 205, 20–25 (2007) Article CAS Google Scholar * Findlay, J. M., Macdonald, R. L., Weir, B. K. & Grace, M.
G. Surgical manipulation of primate cerebral arteries in established vasospasm. _J. Neurosurg._ 75, 425–432 (1991) Article CAS Google Scholar * Kosior, J. C. & Frayne, R. PerfTool: a
software platform for investigating bolus-tracking perfusion imaging quantification strategies. _J. Magn. Reson. Imaging_ 25, 653–659 (2007) Article Google Scholar * Stewart, C. B. &
Disotell, T. R. Primate evolution – in and out of Africa. _Curr. Biol._ 8, R582–R588 (1998) Article CAS Google Scholar * Kobasa, D. et al. Aberrant innate immune response in lethal
infection of macaques with the 1918 influenza virus. _Nature_ 445, 319–323 (2007) Article ADS CAS Google Scholar * Gibbs, R. A. et al. Evolutionary and biomedical insights from the
rhesus macaque genome. _Science_ 316, 222–234 (2007) Article CAS Google Scholar * Draghici, S. et al. A systems biology approach for pathway level analysis. _Genome Res._ 17, 1537–1545
(2007) Article CAS Google Scholar * Marshall, J. W. & Ridley, R. M. Assessment of functional impairment following permanent middle cerebral artery occlusion in a non-human primate
species. _Neurodegeneration_ 5, 275–286 (1996) Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by grants to M.T. from the Canadian Stroke Network
and the Heart and Stroke Foundation of Ontario, grant NA 6988. D.J.C. is a recipient of a Canadian Stroke Network postdoctoral research fellowship. M.T. is a Canada Research Chair (Tier 1)
in Translational Stroke Research. We thank M. Madden, B. Maloo, A. Goldstein, B. Madeira, W. Foltz and Z. Lu for assistance. We thank M. Salter and M. Hill for a review of the manuscript.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Toronto Western Hospital Research Institute, Toronto, Ontario, 2S8, M5T, Canada Douglas J. Cook, Lucy Teves & Michael Tymianski * Department
of Physiology, University of Toronto, Toronto, Ontario, M5S 1A8, Canada, Michael Tymianski * Institute of Medical Science, University of Toronto, Toronto, Ontario, M5S 1A8, Canada, Michael
Tymianski * Department of Surgery, University of Toronto, Toronto, Ontario, M5S 1A8, Canada, Michael Tymianski Authors * Douglas J. Cook View author publications You can also search for this
author inPubMed Google Scholar * Lucy Teves View author publications You can also search for this author inPubMed Google Scholar * Michael Tymianski View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS D.C. and M.T. performed the experimental procedures, collected and analysed the data and drafted the manuscript. L.T. performed
experimental procedures and data collection. CORRESPONDING AUTHOR Correspondence to Michael Tymianski. ETHICS DECLARATIONS COMPETING INTERESTS M.T. is president of NoNO Inc., a biotechnology
company founded to develop PSD-95 inhibitors discovered in his research laboratory. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains Supplementary Figures 1-6 with
legends, Supplementary Methods, a Supplementary Discussion, Supplementary Tables 1-4 and Supplementary References. (PDF 5966 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT
SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Cook, D., Teves, L. &
Tymianski, M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. _Nature_ 483, 213–217 (2012). https://doi.org/10.1038/nature10841 Download citation * Received:
21 November 2011 * Accepted: 11 January 2012 * Published: 29 February 2012 * Issue Date: 08 March 2012 * DOI: https://doi.org/10.1038/nature10841 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative