Play all audios:
ABSTRACT Regulated activation of integrins is critical for cell adhesion, motility and tissue homeostasis. Talin and kindlins activate β1-integrins, but the counteracting inhibiting
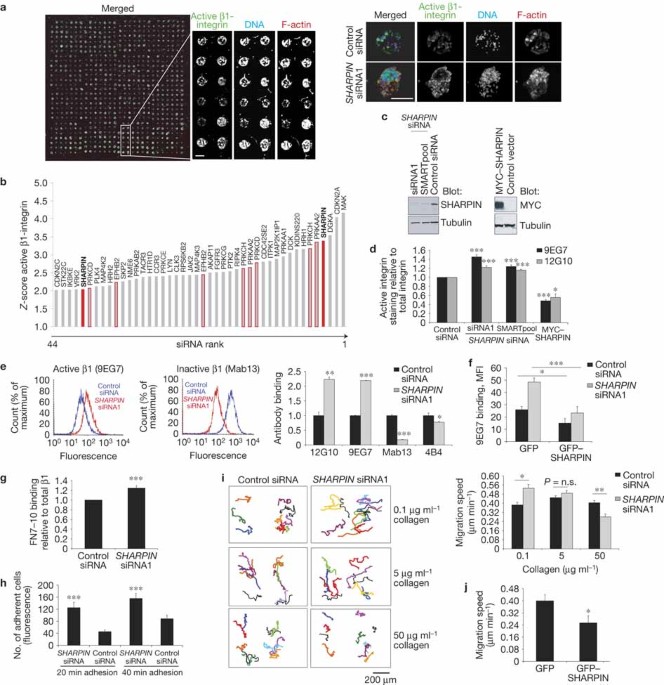
mechanisms are poorly defined. We identified SHARPIN as an important inactivator of β1-integrins in an RNAi screen. SHARPIN inhibited β1-integrin functions in human cancer cells and primary
leukocytes. Fibroblasts, leukocytes and keratinocytes from SHARPIN-deficient mice exhibited increased β1-integrin activity, which was fully rescued by re-expression of SHARPIN. We found that
SHARPIN directly binds to a conserved cytoplasmic region of integrin α-subunits and inhibits recruitment of talin and kindlin to the integrin. Therefore, SHARPIN inhibits the critical
switching of β1-integrins from inactive to active conformations. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution
ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article *
Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn
about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS TALIN AND KINDLIN USE INTEGRIN TAIL ALLOSTERY AND DIRECT BINDING TO
ACTIVATE INTEGRINS Article Open access 12 December 2023 MECHANISM OF INTEGRIN ACTIVATION BY TALIN AND ITS COOPERATION WITH KINDLIN Article Open access 29 April 2022 MIRNA-200C-3P TARGETS
TALIN-1 TO REGULATE INTEGRIN-MEDIATED CELL ADHESION Article Open access 03 November 2021 REFERENCES * Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. _Cell_ 110,
673–687 (2002). Article CAS Google Scholar * Moser, M., Legate, K. R., Zent, R. & Fassler, R. The tail of integrins, talin, and kindlins. _Science_ 324, 895–899 (2009). Article CAS
Google Scholar * Shattil, S. J., Kim, C. & Ginsberg, M. H. The final steps of integrin activation: the end game. _Nat. Rev. Mol. Cell Biol._ 11, 288–300 (2010). Article CAS Google
Scholar * Larjava, H., Plow, E. F. & Wu, C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. _EMBO Rep._ 9, 1203–1208 (2008). Article CAS Google Scholar
* Margadant, C., Charafeddine, R. A. & Sonnenberg, A. Unique and redundant functions of integrins in the epidermis. _FASEB J._ 24, 4133–4152 (2010). Article CAS Google Scholar *
Cantor, J. M., Ginsberg, M. H. & Rose, D. M. Integrin-associated proteins as potential therapeutic targets. _Immunol. Rev._ 223, 236–251 (2008). Article CAS Google Scholar * Hogg, N.
& Bates, P. A. Genetic analysis of integrin function in man: LAD-1 and other syndromes. _Matrix Biol._ 19, 211–222 (2000). Article CAS Google Scholar * Evans, R. et al. Integrins in
immunity. _J. Cell Sci._ 122, 215–225 (2009). Article CAS Google Scholar * Calderwood, D. A. Integrin activation. _J. Cell Sci._ 117, 657–666 (2004). Article CAS Google Scholar * Lim,
S. et al. Sharpin, a novel postsynaptic density protein that directly interacts with the shank family of proteins. _Mol. Cell. Neurosci._ 17, 385–397 (2001). Article CAS Google Scholar *
Byron, A. et al. Anti-integrin monoclonal antibodies. _J. Cell Sci._ 122, 4009–4011 (2009). Article CAS Google Scholar * Taliaferro-Smith, L. et al. LKB1 is required for
adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. _Oncogene_ 28, 2621–2633 (2009). Article CAS Google Scholar * Ewart, M.
A., Kohlhaas, C. F. & Salt, I. P. Inhibition of tumor necrosis factor α-stimulated monocyte adhesion to human aortic endothelial cells by AMP-activated protein kinase. _Arterioscler.
Thromb. Vasc. Biol._ 28, 2255–2257 (2008). Article CAS Google Scholar * Guo, D. L. et al. Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumours.
_Carcinogenesis_ 27, 454–464 (2006). Article CAS Google Scholar * Huusko, P. et al. Nonsense-mediated decay microarray analysis identifies mutations of EPHB2 in human prostate cancer.
_Nat. Genet._ 36, 979–983 (2004). Article CAS Google Scholar * Zou, J. X. et al. An Eph receptor regulates integrin activity through R-Ras. _Proc. Natl Acad. Sci. USA_ 96, 13813–13818
(1999). Article CAS Google Scholar * Ivaska, J. et al. Integrin–protein kinase C relationships. _Biochem. Soc. Trans._ 31, 90–93 (2003). Article CAS Google Scholar * Harper, M. T.
& Poole, A. W. Diverse functions of protein kinase C isoforms in platelet activation and thrombus formation. _J. Thromb. Haemost._ 8, 454–462 (2010). Article CAS Google Scholar *
Jung, J. et al. Newly identified tumor-associated role of human Sharpin. _Mol. Cell. Biochem._ 340, 161–167 (2010). Article CAS Google Scholar * Calderwood, D. A. et al. The
phosphotyrosine binding-like domain of talin activates integrins. _J. Biol. Chem._ 277, 21749–21758 (2002). Article CAS Google Scholar * Schwartz, M. A. & Assoian, R. K. Integrins and
cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. _J. Cell Sci._ 114, 2553–2560 (2001). CAS PubMed Google Scholar * Moser, M., Nieswandt, B.,
Ussar, S., Pozgajova, M. & Fassler, R. Kindlin-3 is essential for integrin activation and platelet aggregation. _Nat. Med._ 14, 325–330 (2008). Article CAS Google Scholar * Palecek,
S. P., Loftus, J. C., Ginsberg, M. H., Lauffenburger, D. A. & Horwitz, A. F. Integrin-ligand binding properties govern cell migration speed through cell–substratum adhesiveness. _Nature_
385, 537–540 (1997). Article CAS Google Scholar * Kiema, T. et al. The molecular basis of filamin binding to integrins and competition with talin. _Mol. Cell_ 21, 337–347 (2006). Article
CAS Google Scholar * Anthis, N. J. et al. β integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. _J. Biol. Chem._ 284, 36700–36710
(2009). Article CAS Google Scholar * Elliott, P. R. et al. The structure of the talin head reveals a novel extended conformation of the FERM domain. _Structure_ 18, 1289–1299 (2010).
Article CAS Google Scholar * Hughes, P. E. et al. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. _J. Biol. Chem._ 271, 6571–6574 (1996).
Article CAS Google Scholar * LaFlamme, S. E., Akiyama, S. K. & Yamada, K. M. Regulation of fibronectin receptor distribution. _J. Cell Biol._ 117, 437–447 (1992). Article CAS Google
Scholar * Chen, Y. P. et al. ‘Inside-out’ signal transduction inhibited by isolated integrin cytoplasmic domains. _J. Biol. Chem._ 269, 18307–18310 (1994). CAS PubMed Google Scholar *
Parsons, M., Messent, A. J., Humphries, J. D., Deakin, N. O. & Humphries, M. J. Quantification of integrin receptor agonism by fluorescence lifetime imaging. _J. Cell Sci._ 121, 265–271
(2008). Article CAS Google Scholar * Tadokoro, S. et al. Talin binding to integrin β tails: a final common step in integrin activation. _Science_ 302, 103–106 (2003). Article CAS Google
Scholar * Montanez, E. et al. Kindlin-2 controls bidirectional signaling of integrins. _Genes Dev._ 22, 1325–1330 (2008). Article CAS Google Scholar * Harburger, D. S., Bouaouina, M.
& Calderwood, D. A. Kindlin-1 and -2 directly bind the C-terminal region of β integrin cytoplasmic tails and exert integrin-specific activation effects. _J. Biol. Chem._ 284, 11485–11497
(2009). Article CAS Google Scholar * Ma, Y. Q., Qin, J., Wu, C. & Plow, E. F. Kindlin-2 (Mig-2): a co-activator of β3 integrins. _J. Cell Biol._ 181, 439–446 (2008). Article CAS
Google Scholar * Gerlach, B. et al. Linear ubiquitination prevents inflammation and regulates immune signalling. _Nature_ 471, 591–596 (2011). Article CAS Google Scholar * Ikeda, F. et
al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-_κ_B activity and apoptosis. _Nature_ 471, 637–641 (2011). Article CAS Google Scholar * Tokunaga, F. et al. SHARPIN is a
component of the NF-_κ_B-activating linear ubiquitin chain assembly complex. _Nature_ 471, 633–636 (2011). Article CAS Google Scholar * Soderberg, O. et al. Direct observation of
individual endogenous protein complexes _in situ_ by proximity ligation. _Nat. Methods_ 3, 995–1000 (2006). Article Google Scholar * Tuomi, S. et al. PKCε regulation of an α5 integrin-ZO-1
complex controls lamellae formation in migrating cancer cells. _Sci. Signal._ 2, ra32 (2009). Article Google Scholar * Askari, J. A. et al. Focal adhesions are sites of integrin
extension. _J. Cell Biol._ 188, 891–903 (2010). Article CAS Google Scholar * Liang, Y., Seymour, R. E. & Sundberg, J. P. Inhibition of NF-κB signaling retards eosinophilic dermatitis
in SHARPIN-deficient mice. _J. Invest. Dermatol._ 131, 141–149 (2011). Article CAS Google Scholar * Seymour, R. E. et al. Spontaneous mutations in the mouse Sharpin gene result in
multiorgan inflammation, immune system dysregulation and dermatitis. _Genes Immun._ 8, 416–421 (2007). Article CAS Google Scholar * Tokunaga, F. et al. Involvement of linear
polyubiquitylation of NEMO in NF-κB activation. _Nat. Cell Biol._ 11, 123–132 (2009). Article CAS Google Scholar * Wegener, K. L. et al. Structural basis of integrin activation by talin.
_Cell_ 128, 171–182 (2007). Article CAS Google Scholar * Millon-Fremillon, A. et al. Cell adaptive response to extracellular matrix density is controlled by ICAP-1-dependent β1-integrin
affinity. _J. Cell Biol._ 180, 427–441 (2008). Article CAS Google Scholar * Nevo, J. et al. Mammary-derived growth inhibitor (MDGI) interacts with integrin α-subunits and suppresses
integrin activity and invasion. _Oncogene_ 29, 6452–6463 (2010). Article CAS Google Scholar * Clark, A. J., Neil, C., Gusterson, B., McWhir, J. & Binas, B. Deletion of the gene
encoding H-FABP/MDGI has no overt effects in the mammary gland. _Transgenic Res._ 9, 439–444 (2000). Article CAS Google Scholar * Legate, K. R., Wickstrom, S. A. & Fassler, R. Genetic
and cell biological analysis of integrin outside-in signaling. _Genes Dev._ 23, 397–418 (2009). Article CAS Google Scholar * He, L., Ingram, A., Rybak, A. P. & Tang, D.
Shank-interacting protein-like 1 promotes tumorigenesis via PTEN inhibition in human tumor cells. _J. Clin. Invest._ 120, 2094–2108 (2010). Article CAS Google Scholar * Landgraf, K. et
al. Sipl1 and Rbck1 are novel Eya1-binding proteins with a role in craniofacial development. _Mol. Cell. Biol._ 30, 5764–5775 (2010). Article CAS Google Scholar * Gustin, J. A., Maehama,
T., Dixon, J. E. & Donner, D. B. The PTEN tumor suppressor protein inhibits tumor necrosis factor-induced nuclear factor κB activity. _J. Biol. Chem._ 276, 27740–27744 (2001). Article
CAS Google Scholar * Kilpinen, S. et al. Systematic bioinformatic analysis of expression levels of 17,330 human genes across 9,783 samples from 175 types of healthy and pathological
tissues. _Genome Biol._ 9, R139 (2008). Article Google Scholar * O’Toole, T. E. et al. Modulation of the affinity of integrin α IIb β3 (GPIIb-IIIa) by the cytoplasmic domain of α IIb.
_Science_ 254, 845–847 (1991). Article Google Scholar * Rantala, J. K. et al. A cell spot microarray method for production of high density siRNA transfection microarrays. _BMC Genomics_
12, 162 (2011). Article CAS Google Scholar * Kraemer, A. et al. Dynamic interaction of cAMP with the Rap guanine-nucleotide exchange factor Epac1. _J. Mol. Biol._ 306, 1167–1177 (2001).
Article CAS Google Scholar * Clark, K. et al. A specific α5β1-integrin conformation promotes directionalintegrin translocation and fibronectin matrix formation. _J. Cell Sci._ 118,
291–300 (2005). Article CAS Google Scholar * Hogan, B. _Manipulating the Mouse Embryo: A Laboratory Manual_ (Cold Spring Harbor Laboratory Press, 1986). Google Scholar * Laukaitis, C.
M., Webb, D. J., Donais, K. & Horwitz, A. F. Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. _J. Cell
Biol._ 153, 1427–1440 (2001). Article CAS Google Scholar * Caswell, P. T. et al. Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. _Dev. Cell_ 13,
496–510 (2007). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank H. Marttila, J. Siivonen, L. Lahtinen, R. Kaukonen, E. Väänänen, K. Silva and A. Arola for
technical assistance. P.R. Elliot is acknowledged for the recombinant talin 1–400 protein. R. Fässler, M. Ginsberg, J. Norman and S. Lee are acknowledged for the plasmids. This study has
been supported by the Academy of Finland, EU-FP06 project ENLIGHT, a European Research Council Starting Grant, the Sigrid Juselius Foundation, the European Molecular Biology Organization
(EMBO) Young Investigator Programme and Finnish Cancer Organizations. J.P. and E.M., Academy of Finland postdoc grant; T.P., Turku Graduate School of Biomedical Sciences; S.V., Alexander von
Humbold foundation and an EMBO long-term fellowship. C.S.P. and J.P.S were supported by the National Institutes of Health (T32 DK07449-28 to C.S.P. and AR49288 to J.P.S.). AUTHOR
INFORMATION Author notes * Juha K. Rantala and Jeroen Pouwels: These authors contributed equally to this work AUTHORS AND AFFILIATIONS * Medical Biotechnology, VTT Technical Research Centre
of Finland, 20521 Turku, Finland Juha K. Rantala, Jeroen Pouwels, Teijo Pellinen, Stefan Veltel, Petra Laasola, Elina Mattila, Olli Kallioniemi & Johanna Ivaska * Centre for
Biotechnology, University of Turku, 20520 Turku, Finland Jeroen Pouwels, Teijo Pellinen, Stefan Veltel, Elina Mattila & Johanna Ivaska * The Jackson Laboratory, Bar Harbor, Maine 04609,
USA Christopher S. Potter, Ted Duffy & John P. Sundberg * Institute for Molecular Medicine Finland (FIMM), Biomedicum 2U, University of Helsinki, 00014 University of Helsinki, Helsinki,
Finland Olli Kallioniemi * Wellcome Trust Centre for Cell-Matrix Research, Faculty of Life Sciences, University of Manchester, Manchester M13 9PT, UK Janet A. Askari & Martin J.
Humphries * Randall Division of Cell and Molecular Biophysics, King’s College London Guy’s Campus, London SE1 1UL, UK Maddy Parsons * MediCity Research Laboratory and Department of Medical
Biochemistry and Genetics, University of Turku, and National Institute for Health and Welfare, FIN-20520 Turku, Finland Marko Salmi * Department of Biochemistry and Food Chemistry,
University of Turku, 20520 Turku, Finland Johanna Ivaska Authors * Juha K. Rantala View author publications You can also search for this author inPubMed Google Scholar * Jeroen Pouwels View
author publications You can also search for this author inPubMed Google Scholar * Teijo Pellinen View author publications You can also search for this author inPubMed Google Scholar * Stefan
Veltel View author publications You can also search for this author inPubMed Google Scholar * Petra Laasola View author publications You can also search for this author inPubMed Google
Scholar * Elina Mattila View author publications You can also search for this author inPubMed Google Scholar * Christopher S. Potter View author publications You can also search for this
author inPubMed Google Scholar * Ted Duffy View author publications You can also search for this author inPubMed Google Scholar * John P. Sundberg View author publications You can also
search for this author inPubMed Google Scholar * Olli Kallioniemi View author publications You can also search for this author inPubMed Google Scholar * Janet A. Askari View author
publications You can also search for this author inPubMed Google Scholar * Martin J. Humphries View author publications You can also search for this author inPubMed Google Scholar * Maddy
Parsons View author publications You can also search for this author inPubMed Google Scholar * Marko Salmi View author publications You can also search for this author inPubMed Google
Scholar * Johanna Ivaska View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.K.R. and O.K. developed cell spot microarrays, J.K.R. and T.P.
carried out the screen. J.K.R., J.P., P.L., S.V. and J.I. carried out the experiments. E.M. immortalized the MEFs. M.P. carried out the FRET-FLIM, C.S.P, T.D. and J.P.S. contributed to the
mouse data, J.A.A. and M.J.H. contributed to the legs together integrin experiments and M.S. contributed to the leukocyte work. J.P., J.I. and M.S. wrote the manuscript. CORRESPONDING AUTHOR
Correspondence to Johanna Ivaska. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
Supplementary Information (PDF 694 kb) SUPPLEMENTARY TABLE 1 Supplementary Information (XLS 31 kb) SUPPLEMENTARY TABLE 2 Supplementary Information (XLS 32 kb) RIGHTS AND PERMISSIONS Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Rantala, J., Pouwels, J., Pellinen, T. _et al._ SHARPIN is an endogenous inhibitor of β1-integrin activation. _Nat Cell Biol_ 13,
1315–1324 (2011). https://doi.org/10.1038/ncb2340 Download citation * Received: 18 May 2011 * Accepted: 09 August 2011 * Published: 25 September 2011 * Issue Date: November 2011 * DOI:
https://doi.org/10.1038/ncb2340 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative