Play all audios:
ABSTRACT Integrins have a critical role in thrombosis and haemostasis1. Antagonists of the platelet integrin αIIbβ3 are potent anti-thrombotic drugs, but also have the life-threatening
adverse effect of causing bleeding2,3. It is therefore desirable to develop new antagonists that do not cause bleeding. Integrins transmit signals bidirectionally4,5. Inside-out signalling
activates integrins through a talin-dependent mechanism6,7. Integrin ligation mediates thrombus formation and outside-in signalling8,9, which requires Gα13 and greatly expands thrombi. Here
we show that Gα13 and talin bind to mutually exclusive but distinct sites within the integrin β3 cytoplasmic domain in opposing waves. The first talin-binding wave mediates inside-out
signalling and also ligand-induced integrin activation, but is not required for outside-in signalling. Integrin ligation induces transient talin dissociation and Gα13 binding to an EXE motif
(in which X denotes any residue), which selectively mediates outside-in signalling and platelet spreading. The second talin-binding wave is associated with clot retraction. An
EXE-motif-based inhibitor of Gα13–integrin interaction selectively abolishes outside-in signalling without affecting integrin ligation, and suppresses occlusive arterial thrombosis without
affecting bleeding time. Thus, we have discovered a new mechanism for the directional switch of integrin signalling and, on the basis of this mechanism, designed a potent new anti-thrombotic
drug that does not cause bleeding. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read
our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS INTEGRIN Β3 DIRECTLY INHIBITS THE GΑ13-P115RHOGEF INTERACTION TO REGULATE G PROTEIN SIGNALING AND PLATELET
EXOCYTOSIS Article Open access 16 August 2023 TARGETING INTEGRIN PATHWAYS: MECHANISMS AND ADVANCES IN THERAPY Article Open access 02 January 2023 PLATELET INTEGRIN ΑIIBΒ3 PLAYS A KEY ROLE IN
A VENOUS THROMBOGENESIS MOUSE MODEL Article Open access 04 October 2024 REFERENCES * Shattil, S. J. & Newman, P. J. Integrins: dynamic scaffolds for adhesion and signaling in platelets.
_Blood_ 104, 1606–1615 (2004) Article CAS Google Scholar * Coller, B. S. Anti-GPIIb/IIIa drugs: current strategies and future directions. _Thromb. Haemost._ 86, 427–443 (2001) Article
CAS Google Scholar * Serebruany, V. L., Malinin, A. I., Eisert, R. M. & Sane, D. C. Risk of bleeding complications with antiplatelet agents: meta-analysis of 338,191 patients enrolled
in 50 randomized controlled trials. _Am. J. Hematol._ 75, 40–47 (2004) Article CAS Google Scholar * Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. _Cell_ 110,
673–687 (2002) Article CAS Google Scholar * Moissoglu, K. & Schwartz, M. A. Integrin signalling in directed cell migration. _Biol. Cell_ 98, 547–555 (2006) Article CAS Google
Scholar * Tadokoro, S. et al. Talin binding to integrin beta tails: a final common step in integrin activation. _Science_ 302, 103–106 (2003) Article ADS CAS Google Scholar * Ye, F.,
Kim, C. & Ginsberg, M. H. Molecular mechanism of inside-out integrin regulation. _J. Thromb. Haemost._ 9 (Suppl. 1). 20–25 (2011) Article CAS Google Scholar * Gong, H. et al. G
protein subunit Gα13 binds to integrin αIIbβ3 and mediates integrin “outside-in” signaling. _Science_ 327, 340–343 (2010) Article ADS CAS Google Scholar * Shen, B., Delaney, M. K. &
Du, X. Inside-out, outside-in, and inside-outside-in: G protein signaling in integrin-mediated cell adhesion, spreading, and retraction. _Curr. Opin. Cell Biol._ 24, 600–606 (2012) Article
CAS Google Scholar * Moser, M., Nieswandt, B., Ussar, S., Pozgajova, M. & Fassler, R. Kindlin-3 is essential for integrin activation and platelet aggregation. _Nature Med._ 14, 325–330
(2008) Article CAS Google Scholar * Ma, Y. Q., Qin, J., Wu, C. & Plow, E. F. Kindlin-2 (Mig-2): a co-activator of β3 integrins. _J. Cell Biol._ 181, 439–446 (2008) Article CAS
Google Scholar * Obergfell, A. et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. _J. Cell Biol._ 157, 265–275 (2002)
Article CAS Google Scholar * Flevaris, P. et al. A molecular switch that controls cell spreading and retraction. _J. Cell Biol._ 179, 553–565 (2007) Article CAS Google Scholar * Patil,
S. et al. Identification of a talin-binding site in the integrin β3 subunit distinct from the NPLY regulatory motif of post-ligand binding functions. The talin N-terminal head domain
interacts with the membrane-proximal region of the β3 cytoplasmic tail. _J. Biol. Chem._ 274, 28575–28583 (1999) Article CAS Google Scholar * Wegener, K. L. et al. Structural basis of
integrin activation by talin. _Cell_ 128, 171–182 (2007) Article CAS Google Scholar * Haling, J. R., Monkley, S. J., Critchley, D. R. & Petrich, B. G. Talin-dependent integrin
activation is required for fibrin clot retraction by platelets. _Blood_ 117, 1719–1722 (2011) Article CAS Google Scholar * Petrich, B. G. et al. Talin is required for integrin-mediated
platelet function in hemostasis and thrombosis. _J. Exp. Med._ 204, 3103–3111 (2007) Article CAS Google Scholar * Coller, B. S. Interaction of normal, thrombasthenic, and Bernard-Soulier
platelets with immobilized fibrinogen: defective platelet-fibrinogen interaction in thrombasthenia. _Blood_ 55, 169–178 (1980) CAS PubMed Google Scholar * Ugarova, T. P. et al.
Conformational changes in fibrinogen elicited by its interaction with platelet membrane glycoprotein GPIIb-IIIa. _J. Biol. Chem._ 268, 21080–21087 (1993) CAS PubMed Google Scholar * Du,
X. et al. Ligands “activate” integrin _α_IIb_β_3 (platelet GPIIb-IIIa). _Cell_ 65, 409–416 (1991) Article CAS Google Scholar * Arias-Salgado, E. G., Lizano, S., Shattil, S. J. &
Ginsberg, M. H. Specification of the direction of adhesive signaling by the integrin β cytoplasmic domain. _J. Biol. Chem._ 280, 29699–29707 (2005) Article CAS Google Scholar * Goksoy, E.
et al. Structural basis for the autoinhibition of talin in regulating integrin activation. _Mol. Cell_ 31, 124–133 (2008) Article CAS Google Scholar * Xi, X., Bodnar, R. J., Li, Z. Y.,
Lam, S. C. T. & Du, X. P. Critical roles for the COOH-terminal NITY and RGT sequences of the integrin β3 cytoplasmic domain in inside-out and outside-in signaling. _J. Cell Biol._ 162,
329–339 (2003) Article CAS Google Scholar * Krishnadas, A., Rubinstein, I. & Onyuksel, H. Sterically stabilized phospholipid mixed micelles: _in vitro_ evaluation as a novel carrier
for water-insoluble drugs. _Pharm. Res._ 20, 297–302 (2003) Article CAS Google Scholar * O’Brien, K. A., Gartner, T. K., Hay, N. & Du, X. ADP-stimulated activation of Akt during
integrin outside-in signaling promotes platelet spreading by inhibiting glycogen synthase kinase-3β. _Arterioscler. Thromb. Vasc. Biol._ 32, 2232–2240 (2012) Article Google Scholar *
Delaney, M. K., Liu, J., Zheng, Y., Berndt, M. C. & Du, X. The role of Rac1 in glycoprotein Ib-IX-mediated signal transduction and integrin activation. _Arterioscler. Thromb. Vasc.
Biol._ 32, 2761–2768 (2012) Article CAS Google Scholar * Cho, J. et al. Protein disulfide isomerase capture during thrombus formation _in vivo_ depends on the presence of β3 integrins.
_Blood_ 120, 647–655 (2012) Article CAS Google Scholar * O’Brien, K. A., Stojanovic-Terpo, A., Hay, N. & Du, X. An important role for Akt3 in platelet activation and thrombosis.
_Blood_ 118, 4215–4223 (2011) Article Google Scholar * Marjanovic, J. A., Li, Z., Stojanovic, A. & Du, X. Stimulatory roles of nitric-oxide synthase 3 and guanylyl cyclase in platelet
activation. _J. Biol. Chem._ 280, 37430–37438 (2005) Article CAS Google Scholar * Nimura, N., Kinoshita, T., Yoshida, T., Uetake, A. & Nakai, C. 1-Pyrenyldiazomethane as a fluorescent
labeling reagent for liquid chromatographic determination of carboxylic acids. _Anal. Chem._ 60, 2067–2070 (1988) Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank
T. Kozasa, B. Kreutz and C. Chow for providing purified recombinant Gα13 protein; and B. Petrich and D. Critchley for providing talin−/− mice. We acknowledge that H. Gong performed
experiments for this project. This work is supported by grants from National Heart, Lung, and Blood Institute (HL080264, HL062350 (X.D.) and HL109439 (J.C.)). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Pharmacology, University of Illinois at Chicago, 835 South Wolcott Avenue, Chicago, Illinois 60612, USA, Bo Shen, Xiaojuan Zhao, Kelly A. O’Brien, Aleksandra
Stojanovic-Terpo, M. Keegan Delaney, Kyungho Kim, Jaehyung Cho, Stephen C.-T. Lam & Xiaoping Du Authors * Bo Shen View author publications You can also search for this author inPubMed
Google Scholar * Xiaojuan Zhao View author publications You can also search for this author inPubMed Google Scholar * Kelly A. O’Brien View author publications You can also search for this
author inPubMed Google Scholar * Aleksandra Stojanovic-Terpo View author publications You can also search for this author inPubMed Google Scholar * M. Keegan Delaney View author publications
You can also search for this author inPubMed Google Scholar * Kyungho Kim View author publications You can also search for this author inPubMed Google Scholar * Jaehyung Cho View author
publications You can also search for this author inPubMed Google Scholar * Stephen C.-T. Lam View author publications You can also search for this author inPubMed Google Scholar * Xiaoping
Du View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.S. performed most of the experiments and participated in experimental design, data
analysis and manuscript writing. X.Z., K.A.O., A.S.-T. and M.K.D. each performed parts of the experiments and participated in aspects of data analyses and manuscript writing. K.K. and J.C.
performed laser-induced thrombosis experiments and data analysis; S.C.-T.L. provided talin constructs and purified proteins, and participated in discussions and data analyses; X.D. designed
and directed the research, analysed data and wrote the paper. CORRESPONDING AUTHOR Correspondence to Xiaoping Du. ETHICS DECLARATIONS COMPETING INTERESTS X.D. holds pending patents on the
EXE-motif-containing peptide inhibitors. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 A SCHEMATIC SHOWING HOW SELECTIVE INHIBITORS OF INTEGRIN OUTSIDE-IN SIGNALLING WORK AS
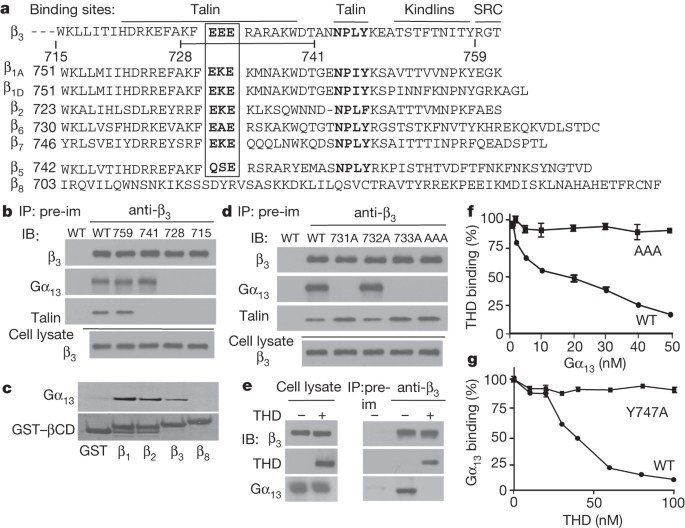
ANTI-THROMBOTICS. Blue arrows indicate steps that are inhibited. EXTENDED DATA FIGURE 2 THE IMPORTANCE OF THE CONSERVED EXE MOTIF IN INTEGRIN–GΑ13 INTERACTIONS. A, B, Lysates from CHO cells
expressing similar levels of wild-type (WT) αIIbβ3, β3 C-terminal truncation mutants (Δ759, Δ741, Δ728 and Δ715) complexed with wild-type αIIb (A), and β3 EXE motif mutants (E731A, E732A,
E733A and EEE to AAA) complexed with wild-type αIIb (B) were immunoprecipitated with anti-Gα13 antibody or equal amount of control rabbit IgG. Immunoprecipitates and lysates (equivalent of
10% used for immunoprecipitation) were immunoblotted with anti-Gα13 and anti-β3 antibodies. C, D, GST–β3CD (WT) or GST–β3(AAA)CD (AAA mutant) proteins immobilized in glutathione-coated
microtitre wells were incubated with increasing concentrations of Gα13 (C), or increasing concentrations of THD (D). After washing, bound Gα13 and THD were respectively detected using
anti-Gα13 or anti-talin (mouse IgG was used as a specificity control) followed by secondary horseradish peroxidase-labelled anti-IgG antibody. E, In addition to the AAA mutation, conserved
mutations of EEE to DED and EEE to QSE (as found in β5) were introduced to the β3 cytoplasmic domain. These mutants were co-transfected with wild-type αIIb into CHO cells, which were sorted
to achieve comparable expression levels with wild-type-αIIbβ3-expressing cells (as shown in Extended Data Fig. 4e). Lysates from these cells were immunoprecipitated with anti-β3 or equal
amount of pre-immune rabbit serum. Lysates (10%) and immunoprecipitates were immunoblotted with anti-Gα13 or anti-β3. F, Lysates from human platelets (with or without stimulation with 0.025
U ml−1thrombin) were immunoprecipitated with anti-Gα13 antibody or equal amount of control rabbit IgG. Immunoprecipitates were immunoblotted with anti-Gα13 and anti-β1 antibodies. Gα13 is
associated with β1, which is increased after thrombin stimulation. EXTENDED DATA FIGURE 3 LIGAND OCCUPANCY INDUCES SWITCH OF INTEGRIN ΑIIBΒ3 FROM THE TALIN-BOUND TO THE GΑ13-BOUND STATE. A,
To determine the effect of integrin activation and ligand occupancy on Gα13–β3 association, human platelets were incubated with or without 1 mM MnCl2 and 30 μg ml−1 fibrinogen for 5 min at
22 °C. Platelet lysates were then immunoprecipitated with anti-β3 or pre-immune rabbit serum. Lysates (10%) and immunoprecipitates were immunoblotted with anti-β3 or anti-Gα13. B, C, Washed
human platelets were stimulated with 0.025 U ml−1 α-thrombin with or without adding 2 mM EDTA (an inhibitor of the ligand binding function of integrins), stirred (1,000 r.p.m.) at 37 °C,
solubilized at various time points, and immunoprecipitated with anti-β3 or equal amounts of pre-immune rabbit serum. Lysates (10%) and immunoprecipitates were immunoblotted with anti-Gα13,
anti-talin or anti-β3 antibodies. B, Western blot results. C, Turbidity changes in platelet suspension indicating integrin-dependent platelet aggregation. Note the inhibitory effect of EDTA
on talin dissociation and Gα13 binding to β3. D, As additional controls for Fig. 2a to exclude the possibility of loss of talin and β3 in platelet lysates to insoluble fraction during
integrin signalling, washed human platelets were stimulated with 0.025 U ml−1 α-thrombin in the absence or presence of 2 mM integrin inhibitor RGDS, stirred (1,000 r.p.m.) at 37 °C, and then
solubilized at various time points as in Fig. 2a. Solubilized platelets were centrifuged at 14,000_g_ for 10 min to separate lysates from insoluble pellets. Pellets were dissolved in SDS
sample buffer to the same volume as the lysates after diluting them 1:1 with 2× SDS sample buffer, and both were immunoblotted with anti-β3 and anti-talin antibodies. Note that the levels of
talin and β3 in platelet lysates kept essentially constant during the course of platelet aggregation and, with low concentrations of thrombin used to stimulate platelets, very little
insoluble β3 and talin were present in the pellet, which were detectable only after prolonged exposure (5-min exposure compared to 10 s of normal exposure time) and with no obvious variation
during the course of platelet aggregation. EXTENDED DATA FIGURE 4 EFFECTS OF SHRNA-INDUCED TALIN KNOCKDOWN AND TALIN KNOCKOUT ON INTEGRIN SIGNALLING. A, Western blot comparison of talin 1
expression levels in mouse platelets derived from control shRNA- or talin-shRNA-transfected bone marrow stem cells. Western blots of Gα13, and integrin β1 and β3 are also shown. B, Adhesion
of unstimulated mouse platelets to immobilized fibrinogen for 1 h. Adherent platelets were quantified as percentage of total platelets loaded (mean ± s.d., _n_ = 4). C, Turbidity changes in
mouse platelet suspension stimulated with 5 μM ADP in the presence of 20 μg ml−1 fibrinogen, with or without 1 mM MnCl2, as detected using an aggregometer. D, Fluorescence microscopy images
of phalloidin-stained mouse platelet spreading on fibrinogen for 1 h, with or without 1 mM MnCl2. E, Quantification of surface areas of individual adherent platelets as shown in Fig. 2g
(mean ± s.e.m.). EXTENDED DATA FIGURE 5 EFFECTS OF AAA MUTATION ON INTEGRIN OUTSIDE-IN SIGNALLING IN PLATELETS. A, Flow cytometric analysis of integrin αIIbβ3 expression levels in β3−/−
mouse platelets transfected with wild-type or AAA mutant β3 using bone marrow stem cell transplantation technology in comparison with C57BL/6 mouse platelets. β3−/− platelets were used as a
negative control. αIIbβ3 complex was detected using an anti-mouse αIIb antibody. B, Mouse platelets expressing recombinant wild-type or AAA mutant β3 as in A were lysed and
immunoprecipitated with anti-β3 or equal amounts of pre-immune rabbit serum. Lysates (10%) and immunoprecipitates were immunoblotted with anti-SRC or anti-β3 antibodies. C, D, Spreading of
phalloidin-stained wild-type platelets (EEE), AAA mutant platelets and AAA mutant platelets incubated with mP6Scr or mP6 on immobilized fibrinogen for 1 h. C, Typical fluorescence microscopy
images. D, Quantification of surface areas of individual platelets (mean ± s.e.m.). EXTENDED DATA FIGURE 6 EFFECTS OF MUTATIONAL DISRUPTION OF THE EXE MOTIF ON INTEGRIN OUTSIDE-IN
SIGNALLING. A, Expression levels of wild-type or the EXE motif (QSE, DED or AAA) mutants of β3 in complex with wild-type αIIb in CHO cells, as determined by flow cytometry. Mouse IgG was
used as a negative control. B, C, Spreading of CHO-1b9 cells expressing wild-type αIIbβ3, and QSE, DED or AAA mutant αIIbβ3 on fibrinogen for 1 h. B, Quantification of surface areas of
individual cells (mean ± s.e.m.). C, Typical microscopy images. D, Flow cytometric analysis of wild-type αIIbβ3, AAA or Y747A mutant αIIbβ3 expression in CHO cells. Mouse IgG was used as a
control. E, CHO cells expressing wild-type, AAA or Y747A β3 without (top panels) or with (bottom panels) co-expression of recombinant THD were solubilized and immunoprecipitated with anti-β3
or pre-immune serum. 10% lysates and immunoprecipitates were immunoblotted with anti-talin, anti-Gα13 or anti-β3 antibodies. F, Typical western blots for Fig. 3g. Wild-type or
AAA-mutant-αIIbβ3-expressing CHO-1b9 cells were allowed to adhere to immobilized fibrinogen, solubilized at various time points, and analysed for RHOA activation and SRC Tyr 416
phosphorylation. EXTENDED DATA FIGURE 7 MP6 SELECTIVELY INHIBITS INTEGRIN OUTSIDE-IN SIGNALLING WITHOUT AFFECTING INSIDE-OUT SIGNALLING. A–D, Washed human platelets were stimulated with
0.025 U ml−1 α-thrombin in the absence or presence of 250 μM myristoylated peptides, mP13 (A, B) and mP6 (C, D) with stirring (1,000 r.p.m.) at 37 °C, and then solubilized at various time
points. Lysates were immunoprecipitated with anti-β3 rabbit serum or equal amounts of pre-immune serum. Lysates (10%) and immunoprecipitates were immunoblotted with anti-Gα13, anti-talin or
anti-β3 antibodies. A, C, Typical western blot results. B, D, Typical turbidity changes in platelet suspension indicating integrin-dependent platelet aggregation. E, Quantification of human
platelet spreading on immobilized fibrinogen for 1 h, without or with treatment with DMSO, mP6Scr, or mP6 as shown in Fig. 4b (mean surface area ± s.e.m.). F, Flow cytometric analysis of
PAR4-AP-induced Oregon Green-labelled soluble fibrinogen binding to human platelets pre-treated with 100 μM mP6Scr or 100 μM mP6 stimulated with increasing concentrations of PAR4-AP.
Integrilin-treated platelets were used as a negative control. G, Flow cytometric analysis of 100 μM PAR4-AP-induced PAC1 binding to human platelets pre-treated with 100 μM mP6Scr or mP6.
Integrilin-treated platelets were used as negative control. H, Flow cytometric analysis of PAR4-AP-induced Oregon Green-labelled soluble fibrinogen binding to human platelets pre-treated
with solvent DMSO, mP13Scr or mP13. Resting platelets were used as a negative control. EXTENDED DATA FIGURE 8 THE _IN VIVO_ EFFECT OF MP6: SELECTIVE INHIBITION OF THROMBOSIS BUT NOT
HAEMOSTASIS. A, Representative images of laser-induced mouse cremaster arteriolar thrombosis (red) in the context of the bright-field microvascular histology, visualized by infusion of
nonblocking rat anti-mouse GPIbβ antibody conjugated to DyLight 649. The C57BL/6 mice were injected with 5 μmol kg−1micellar formulated mP6 or mP6Src (negative control), 12 μmol
kg−1Integrillin or buffer, 3 min before laser-induced arteriolar wall injury. White arrows indicate the directions of the blood flow. B, The mean platelet fluorescence intensity for 30
thrombi (performed in three mice) for each treatment at selected time points (mean ± s.e.m., _n_ = 30, _t_-test). Fluorescence in mP6- and Integrilin-treated mice is minimal. C, Comparison
of mP6 (5 μmol kg−1) with the same dose of Integrilin and their respective controls in occlusion time of FeCl3-induced carotid artery thrombosis in mice. Typical arterial blood flow charts
of FeCl3-induced occlusive thrombosis are shown. D, Comparison of mP6 (5 μmol kg−1) with the same dose of Integrilin and controls in mouse tail bleeding analysis. Released haemoglobin levels
were used as a parameter to assess blood loss (mean ± s.d., _n_ = 10). EXTENDED DATA FIGURE 9 PLATELET UPTAKE OF MP6 AND MP6SCR, AND NO EFFECT OF MP6 ON HEMOGRAM. A, Estimation of
intracellular levels of 1-pyrenyldiazomethane (PDAM)-conjugated mP6 and mP6Scr following incubation with platelets for 5 min. Platelets were pelleted by centrifugation, and the amounts of
PDAM-conjugated peptides in platelet lysates were estimated (mean ± s.d., _n_ = 3). B, Haemogram of mouse whole blood before or 1 h after injection of mP6 or mP6Scr (5 μmol kg−1), showing no
significant differences. SUPPLEMENTARY INFORMATION ARTERIOLAR THROMBOSIS: BUFFER CONTROL This file shows Intravital videomicroscopy of the cremaster muscle arteriolar circulation and
platelet thrombus formation (red) after laser injury, with HEPES buffer injection. (MOV 8136 kb) ARTERIOLAR THROMBOSIS: INTEGRILIN TREATMENT This file shows Intravital videomicroscopy of the
cremaster muscle arteriolar circulation and thrombus formation (red) after laser injury, with Integrilin (12 µM per kg) injection. (MOV 9312 kb) ARTERIOLAR THROMBOSIS: MP6SRC CONTROL This
file shows Intravital videomicroscopy of the cremaster muscle arteriolar circulation and thrombus formation (red) after laser injury, with mP6Scr micelle (5 µM per kg) injection. (MOV 8138
kb) ARTERIOLAR THROMBOSIS: MP6SRC CONTROL This file shows Intravital videomicroscopy of the cremaster muscle arteriolar circulation and thrombus formation (red) after laser injury, with mP6
micelle (5 µM per kg) injection. (MOV 7981 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shen, B., Zhao, X., O’Brien, K. _et al._ A directional switch of integrin signalling and a new anti-thrombotic
strategy. _Nature_ 503, 131–135 (2013). https://doi.org/10.1038/nature12613 Download citation * Received: 08 November 2012 * Accepted: 28 August 2013 * Published: 27 October 2013 * Issue
Date: 07 November 2013 * DOI: https://doi.org/10.1038/nature12613 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative