Play all audios:
ABSTRACT Single particle electron cryomicroscopy (cryo-EM) has recently made significant progress in high-resolution structure determination of macromolecular complexes due to improvements
in electron microscopic instrumentation and computational image analysis. However, cryo-EM structures can be highly non-uniform in local resolution1,2 and all structures available to date
have been limited to resolutions above 3 Å3,4. Here we present the cryo-EM structure of the 70S ribosome from _Escherichia coli_ in complex with elongation factor Tu, aminoacyl-tRNA and the
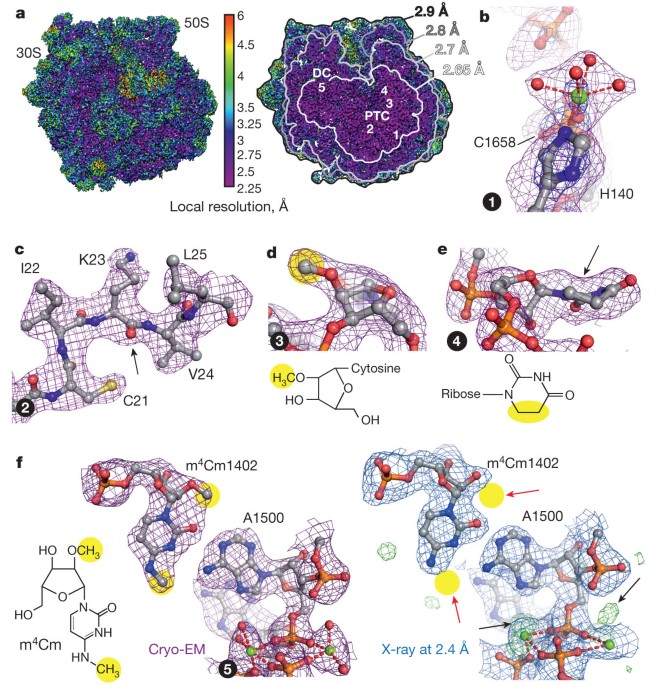
antibiotic kirromycin at 2.65–2.9 Å resolution using spherical aberration (Cs)-corrected cryo-EM. Overall, the cryo-EM reconstruction at 2.9 Å resolution is comparable to the best-resolved
X-ray structure of the _E. coli_ 70S ribosome5 (2.8 Å), but provides more detailed information (2.65 Å) at the functionally important ribosomal core. The cryo-EM map elucidates for the first
time the structure of all 35 rRNA modifications in the bacterial ribosome, explaining their roles in fine-tuning ribosome structure and function and modulating the action of antibiotics. We
also obtained atomic models for flexible parts of the ribosome such as ribosomal proteins L9 and L31. The refined cryo-EM-based model presents the currently most complete high-resolution
structure of the _E. coli_ ribosome, which demonstrates the power of cryo-EM in structure determination of large and dynamic macromolecular complexes. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online
access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which
are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS
THE TRANSLATING BACTERIAL RIBOSOME AT 1.55 Å RESOLUTION GENERATED BY CRYO-EM IMAGING SERVICES Article Open access 25 February 2023 HIGH-RESOLUTION STRUCTURES OF A THERMOPHILIC EUKARYOTIC
80S RIBOSOME REVEAL ATOMISTIC DETAILS OF TRANSLOCATION Article Open access 25 January 2022 CRYO-ELECTRON MICROSCOPY STRUCTURE OF THE 70S RIBOSOME FROM _ENTEROCOCCUS FAECALIS_ Article Open
access 01 October 2020 ACCESSION CODES PRIMARY ACCESSIONS ELECTRON MICROSCOPY DATA BANK * 2847 PROTEIN DATA BANK * 5AFI DATA DEPOSITS The 2.9 Å cryo-EM map of the _E. coli_ ribosome–EF-Tu
complex has been deposited in the Electron Microscopy Data Bank with accession code EMD-2847, the coordinates of the atomic model have been deposited in the Protein Data Bank under accession
code 5AFI. REFERENCES * Leschziner, A. E. & Nogales, E. Visualizing flexibility at molecular resolution: analysis of heterogeneity in single-particle electron microscopy
reconstructions. _Annu. Rev. Biophys. Biomol. Struct._ 36, 43–62 (2007) Article CAS PubMed Google Scholar * Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local
resolution of cryo-EM density maps. _Nature Methods_ 11, 63–65 (2014) Article CAS PubMed Google Scholar * Yu, X., Ge, P., Jiang, J. S., Atanasov, I. & Zhou, Z. H. Atomic model of CPV
reveals the mechanism used by this single-shelled virus to economically carry out functions conserved in multishelled reoviruses. _Structure_ 19, 652–661 (2011) Article CAS PubMed PubMed
Central Google Scholar * Wong, W. et al. Cryo-EM structure of the _Plasmodium falciparum_ 80S ribosome bound to the anti-protozoan drug emetine. _eLife_ 3, e03080 (2014) Article PubMed
Central CAS Google Scholar * Noeske, J. et al. Synergy of streptogramin antibiotics occurs independently of their effects on translation. _Antimicrob. Agents Chemother._ 58, 5269–5279
(2014) Article PubMed PubMed Central CAS Google Scholar * Schmeing, T. M., Voorhees, R. M., Kelley, A. C. & Ramakrishnan, V. How mutations in tRNA distant from the anticodon affect
the fidelity of decoding. _Nature Struct. Mol. Biol._ 18, 432–436 (2011) Article CAS Google Scholar * Fischer, N., Konevega, A. L., Wintermeyer, W., Rodnina, M. V. & Stark, H.
Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. _Nature_ 466, 329–333 (2010) Article CAS PubMed ADS Google Scholar * Karplus, P. A. & Diederichs, K.
Linking crystallographic model and data quality. _Science_ 336, 1030–1033 (2012) Article CAS PubMed PubMed Central ADS Google Scholar * Polikanov, Y. S. et al. Amicoumacin A inhibits
translation by stabilizing mRNA interaction with the ribosome. _Mol. Cell_ 56, 531–540 (2014) Article CAS PubMed PubMed Central Google Scholar * Schmeing, T. M., Huang, K. S., Kitchen,
D. E., Strobel, S. A. & Steitz, T. A. Structural insights into the roles of water and the 2′ hydroxyl of the P site tRNA in the peptidyl transferase reaction. _Mol. Cell_ 20, 437–448
(2005) Article CAS PubMed Google Scholar * Sergiev, P. et al. in _Ribosomes_ (eds Rodnina, M. V., Wintermeyer, W. & Green, R. ) Ch. 9 97–110 (Springer Vienna, 2011) * Burakovsky, D.
E. et al. Impact of methylations of m2G966/m5C967 in 16S rRNA on bacterial fitness and translation initiation. _Nucleic Acids Res._ 40, 7885–7895 (2012) Article CAS PubMed PubMed Central
Google Scholar * Das, G. et al. Role of 16S ribosomal RNA methylations in translation initiation in _Escherichia coli_. _EMBO J._ 27, 840–851 (2008) Article CAS PubMed PubMed Central
Google Scholar * Kimura, S. & Suzuki, T. Fine-tuning of the ribosomal decoding center by conserved methyl-modifications in the _Escherichia coli_ 16S rRNA. _Nucleic Acids Res._ 38,
1341–1352 (2010) Article CAS PubMed Google Scholar * Schuwirth, B. S. et al. Structural analysis of kasugamycin inhibition of translation. _Nature Struct. Mol. Biol._ 13, 879–886 (2006)
Article CAS Google Scholar * Gutierrez, B. et al. Fitness cost and interference of Arm/Rmt aminoglycoside resistance with the RsmF housekeeping methyltransferases. _Antimicrob. Agents
Chemother._ 56, 2335–2341 (2012) Article CAS PubMed PubMed Central Google Scholar * Green, R. & Noller, H. F. _In vitro_ complementation analysis localizes 23S rRNA
posttranscriptional modifications that are required for _Escherichia coli_ 50S ribosomal subunit assembly and function. _RNA_ 2, 1011–1021 (1996) CAS PubMed PubMed Central Google Scholar
* LaMarre, J. M., Howden, B. P. & Mankin, A. S. Inactivation of the indigenous methyltransferase RlmN in _Staphylococcus aureus_ increases linezolid resistance. _Antimicrob. Agents
Chemother._ 55, 2989–2991 (2011) Article CAS PubMed PubMed Central Google Scholar * Toh, S.-M. & Mankin, A. S. An indigenous posttranscriptional modification in the ribosomal
peptidyl transferase center confers resistance to an array of protein synthesis inhibitors. _J. Mol. Biol._ 380, 593–597 (2008) Article CAS PubMed PubMed Central Google Scholar *
Osterman, I. A. et al. Methylated 23S rRNA nucleotide m2G1835 of _Escherichia coli_ ribosome facilitates subunit association. _Biochimie_ 93, 725–729 (2011) Article CAS PubMed Google
Scholar * Sergiev, P. V., Serebryakova, M. V., Bogdanov, A. A. & Dontsova, O. A. The ybiN gene of _Escherichia coli_ encodes adenine-N6 methyltransferase specific for modification of
A1618 of 23S ribosomal RNA, a methylated residue located close to the ribosomal exit tunnel. _J. Mol. Biol._ 375, 291–300 (2008) Article CAS PubMed Google Scholar * David-Eden, H.,
Mankin, A. S. & Mandel-Gutfreund, Y. Structural signatures of antibiotic binding sites on the ribosome. _Nucleic Acids Res._ 38, 5982–5994 (2010) Article CAS PubMed PubMed Central
Google Scholar * Burnley, B. T., Afonine, P. V., Adams, P. D. & Gros, P. Modelling dynamics in protein crystal structures by ensemble refinement. _eLife_ 1, e00311 (2012) Article
PubMed PubMed Central CAS Google Scholar * Schröder, G. F., Levitt, M. & Brunger, A. T. Deformable elastic network refinement for low-resolution macromolecular crystallography. _Acta
Crystallogr. D_ 70, 2241–2255 (2014) Article PubMed PubMed Central CAS Google Scholar * Kleywegt, G. J. Crystallographic refinement of ligand complexes. _Acta Crystallogr. D_ 63,
94–100 (2007) Article CAS PubMed Google Scholar * Schmeing, T. M. et al. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. _Science_ 326, 688–694 (2009) Article
CAS PubMed PubMed Central ADS Google Scholar * Brandt, F. et al. The native 3D organization of bacterial polysomes. _Cell_ 136, 261–271 (2009) Article CAS PubMed Google Scholar *
Akanuma, G., Nanamiya, H., Natori, Y., Nomura, N. & Kawamura, F. Liberation of zinc-containing L31 (RpmE) from ribosomes by its paralogous gene product, YtiA, in _Bacillus subtilis_. _J.
Bacteriol._ 188, 2715–2720 (2006) Article CAS PubMed PubMed Central Google Scholar * Samaha, R. R., Green, R. & Noller, H. F. A base pair between tRNA and 23S rRNA in the peptidyl
transferase centre of the ribosome. _Nature_ 377, 309–314 (1995) Article CAS PubMed ADS Google Scholar * Ippolito, J. A. et al. Crystal structure of the oxazolidinone antibiotic
linezolid bound to the 50S ribosomal subunit. _J. Med. Chem._ 51, 3353–3356 (2008) Article CAS PubMed Google Scholar * Milon, P. et al. Transient kinetics, fluorescence, and FRET in
studies of initiation of translation in bacteria. _Methods Enzymol._ 430, 1–30 (2007) Article CAS PubMed Google Scholar * Rodnina, M. V. et al. Thiostrepton inhibits the turnover but not
the GTPase of elongation factor G on the ribosome. _Proc. Natl Acad. Sci. USA_ 96, 9586–9590 (1999) Article CAS PubMed ADS PubMed Central Google Scholar * Rodnina, M. V. &
Wintermeyer, W. GTP consumption of elongation factor Tu during translation of heteropolymeric messenger-RNAs. _Proc. Natl Acad. Sci. USA_ 92, 1945–1949 (1995) Article CAS PubMed ADS
PubMed Central Google Scholar * Sander, B., Golas, M. M. & Stark, H. Automatic CTF correction for single particles based upon multivariate statistical analysis of individual power
spectra. _J. Struct. Biol._ 142, 392–401 (2003) Article CAS PubMed Google Scholar * Valle, M. et al. Cryo-EM reveals an active role for aminoacyl-tRNA in the accommodation process. _EMBO
J._ 21, 3557–3567 (2002) Article CAS PubMed PubMed Central Google Scholar * Stark, H. et al. Ribosome interactions of aminoacyl-tRNA and elongation factor Tu in the codon-recognition
complex. _Nature Struct. Mol. Biol._ 9, 849–854 (2002) CAS Google Scholar * Scheres, S. H. W. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. _J. Struct.
Biol._ 180, 519–530 (2012) Article CAS PubMed PubMed Central Google Scholar * Bock, L. V. et al. Energy barriers and driving forces in tRNA translocation through the ribosome. _Nature
Struct. Mol. Biol._ 20, 1390–1396 (2013) Article CAS Google Scholar * Pronk, S. et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit.
_Bioinformatics_ 29, 845–854 (2013) Article CAS PubMed PubMed Central Google Scholar * Kleywegt, G. J. & Jones, T. A. Software for handling macromolecular envelopes. _Acta
Crystallogr. D_ 55, 941–944 (1999) Article CAS PubMed Google Scholar * Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. _J. Comput.
Chem._ 25, 1605–1612 (2004) Article CAS PubMed Google Scholar * Brunger, A. T. Version 1.2 of the Crystallography and NMR system. _Nature Protocols_ 2, 2728–2733 (2007) Article CAS
PubMed Google Scholar * Brünger, A. T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. _Acta Crystallogr. D_ 54, 905–921 (1998)
Article PubMed Google Scholar * Greber, B. J. et al. The complete structure of the large subunit of the mammalian mitochondrial ribosome. _Nature_ 515, 283–286 (2014) Article CAS PubMed
ADS Google Scholar * Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. _Acta Crystallogr. D Biol. Crystallogr._ 66, 213–221 (2010)
Article CAS PubMed PubMed Central Google Scholar * DiMaio, F. et al. Improved molecular replacement by density-and energy-guided protein structure optimization. _Nature_ 473, 540–543
(2011) Article CAS PubMed PubMed Central ADS Google Scholar * DiMaio, F., Tyka, M. D., Baker, M. L., Chiu, W. & Baker, D. Refinement of protein structures into low-resolution
density maps using rosetta. _J. Mol. Biol._ 392, 181–190 (2009) Article CAS PubMed PubMed Central Google Scholar * Söding, J., Biegert, A. & Lupas, A. N. The HHpred interactive
server for protein homology detection and structure prediction. _Nucleic Acids Res._ 33, W244–W248 (2005) Article PubMed PubMed Central CAS Google Scholar * Dunkle, J. A. et al.
Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. _Science_ 332, 981–984 (2011) Article CAS PubMed PubMed Central ADS Google Scholar * Selmer, M. et
al. Structure of the 70S ribosome complexed with mRNA and tRNA. _Science_ 313, 1935–1942 (2006) Article CAS PubMed ADS Google Scholar * Byrne, R. T., Konevega, A. L., Rodnina, M. V.
& Antson, A. A. The crystal structure of unmodified tRNAPhe from _Escherichia coli_. _Nucleic Acids Res._ 38, 4154–4162 (2010) Article CAS PubMed PubMed Central Google Scholar *
Schröder, G. F., Levitt, M. & Brunger, A. T. Super-resolution biomolecular crystallography with low-resolution data. _Nature_ 464, 1218–1222 (2010) Article PubMed PubMed Central ADS
CAS Google Scholar * Emsley, P., Lohkamp, B., Scott, W. & Cowtan, K. Features and development of Coot. _Acta Crystallogr. D_ 66, 486–501 (2010) Article CAS PubMed PubMed Central
Google Scholar * Terwilliger, T. C. Maximum-likelihood density modification. _Acta Crystallogr. D_ 56, 965–972 (2000) Article CAS PubMed PubMed Central Google Scholar * The CCP4 Suite:
programs for protein crystallography. _Acta Crystallogr. D_ 50, 760–763 (1994) * Chou, F.-C., Sripakdeevong, P., Dibrov, S. M., Hermann, T. & Das, R. Correcting pervasive errors in RNA
crystallography through enumerative structure prediction. _Nature Methods_ 10, 74–76 (2013) Article CAS PubMed Google Scholar * Afonine, P. V., Headd, J. J., Terwilliger, T. C. &
Adams, P. D. New tool: phenix.real_space_refine. _Computational Crystallography Newsletter_ 4, 43–44 (2013) Google Scholar * Jenner, L., Demeshkina, N., Yusupova, G. & Yusupov, M.
Structural rearrangements of the ribosome at the tRNA proofreading step. _Nature Struct. Mol. Biol._ 17, 1072–1078 (2010) Article CAS Google Scholar * Jones, T. A. & Kjeldgaard, M.
Electron-density map interpretation. _Methods Enzymol._ 277, 173–208 (1997) Article CAS PubMed Google Scholar * Allegretti, M., Mills, D. J., McMullan, G., Kühlbrandt, W. & Vonck, J.
Atomic model of the F420-reducing [NiFe] hydrogenase by electron cryo-microscopy using a direct electron detector. _eLife_ 3, e01963 (2014) Article PubMed PubMed Central Google Scholar
* Bartesaghi, A., Matthies, D., Banerjee, S., Merk, A. & Subramaniam, S. Structure of β-galactosidase at 3.2-Å resolution obtained by cryo-electron microscopy. _Proc. Natl Acad. Sci.
USA_ 111, 11709–11714 (2014) Article CAS PubMed ADS PubMed Central Google Scholar * Burmeister, W. P. Structural changes in a cryo-cooled protein crystal owing to radiation damage.
_Acta Crystallogr. D_ 56, 328–341 (2000) Article CAS PubMed Google Scholar * Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and
contrast loss in single-particle electron cryomicroscopy. _J. Mol. Biol._ 333, 721–745 (2003) Article CAS PubMed Google Scholar * Grosse-Kunstleve, R. W., Sauter, N. K., Moriarty, N. W.
& Adams, P. D. The Computational Crystallography Toolbox: crystallographic algorithms in a reusable software framework. _J. Appl. Crystallogr._ 35, 126–136 (2002) Article CAS Google
Scholar * Demirci, H. et al. Modification of 16S ribosomal RNA by the KsgA methyltransferase restructures the 30S subunit to optimize ribosome function. _RNA_ 16, 2319–2324 (2010) Article
CAS PubMed PubMed Central Google Scholar * Helser, T. L., Davies, J. E. & Dahlberg, J. E. Mechanism of kasugamycin resistance in _Escherichia coli_. _Nature_ 235, 6–9 (1972) CAS
Google Scholar * O’Connor, M., Thomas, C. L., Zimmermann, R. A. & Dahlberg, A. E. Decoding fidelity at the ribosomal A and P sites: influence of mutations in three different regions of
the decoding domain in 16S rRNA. _Nucleic Acids Res._ 25, 1185–1193 (1997) Article PubMed PubMed Central Google Scholar * François, B. et al. Crystal structures of complexes between
aminoglycosides and decoding A site oligonucleotides: role of the number of rings and positive charges in the specific binding leading to miscoding. _Nucleic Acids Res._ 33, 5677–5690 (2005)
Article PubMed PubMed Central Google Scholar * Jiang, J., Aduri, R., Chow, C. S. & SantaLucia, J. Structure modulation of helix 69 from _Escherichia coli_ 23S ribosomal RNA by
pseudouridylations. _Nucleic Acids Res._ 42, 3971–3981 (2013) Article PubMed PubMed Central CAS Google Scholar * Davis, D. R. Stabilization of RNA stacking by pseudouridine. _Nucleic
Acids Res._ 23, 5020–5026 (1995) Article CAS PubMed PubMed Central Google Scholar * Ortiz-Meoz, R. F. & Green, R. Helix 69 is key for uniformity during substrate selection on the
ribosome. _J. Biol. Chem._ 286, 25604–25610 (2011) Article CAS PubMed PubMed Central Google Scholar * Seidelt, B. et al. Structural insight into nascent polypeptide chain–mediated
translational stalling. _Science_ 326, 1412–1415 (2009) Article CAS PubMed PubMed Central ADS Google Scholar * Pulk, A. & Cate, J. H. Control of ribosomal subunit rotation by
elongation factor G. _Science_ 340, 1235970 (2013) Article PubMed PubMed Central CAS Google Scholar * Vorstenbosch, E., Pape, T., Rodnina, M., Kraal, B. & Wintermeyer, W. The G222D
mutation in elongation factor Tu inhibits the codon-induced conformational changes leading to GTPase activation on the ribosome. _EMBO J._ 15, 6766–6774 (1996) Article CAS PubMed PubMed
Central Google Scholar * Daviter, T., Wieden, H.-J. & Rodnina, M. V. Essential role of histidine 84 in elongation factor Tu for the chemical step of GTP hydrolysis on the ribosome. _J.
Mol. Biol._ 332, 689–699 (2003) Article CAS PubMed Google Scholar * Voorhees, R. M., Schmeing, T. M., Kelley, A. C. & Ramakrishnan, V. The mechanism for activation of GTP hydrolysis
on the ribosome. _Science_ 330, 835–838 (2010) Article CAS PubMed PubMed Central ADS Google Scholar * Pintilie, G. D., Zhang, J., Goddard, T. D., Chiu, W. & Gossard, D. C.
Quantitative analysis of cryo-EM density map segmentation by watershed and scale-space filtering, and fitting of structures by alignment to regions. _J. Struct. Biol._ 170, 427–438 (2010)
Article CAS PubMed PubMed Central Google Scholar * Selmer, M., Gao, Y.-G., Weixlbaumer, A. & Ramakrishnan, V. Ribosome engineering to promote new crystal forms. _Acta Crystallogr.
D_ 68, 578–583 (2012) Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank F. Würriehausen for expert technical assistance and M. Lüttich and
B. Busche for support in high-performance computation and programming. The work was supported by the Deutsche Forschungsgemeinschaft Grant FOR 1805 (to H.S. and M.V.R.) and by the
Sonderforschungsbereich 860 (to H.S., R. F., and M.V.R.). AUTHOR INFORMATION Author notes * Niels Fischer and Piotr Neumann: These authors contributed equally to this work. AUTHORS AND
AFFILIATIONS * 3D Electron Cryomicroscopy Group, Max-Planck-Institute for Biophysical Chemistry, Am Fassberg 11, 37077 Göttingen, Germany, Niels Fischer & Holger Stark * Abteilung
Molekulare Strukturbiologie, Institut für Mikrobiologie und Genetik, GZMB, Georg-August Universität Göttingen, Justus-von Liebig Weg 11, 37077 Göttingen, Germany, Piotr Neumann & Ralf
Ficner * Molecular and Radiation Biophysics Department, B.P. Konstantinov Petersburg Nuclear Physics Institute of National Research Centre ‘Kurchatov Institute’, 188300 Gatchina, Russia,
Andrey L. Konevega * St Petersburg Polytechnic University, Polytechnicheskaya, 29, 195251 St Petersburg, Russia, Andrey L. Konevega * Department of Physical Biochemistry, Max Planck
Institute for Biophysical Chemistry, Am Fassberg 11, 37077 Göttingen, Germany, Andrey L. Konevega & Marina V. Rodnina * Department of Theoretical and Computational Biophysics, Max Planck
Institute for Biophysical Chemistry, Am Fassberg 11, 37077 Göttingen, Germany, Lars V. Bock * Department of 3D Electron Cryomicroscopy, Institute of Microbiology and Genetics, Georg-August
Universität, 37077 Göttingen, Germany, Holger Stark Authors * Niels Fischer View author publications You can also search for this author inPubMed Google Scholar * Piotr Neumann View author
publications You can also search for this author inPubMed Google Scholar * Andrey L. Konevega View author publications You can also search for this author inPubMed Google Scholar * Lars V.
Bock View author publications You can also search for this author inPubMed Google Scholar * Ralf Ficner View author publications You can also search for this author inPubMed Google Scholar *
Marina V. Rodnina View author publications You can also search for this author inPubMed Google Scholar * Holger Stark View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS N.F. designed and performed cryo-EM experiments and data analysis. P.N. conceived and performed pseudo-crystallographic refinement and model validation and
analysed data. A.L.K. prepared ribosome complexes. L.V.B. performed and analysed molecular dynamics simulations. All authors discussed the results. H.S. and N.F. conceived the project and
wrote the paper with input from all authors. CORRESPONDING AUTHORS Correspondence to Niels Fischer or Holger Stark. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
financial interests. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 ABERRATION-CORRECTED CRYO-EM. A, Exemplary Zemlin tableau (left) and phase diagram (right) as obtained for the
present data set with the CEOS software by correcting electron optical aberrations using the Cs corrector. The resulting phase errors were less than 45° at ≤2.1 Å (that is, at scattering
angles of 12 to 15 mrad) over up to 36 h of image acquisition. The main limiting aberration is axial coma (B2) and the next limiting aberration would be threefold astigmatism (A3). B, Local
correction for the contrast transfer function. From micrographs (left) areas with individual ribosome particles (yellow frames) were extracted and local power spectra were computed for each
of these areas by fast Fourier transform algorithms (FFT). Local power spectra were subjected to principal component analysis (PCA) and classification to average power spectra with similar
contrast transfer function parameters that were obtained from different micrographs. Class averages of power spectra reveal an improved signal-to-noise ratio in Thon rings which are clearly
visible up to 2.4 Å (right). C, Global power spectrum from a single micrograph showing Thon rings up to 3.5 Å. EXTENDED DATA FIGURE 2 HIERARCHICAL SORTING OF RIBOSOME PARTICLE IMAGES.
Ribosome particles were sorted in three steps according to: (1) global ribosome conformation (C1), that is, 30S body rotation7; and (2) ligand occupancy (C2)35 and particle quality
(C3)37(Methods). The asterisk denotes particles assigned to the largest 30S body rotations ≤−10° and ≥10° which contain particles with extreme 30S rotation angles, but also low-quality
particle images. EXTENDED DATA FIGURE 3 RESOLUTION CURVES AND MODEL VALIDATION OF THE _E. COLI_ 70S RIBOSOME–EF-TU CRYO-EM STRUCTURE. A, Fourier-shell correlation (FSC) curve (black) for the
70S ribosome cryo-EM reconstruction computed between the masked independent half-maps (half1 and half2) that were obtained by so-called ‘gold-standard’ refinement in RELION37. The
resolution of the cryo-EM reconstruction is ∼2.9 Å according to the 0.143 criterion63 (black dashed line). B, FSC curves computed between cryo-EM maps and model maps generated from refined
atomic coordinates. The vertical black dashed line indicates the maximum resolution at which the full atomic models were refined. Black, the FSC curve between the final cryo-EM map (map) and
the final model (model); blue, the FSC curve between half map 1 (half1) and the model obtained by refinement only against half map 1 (model1); red, the FSC curve between half map 1 and the
model obtained by refinement only against half map 2 (model2). C, FSC curves (FSCwork) between reflections from solvent-flattened cryo-EM map and model as obtained by pseudo-crystallographic
refinement of the complete ribosome model (mask1) and three sub-models using different masks corresponding to local variations in resolution (mask2–4; Methods) as shown in H. Coloured
numbers indicate the highest resolution used in refinement with the respective mask as indicated by the colour code. For all refinements, the FSC is above the 0.5 threshold (black dashed
line) in the highest-resolution shell. Differences to B result largely from solvent-flattening before Fourier transformation for refinement (Methods). D–G, CCwork and Rwork as obtained by
refinement using the respective mask (see labels). For a reliable resolution estimate CCwork (ref. 8) is expected to be >0.2 and Rwork <0.51 in the highest-resolution shell. H,
Isosurface representations of the mask used for local refinements; ‘%’ indicates the fraction of atoms of the complete model entailed in the refinement with the respective mask. EXTENDED
DATA FIGURE 4 MODIFICATIONS IN RRNA. COMPARISON BETWEEN CRYO-EM AND X-RAY CRYSTALLOGRAPHY. A, Experimental densities. In each row density maps for the same type of rRNA modification are
shown (from left to right): for the present cryo-EM map and for the current best resolved bacterial and archaeal ribosome maps determined by X-ray crystallography, that is, the bacterial 70S
ribosome from _E. coli_ (_Eco_70S) at 2.8 Å resolution5 (PDB IDs: 4TPA and 4TPB); the bacterial 70S ribosome from _T. thermophilus_ (_Tth_70S) at 2.4 Å resolution9 (PDB IDs: 4RB5 and 4RB6);
and the archaeal 50S subunit from _Haloarcula marismortui_ (_Hma_70S) at 2.2 Å resolution10 (PDB ID: 1VQ0). _E. coli_ numbering is used for bacterial ribosome structures. Locations of rRNA
modifications as determined by biochemical data are marked by yellow circles, modifications not observed in the density maps are denoted by red arrows and the black arrow designates the
non-planarity of dihydrouridine observed in the cryo-EM map. B, Model-based densities for m62A1518 and m62A1519 showing slight differences due to scattering properties. Densities were
computed in CCTBX64 at 2.65 Å resolution from our final model with atomic-displacement factors kept unchanged using electron (e− scattering, purple) and X-ray scattering factors (X-ray
scattering, blue), respectively. Map thresholds were normalized to show similar density levels for the electron-rich phosphate groups. Accordingly, the absence of densities for modifications
in crystallographic maps also at higher resolutions may result from differences in electron and X-ray scattering and in data quality which is affected, for example, by local and global
disorder. EXTENDED DATA FIGURE 5 RRNA MODIFICATIONS IN THE _E. COLI_ 70S RIBOSOME. A, Stabilizing effects of 16S rRNA methyl groups in the P site of the decoding centre. Numbers in the
overview (top left) mark the positions of close-ups (1–3), which show the interactions of the rRNA methyl groups with distances colour-encoded by dashed lines. Close-up 1: Stacking network
of m2G966 and m5C967 stabilizing binding of initiator tRNA. Close-ups 2, 3: rRNA modifications impacting mRNA binding. The universally conserved bulky dimethylamine groups of m62A1518 and
m62A1519 stabilize their direct environment by steric encumbrance explaining their requirement for correct packing of 16S rRNA helices 24a, 44 and 4565. In particular, the dimethylamine of
m62A1519 is involved in medium and long-range repulsive interactions with the backbone of m3U1498 and the 2' O of C1520, while its conformation is mostly determined by the dimethylamine
moiety of the adjacent m62A1518 which, in turn, is fixed by short repulsive interactions with O6 of G1517 and O4 of U793. The additional methyl groups of m62A1519 interact with A792, which
provides part of the binding site for the antibiotic kasugamycin15, accounting for the resistance against kasugamycin upon demethylation of m62A151966. Furthermore, m62A1518 and m62A1519
impact initiation67 possibly via m3U1498 whose backbone interacts with m62A1519, while its modified base contacts the mRNA backbone. The methyl groups of m3U1498 and m4Cm1402 form part of
the binding site for the initiation codon and modulate translation initiation13,14 by steric encumbrance and/or by preventing direct hydrogen bonds with the mRNA backbone. B, Constitutive
rRNA modifications in the _E. coli_ 70S ribosome (list adapted from ref. 11 and references therein). C, rRNA modifications in the A site of the decoding centre and helix 69 of 23S rRNA
(H69). The binding site of aminoglycosides in helix 44 of 16S rRNA (h44)—including N4 of m5C1407—is indicated for neomycin B (magenta, superposition from PDB ID: 2ET4)68. The three
pseudouridines stabilizing H6969 by enhancing base stacking70 are depicted in blue. The methyl group (yellow) on m3Ψ1917 in H69 prevents potential base-pairing with A1913, a residue
important for uniform tRNA selection71. Note the flipped out conformation of A1913 facilitating interaction with the 2′ OH of m2s6iA37 of the distorted Phe–tRNAPhe (purple), which, in turn,
stacks onto A36 of the tRNA anticodon. D, Methyl group on m2G1835 of 23S rRNA enhancing subunit association. The four helices of 23S rRNA that intersect around residue m2G1835 and form
intersubunit bridges B2b and B2c with helices 24 (h24) and 45 (h45) of 16S rRNA (dark grey) are denoted in different colours: helix 67 (H67), light blue; helix 68 (H68), blue; helix 69
(H69), teal; helix 70 (H70), purple. Inset, contacts of the methyl group on m2G1835 with adjacent residues which, in turn, interact with 16S rRNA. E, Cluster of 23S rRNA modifications in the
peptide exit tunnel. The modified rRNA residues, the functionally important nearby tip of protein L22 (teal), P-site fMet–tRNAfMet (green) and a model of the nascent peptide chain (pink,
superposition from PDB ID: 2WWL)72 are indicated. EXTENDED DATA FIGURE 6 VISUALIZATION OF STRUCTURAL DYNAMICS OF THE RIBOSOME BY DIFFERENT APPROACHES. A–D, In each panel, the ribosome is
shown from the factor binding site on the left and in cut-away view on the right; h denotes the head and b the body of the 30S ribosomal subunit. A, Present cryo-EM map coloured according to
local resolution as determined by Resmap2. B, Present cryo-EM map coloured according to the B factors obtained from the pseudo-crystallographic atomic model refinement (Methods). C, Model
map of the 2.95 Å crystal structure of the _E. coli_ 70S ribosome73 (PDB IDs: 4KJ1 and 4KJ2) coloured according to respective B factors. The black arrow denotes the stabilization of the 30S
head region by crystal contacts, whereas the 30S body (white arrow) is less constrained by crystal contacts and shows higher B factors, indicating larger flexibility for this region. D,
Snapshot from molecular dynamics trajectory of the _E. coli_ 70S ribosome coloured according to root mean squared fluctuations (RMSFs) obtained from the full 2 µs explicit solvent molecular
dynamics simulation (Methods). Note the large fluctuations of the 30S head and body (white arrows) of the ribosome in solution not constrained by crystal contacts. EXTENDED DATA FIGURE 7
STRUCTURE OF _E. COLI_ EF-TU–PHE–TRNAPHE BOUND TO THE RIBOSOME. A, Detailed comparison of the distorted A/T-site–tRNA interactions between the _E. coli_ and _T. thermophilus_
ribosome–EF-Tu–kirromycin complexes6,26 and the free _E. coli_ tRNAPhe51. We found significant differences in tRNA conformation and interactions implicated in the GTPase activation
mechanism26 that correlate with ribosome binding and differences in organism and tRNA species. Here and below residue numbers refer to _E. coli_. B, Overview of the _E. coli_ ribosome–EF-Tu
structure. The residues interacting with the EF-Tu ternary complex (depicted in stick representation) generally agree with those seen in the _T. thermophilus_ structures6,26. rRNA helices
are denoted as: h44, helix 44 of 16S rRNA; H69, helix 69; and SRL, sarcin–ricin loop of 23S rRNA. The dashed boxes indicate the parts of the structure magnified in C and D. C, Structural
differences in an important ribosome–EF-Tu interaction. In the _E. coli_ structure (_Eco_, left panel) residues A55 and A368 of 16S rRNA assume different conformations (cyan arrows) and
interact differently with the ribosome and EF-Tu–tRNA complex than in the _T. thermophilus_ structure (_Tth_, right panel, PDB ID: 2WRN)26. Furthermore, the differences in EF-Tu sequence
result in slightly different ribosome–EF-Tu interactions, for example, the ε-amino group of lysine 282 in _E. coli_ EF-Tu is within hydrogen-bonding distance of G382 in 16S rRNA, but not the
serine at this position in _T. thermophilus_ EF-Tu. Inset on left panel, overlay of the crucial β-turn26,74 in EF-Tu domain 2 in the free (PDB ID: 1OB2, R. C. Nielsen _et al._, unpublished
data) and ribosome-bound state from _E. coli_ and _T. thermophilus_. D, Dynamics of the catalytic histidine 84 (ref. 75) of EF-Tu. A split density for the side chain of histidine 84 (data
not shown) indicates the presence of two rotamers (rot1 and rot2, panel 1) in the present _E. coli_ complex. In D, the _T. thermophilus_ ribosome-bound EF-Tu structures6,26,76 (dark grey,
complex as indicated) are shown with the corresponding rotamer of the present structure (red). Residues valine 20 and isoleucine 60 of the hydrophobic gate76 are denoted; isoleucine 60 is
not resolved in the kirromycin-stalled ribosome–EF-Tu complexes. EXTENDED DATA FIGURE 8 CRYO-EM DENSITIES FOR MOBILE PROTEINS L9 AND L31 AND THE ARRANGEMENT OF L9 IN POLYSOMES. Densities in
A and B were obtained by semi-automatic segmentation using the ‘segger’ tool in UCSF CHIMERA41,77 and normalized and low-pass filtered according to local resolution estimates. A, Cryo-EM map
and models of protein L9 (∼4 Å local resolution, rendered at 1_σ_). B, Cryo-EM maps and models of protein L31 in the ground-state of the ribosome (left, ∼4.3 Å local resolution) and in the
rotated state (right, ∼6 Å local resolution); maps were rendered at 1.5_σ_. C, Model of protein L9 in the context of polysomes. Overviews show the arrangement of neighbouring ribosomes (i-1
and i) in the major t-t form of _E. coli_ polysomes as obtained by fitting the present 70S ribosome structure into the cryo-electron tomography reconstruction27 in UCSF CHIMERA41. Left
close-up, the conformer of L9 as seen in the present cryo-EM map (L9 cryo-EM, blue) is located close to protein S4 of the neighbouring 30S subunit (30S i) according to the polysome model.
The purple arrow indicates the rearrangement of L9 from the cryo-EM conformation to that seen in crystals. The black arrow denotes the location of the mRNA entry channel in the 30S subunit
i. Right close-up, the conformer of L9 as seen in the context of ribosome crystals (L9 X-ray, pink) reaches into the ribosomal A-site of the neighbouring 30S subunit and would be compatible
with the simultaneous binding of elongation factors in the polysome model. In crystals, protein L9 precludes the binding of elongation factors due to the tighter packing of ribosomes78. The
model of L9 was obtained by superposition of the _E. coli_ 70S ribosome X-ray structure5 (PDB IDs: 4TP8 and 4TP9) onto ribosome i-1 in the polysome model using UCSF CHIMERA41. POWERPOINT
SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Fischer, N.,
Neumann, P., Konevega, A. _et al._ Structure of the _E. coli_ ribosome–EF-Tu complex at <3 Å resolution by Cs-corrected cryo-EM. _Nature_ 520, 567–570 (2015).
https://doi.org/10.1038/nature14275 Download citation * Received: 21 November 2014 * Accepted: 30 January 2015 * Published: 23 February 2015 * Issue Date: 23 April 2015 * DOI:
https://doi.org/10.1038/nature14275 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative