Play all audios:
ABSTRACT The cannabinoid receptor 1 (CB1) is the principal target of the psychoactive constituent of marijuana, the partial agonist Δ9-tetrahydrocannabinol (Δ9-THC)1. Here we report two
agonist-bound crystal structures of human CB1 in complex with a tetrahydrocannabinol (AM11542) and a hexahydrocannabinol (AM841) at 2.80 Å and 2.95 Å resolution, respectively. The two
CB1–agonist complexes reveal important conformational changes in the overall structure, relative to the antagonist-bound state2, including a 53% reduction in the volume of the ligand-binding
pocket and an increase in the surface area of the G-protein-binding region. In addition, a ‘twin toggle switch’ of Phe2003.36 and Trp3566.48 (superscripts denote Ballesteros–Weinstein
numbering3) is experimentally observed and appears to be essential for receptor activation. The structures reveal important insights into the activation mechanism of CB1 and provide a
molecular basis for predicting the binding modes of Δ9-THC, and endogenous and synthetic cannabinoids. The plasticity of the binding pocket of CB1 seems to be a common feature among certain
class A G-protein-coupled receptors. These findings should inspire the design of chemically diverse ligands with distinct pharmacological properties. Access through your institution Buy or
subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get
Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90
per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURAL BASIS OF THC ANALOG
ACTIVITY AT THE CANNABINOID 1 RECEPTOR Article Open access 08 January 2025 STRUCTURAL BASIS FOR ACTIVATION OF CB1 BY AN ENDOCANNABINOID ANALOG Article Open access 09 May 2023 CANNABIDIOL
INHIBITS NAV CHANNELS THROUGH TWO DISTINCT BINDING SITES Article Open access 17 June 2023 ACCESSION CODES PRIMARY ACCESSIONS PROTEIN DATA BANK * 5XR8 * 5XRA REFERENCES * Mechoulam, R.,
Hanuš, L. O., Pertwee, R. & Howlett, A. C. Early phytocannabinoid chemistry to endocannabinoids and beyond. _Nat. Rev. Neurosci._ 15, 757–764 (2014) Article CAS Google Scholar * Hua,
T. et al. Crystal structure of the human cannabinoid receptor CB1. _Cell_ 167, 750–762 e714 (2016) Article CAS Google Scholar * Ballesteros, J. A. & Weinstein, H. in _Methods in
Neurosciences_ Vol. 25 (ed. Sealfon Stuart, C. ) 366–428 (Academic, 1995) * Lemberger, L. Potential therapeutic usefulness of marijuana. _Annu. Rev. Pharmacol. Toxicol._ 20, 151–172 (1980)
Article CAS Google Scholar * Li, H.-L. An archaeological and historical account of cannabis in China. _Econ. Bot._ 28, 437–448 (1973) Article Google Scholar * Makriyannis, A. 2012
Division of Medicinal Chemistry Award Address. Trekking the cannabinoid road: a personal perspective. _J. Med. Chem._ 57, 3891–3911 (2014) Article CAS Google Scholar * Shao, Z. et al.
High-resolution crystal structure of the human CB1 cannabinoid receptor. _Nature_ 540, 602–606 (2016) Article CAS ADS Google Scholar * Nikas, S. P. et al. The role of halogen
substitution in classical cannabinoids: a CB1 pharmacophore model. _AAPS J._ 6, e30 (2004) Article Google Scholar * Nikas, S. P. et al. Novel 1′,1′-chain substituted hexahydrocannabinols:
9β-hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol (AM2389) a highly potent cannabinoid receptor 1 (CB1) agonist. _J. Med. Chem._ 53, 6996–7010 (2010) Article CAS Google Scholar *
Xie, X. Q., Melvin, L. S. & Makriyannis, A. The conformational properties of the highly selective cannabinoid receptor ligand CP-55,940. _J. Biol. Chem._ 271, 10640–10647 (1996) Article
CAS Google Scholar * Makriyannis, A. & Rapaka, R. S. The medicinal chemistry of cannabinoids: an overview. _NIDA Res. Monogr._ 79, 204–210 (1987) CAS PubMed Google Scholar * Ahn,
K. H., Bertalovitz, A. C., Mierke, D. F. & Kendall, D. A. Dual role of the second extracellular loop of the cannabinoid receptor 1: ligand binding and receptor localization. _Mol.
Pharmacol._ 76, 833–842 (2009) Article CAS Google Scholar * Feigenbaum, J. J. et al. Nonpsychotropic cannabinoid acts as a functional _N_-methyl-d-aspartate receptor blocker. _Proc. Natl
Acad. Sci. USA_ 86, 9584–9587 (1989) Article CAS ADS Google Scholar * Mechoulam, R. et al. Enantiomeric cannabinoids: stereospecificity of psychotropic activity. _Experientia_ 44,
762–764 (1988) Article CAS Google Scholar * Hanson, M. A. et al. Crystal structure of a lipid G protein-coupled receptor. _Science_ 335, 851–855 (2012) Article CAS ADS Google Scholar
* Rasmussen, S. G. et al. Crystal structure of the β2 adrenergic receptor–Gs protein complex. _Nature_ 477, 549–555 (2011) Article CAS ADS Google Scholar * Singh, R. et al. Activation of
the cannabinoid CB1 receptor may involve a W6.48/F3.36 rotamer toggle switch. _J. Pept. Res._ 60, 357–370 (2002) Article CAS Google Scholar * Tiburu, E. K. et al. Structural biology of
human cannabinoid receptor-2 helix 6 in membrane-mimetic environments. _Biochem. Biophys. Res. Commun._ 384, 243–248 (2009) Article CAS Google Scholar * Zhang, K. et al. Structure of the
human P2Y12 receptor in complex with an antithrombotic drug. _Nature_ 509, 115–118 (2014) Article CAS ADS Google Scholar * Zhang, J. et al. Agonist-bound structure of the human P2Y12
receptor. _Nature_ 509, 119–122 (2014) Article CAS ADS Google Scholar * Nikas, S. P. et al. A concise methodology for the synthesis of (−)-Δ9-tetrahydrocannabinol and
(−)-Δ9-tetrahydrocannabivarin metabolites and their regiospecifically deuterated analogs. _Tetrahedron_ 63, 8112–8113 (2007) Article CAS Google Scholar * Kulkarni, S. et al. Novel
C-ring-hydroxy-substituted controlled deactivation cannabinergic analogues. _J. Med. Chem._ 59, 6903–6919 (2016) Article CAS Google Scholar * D’Antona, A. M., Ahn, K. H. & Kendall, D.
A. Mutations of CB1 T210 produce active and inactive receptor forms: correlations with ligand affinity, receptor stability, and cellular localization. _Biochemistry_ 45, 5606–5617 (2006)
Article Google Scholar * Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. _Nat. Protocols_ 4, 706–731 (2009) Article CAS Google Scholar *
Cherezov, V. et al. Rastering strategy for screening and centring of microcrystal samples of human membrane proteins with a sub-10 microm size X-ray synchrotron beam. _J. R. Soc. Interface_
6 (suppl. 5), S587–S597 (2009) Article CAS Google Scholar * Chun, E. et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. _Structure_
20, 967–976 (2012) Article CAS Google Scholar * Kabsch, W. Xds. _Acta Crystallogr. D_ 66, 125–132 (2010) Article CAS Google Scholar * McCoy, A. J. et al. Phaser crystallographic
software. _J. Appl. Crystallogr._ 40, 658–674 (2007) Article CAS Google Scholar * Adams, P. D . et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution.
_Acta Crystallogr. D_ 66, 213–221 (2010) Article CAS Google Scholar * Smart, O. S. et al. Exploiting structure similarity in refinement: automated NCS and target-structure restraints in
BUSTER. _Acta Crystallogr. D_ 68, 368–380 (2012) Article CAS Google Scholar * Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. _Acta Crystallogr.
D_ 66, 486–501 (2010) Article CAS Google Scholar * Friesner, R. A . et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. _J.
Med. Chem._ 47, 1739–1749 (2004) Article CAS Google Scholar * Friesner, R. A. et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for
protein-ligand complexes. _J. Med. Chem._ 49, 6177–6196 (2006) Article CAS Google Scholar * Halgren, T. A . et al. Glide: a new approach for rapid, accurate docking and scoring. 2.
Enrichment factors in database screening. _J. Med. Chem._ 47, 1750–1759 (2004) Article CAS Google Scholar * Abraham, M. J. et al. GROMACS: High performance molecular simulations through
multi-level parallelism from laptops to supercomputers. _SoftwareX_ 1–2, 19–25 (2015) Article ADS Google Scholar * Pettersen, E. F. et al. UCSF Chimera—a visualization system for
exploratory research and analysis. _J. Comput. Chem._ 25, 1605–1612 (2004) Article CAS Google Scholar * Sousa da Silva, A. W. & Vranken, W. F. ACPYPE – AnteChamber PYthon Parser
interfacE. _BMC Res. Notes_ 5, 367 (2012) Article Google Scholar * Berman, H. M. et al. The Protein Data Bank. _Nucleic Acids Res._ 28, 235–242 (2000) Article CAS ADS Google Scholar *
Skjærven, L., Yao, X. Q., Scarabelli, G. & Grant, B. J. Integrating protein structural dynamics and evolutionary analysis with Bio3D. _BMC Bioinformatics_ 15, 399 (2014) Article Google
Scholar Download references ACKNOWLEDGEMENTS This work was supported by the NSF of China grant 31330019 (Z.-J.L.), the MOST of China grants 2014CB910400 (Z.-J.L.) and 2015CB910104
(Z.-J.L.), NSF of Shanghai 16ZR1448500 grant (S.Z.), Key R&D Program of China grant 2016YCF0905902 (S.Z.), NIH grants R01DA041435 (R.C.S., A.M.), P01DA009158 (A.M., L.M.B.), R37DA023142
(A.M.), NSF grants, Shanghai Municipal Government, ShanghaiTech University and GPCR Consortium. The diffraction data were collected at GM/CA@APS of Argonne National Laboratory, X06SA@SLS of
the Paul Scherrer Insitute, and BL41XU@Spring-8 with JASRI proposals 2015B1031 and 2016A2731. We thank M. Wang, C.-Y. Huang, V. Olieric, M. Audet and M.-Y. Lee for their help with data
collection, A. Walker for critical review of the manuscript, and F. Sun for high-resolution mass spectrometry analysis. AUTHOR INFORMATION Author notes * Kiran Vemuri and Spyros P. Nikas:
These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * iHuman Institute, ShanghaiTech University, Shanghai, 201210, China Tian Hua, Yiran Wu, Lu Qu, Mengchen Pu, Kang
Ding, Suwen Zhao, Raymond C. Stevens & Zhi-Jie Liu * National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, 100101, China Tian Hua, Lu
Qu & Zhi-Jie Liu * University of Chinese Academy of Sciences, Beijing, 100049, China Tian Hua, Lu Qu & Kang Ding * Center for Drug Discovery, Department of Pharmaceutical Sciences,
Kiran Vemuri, Spyros P. Nikas, Anisha Korde, Shan Jiang & Alexandros Makriyannis * Department of Chemistry and Chemical Biology, Northeastern University, Boston, 02115, Massachusetts,
USA Kiran Vemuri, Spyros P. Nikas, Anisha Korde, Shan Jiang & Alexandros Makriyannis * Departments of Molecular Medicine and Neuroscience, The Scripps Research Institute, Jupiter, 33458,
Florida, USA Robert B. Laprairie, Jo-Hao Ho & Laura M. Bohn * Departments of Biological Sciences and Chemistry, Bridge Institute, University of Southern California, Los Angeles, 90089,
California, USA Gye Won Han & Raymond C. Stevens * School of Life Science and Technology, ShanghaiTech University, Shanghai, 201210, China Kang Ding, Suwen Zhao, Raymond C. Stevens &
Zhi-Jie Liu * Complex Systems Division, Beijing Computational Science Research Center, Beijing, 100193, China Xuanxuan Li & Haiguang Liu * GPCR Consortium, San Marcos, 92078,
California, USA Michael A. Hanson Authors * Tian Hua View author publications You can also search for this author inPubMed Google Scholar * Kiran Vemuri View author publications You can also
search for this author inPubMed Google Scholar * Spyros P. Nikas View author publications You can also search for this author inPubMed Google Scholar * Robert B. Laprairie View author
publications You can also search for this author inPubMed Google Scholar * Yiran Wu View author publications You can also search for this author inPubMed Google Scholar * Lu Qu View author
publications You can also search for this author inPubMed Google Scholar * Mengchen Pu View author publications You can also search for this author inPubMed Google Scholar * Anisha Korde
View author publications You can also search for this author inPubMed Google Scholar * Shan Jiang View author publications You can also search for this author inPubMed Google Scholar *
Jo-Hao Ho View author publications You can also search for this author inPubMed Google Scholar * Gye Won Han View author publications You can also search for this author inPubMed Google
Scholar * Kang Ding View author publications You can also search for this author inPubMed Google Scholar * Xuanxuan Li View author publications You can also search for this author inPubMed
Google Scholar * Haiguang Liu View author publications You can also search for this author inPubMed Google Scholar * Michael A. Hanson View author publications You can also search for this
author inPubMed Google Scholar * Suwen Zhao View author publications You can also search for this author inPubMed Google Scholar * Laura M. Bohn View author publications You can also search
for this author inPubMed Google Scholar * Alexandros Makriyannis View author publications You can also search for this author inPubMed Google Scholar * Raymond C. Stevens View author
publications You can also search for this author inPubMed Google Scholar * Zhi-Jie Liu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
T.H.: crystallization, data collection, structure determination and analysis; K.V., S.P.N., S.J.: design, synthesis and characterization of ligands; Y.W.: docking, molecular dynamics
simulation; L.Q., M.P.: data collection and processing, structure refinement; G.W.H., M.A.H.: structure refinement and data analysis. R.B.L. and J.-H.H.: functional studies, mutations; A.K.:
radioligand binding assays; K.D.: structure analysis; X.L. and H.L.: molecular dynamics simulations; S.Z.: supervision of structure and simulation analysis; L.M.B.: design and supervision
of functional and kinetic studies; A.M.: supervision on agonist conceptual design, synthesis and characterization; R.C.S.: project conception, data analysis supervision; Z.J.L.: design and
supervision of experiments, data analysis; Z.J.L., T.H., R.C.S., A.M., L.M.B. and S.Z. wrote the manuscript with discussions and improvements from M.A.H., K.V., S.P.N. and Y.W. CORRESPONDING
AUTHORS Correspondence to Suwen Zhao, Laura M. Bohn, Alexandros Makriyannis or Zhi-Jie Liu. ETHICS DECLARATIONS COMPETING INTERESTS A.M. is a founder of MAKScientific, LLC. R.C.S. is a
board member and shareholder with Birdrock Bio. The remaining authors declare no competing financial interests. ADDITIONAL INFORMATION REVIEWER INFORMATION _Nature_ thanks G. Kunos and the
other anonymous reviewer(s) for their contribution to the peer review of this work. Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published
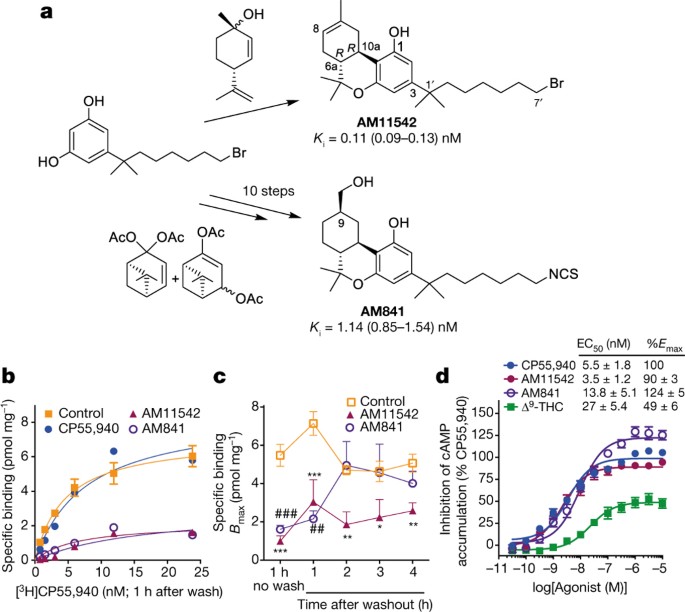
maps and institutional affiliations. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 SYNTHESIS OF AM841 AND AM11542. Reagents and conditions: (a) CH3I, NaH, DMF, 0 °C to room
temperature, 2 h, 95%; (b) DIBAL-H, CH2Cl2, −78 °C, 0.5 h, 87%; (c) Br− P+Ph3(CH2)5OPh, (Me3Si)2NK, THF, 0–10 °C, 30 min, then addition to 3, 0 °C to room temperature, 2 h, 96%; (d) H2, 10%
Pd/C, AcOEt, room temperature, 2.5 h, 89%; (e) BBr3, CH2Cl2, −78 °C to room temperature, 6 h, 85%; (f) diacetates, _p_-TSA, CHCl3, 0 °C to room temperature, 4 days, 64%; (g) TMSOTf,
CH2Cl2/MeNO2, 0 °C to room temperature, 3 h, 71%; (h) TBDMSCl, imidazole, DMAP, DMF, room temperature, 12 h, 85%; (i) Cl− Ph3P+CH2OMe, (Me3Si)2NK, THF, 0 °C to room temperature, 1 h, then
addition to 9, 0 °C to room temperature, 1.5 h, 73%; (j) Cl3CCOOH, CH2Cl2, room temperature, 50 min, 95%; (k) K2CO3, EtOH, room temperature, 3 h, 84%; (l) NaBH4, EtOH, 0 °C, 30 min, 98%; (m)
TBAF, THF, −40 °C, 30 min, 96%; (n) TMG-N3, CHCl3/MeNO2, room temperature, 18 h, 84%; (o) PPh3, CS2, THF, room temperature, 10 h, 76%; (p) (+)-_cis_/_trans_-_p_-mentha-2,8-dien-1-ol,
_p_-TSA, benzene, reflux 4 h, 65%. EXTENDED DATA FIGURE 2 ANALYTICAL SIZE EXCLUSION CHROMATOGRAPHY PROFILE AND CRYSTALS OF CB1–AM11542/AM841 COMPLEX. A, Analytical size exclusion
chromatography and crystal image of the CB1–AM11542 complex. Scale bar, 70 μm. B, Analytical size exclusion chromatography and crystal image of the CB1–AM841 complex. Scale bar, 70 μm. C,
The overall structures of CB1–AM11542 and CB1–AM841 complexes and crystal packing of CB1–AM11542; receptor is in orange (AM11542)/green (AM841) colour and the flavodoxin fusion protein is in
purple-blue colour. The agonists AM11542 (yellow) and AM841 (pink) are shown in sticks representation. The four single mutations T2103.46A, E2735.37K, T2835.47V and R3406.32E are shown as
green spheres in the CB1–AM11542 structure. EXTENDED DATA FIGURE 3 REPRESENTATIVE ELECTRON DENSITY OF THE CB1 AGONISTS-BOUND STRUCTURES AND CHOLESTEROL BINDING SITES. A, The |_F_o| − |_F_c|
omit maps of AM11542 and AM841 contoured at 3.0_σ_ at 2.80 Å and 2.95 Å, respectively. B, The cholesterol binding site in the CB1–AM11542 structure (orange) with CB1–AM6538 structure (blue)
superposed. EXTENDED DATA FIGURE 4 MUTATIONS OF THE CB1 RECEPTOR AND THE EFFECTS ON AGONIST-INDUCED ACTIVITY AS ASSESSED BY THE FORSKOLIN-STIMULATED ACCUMULATION OF CAMP. A, Primers used to
generate mutations in 3×HA–CB1 and validation of cell-surface expression of wild-type and mutant CB1 in CHO-K1 cell lines quantitative flow cytometry. B, Dose response studies of agonist
(AM11542, AM841 and CP55,940) activity for each mutant compared to wild type (in blue filled circles) from Fig. 3c. C, Assessment of the effect of the individual point mutations that were
made to stabilize the receptor, in absence of the flavodoxin insert, on receptor activity. All experiments were repeated at least three times, and error bars denote s.e.m. of duplicate
measurements (parameters are in Extended Data Table 2). EXTENDED DATA FIGURE 5 DOCKING POSES OF DIFFERENT CANNABINOID RECEPTOR AGONISTS AND MD VALIDATION. A–F, The r.m.s.d. values of ligand
heavy atoms show that the docked poses are stable during the 1 μs molecular dynamics simulations: Δ9-THC (A), AEA (B), JWH-018 (C), HU-210 (D), 2-AG (E), WIN 55,212-2 (F). G, H, J, K, The
poses of HU-210 (G), JWH-018 (H), 2-AG (J) and WIN 55,212-2 (K) are shown. I, The superimposition of HU-210 (yellow sticks) and HU-211 (blue sticks) in the binding pocket. The binding pose
of HU-210 explains why HU-211, the enantiomer of HU-210, failed to stimulate CB1 because superimposed HU-211 on HU-210 shows severe clashes with H1782.65 in CB1. EXTENDED DATA FIGURE 6
STRUCTURAL CONFORMATION CHANGES OF SOLVED AGONIST- AND ANTAGONIST-BOUND CLASS A GPCRS. A, The pattern of r.m.s.d. values of transmembrane helices between agonist- and antagonist-bound class
A GPCR structures. The structures used for analysis are the same as described in Extended Data Table 3. B, Measurement of the degree of helix VI bending observed in class A GPCRs structures.
All structures were superimposed onto inactive-state β2-adrenergic receptor by UCSF Chimera. The direction of helices VI were defined by vectors _Η__I_ which starts from the centre of Cα of
residues 6.45–6.48 to the centre of Cα of residues 6.29-30–6.32-33. The two vectors _Η__0_ and _Η__1_ of helices VI in the inactive-state and active-state β2-adrenergic receptor were
selected as reference to form a plane α. The vector _Η__I_ of helix VI of other structure was projected to the plane α as a new vector _Η__I__′_. The bending angle of each helix VI was then
defined by the angle between _Η__I′_ and _Η__0_. The structures are: ETB (PDB code 5GLH), β1-adrenergic receptor (PDB code 2Y02), P2Y12 (PDB code 4PXZ), β2-adrenergic receptor (PDB code
3PDS), FFA1 (PDB code 4PHU), 5HT2B (PDB code 4IB4), 5HT1B (PDB code 4IAR), Rho (PDB code 2HPY), A2A (PDB code 3QAK), NTS1 (PDB code 4BUO), CB1 (bound to AM11542; PDB code 5XRA), μ-opioid
receptor + nanobody (NB) (PDB code 5C1M), Rho + NB (PDB code 2X72), Rho + arrestin (PDB code 4ZWJ), M2R + NB (PDB code 4MQS), β2-adrenergic receptor + NB (PDB code 4LDL), A2A + mini-Gs (PDB
code 5G53), β2-adrenergic receptor + Gs (PDB code 3SN6). SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains Supplementary Figures 1-2. (PDF 1249 kb) POWERPOINT SLIDES
POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Hua, T., Vemuri, K., Nikas, S. _et al._ Crystal structures of agonist-bound human cannabinoid receptor CB1. _Nature_ 547, 468–471 (2017). https://doi.org/10.1038/nature23272 Download
citation * Received: 06 April 2017 * Accepted: 15 June 2017 * Published: 05 July 2017 * Issue Date: 27 July 2017 * DOI: https://doi.org/10.1038/nature23272 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative