Play all audios:
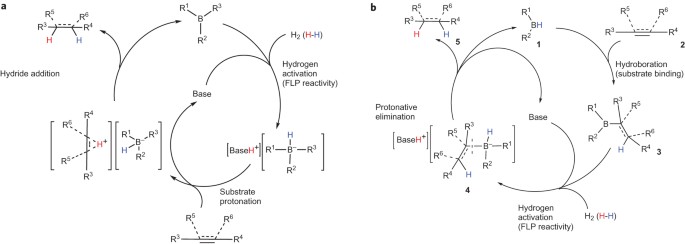
ABSTRACT Frustrated Lewis pairs are compounds containing both Lewis acidic and Lewis basic moieties, where the formation of an adduct is prevented by steric hindrance. They are therefore
highly reactive, and have been shown to be capable of heterolysis of molecular hydrogen, a property that has led to their use in hydrogenation reactions of polarized multiple bonds. Here, we
describe a general approach to the hydrogenation of alkynes to _cis_-alkenes under mild conditions using the unique _ansa_-aminohydroborane as a catalyst. Our approach combines several
reactions as the elementary steps of the catalytic cycle: hydroboration (substrate binding), heterolytic hydrogen splitting (typical frustrated-Lewis-pair reactivity) and facile
intramolecular protodeborylation (product release). The mechanism is verified by experimental and computational studies. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only
$21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS REGIOSELECTIVE ALIPHATIC C–H
FUNCTIONALIZATION USING FRUSTRATED RADICAL PAIRS Article 05 July 2023 COOPERATIVE TRIPLE CATALYSIS ENABLES REGIOIRREGULAR FORMAL MIZOROKI–HECK REACTIONS Article Open access 13 July 2022
REGIOIRREGULAR AND CATALYTIC MIZOROKI–HECK REACTIONS Article 15 April 2021 REFERENCES * Welch, G. C., San Juan, R. R., Masuda, J. D. & Stephan, D. W. Reversible, metal-free hydrogen
activation. _Science_ 314, 1124–1126 (2006). CAS PubMed Google Scholar * Stephan, D. W. ‘Frustrated Lewis pairs’: a concept for new reactivity and catalysis. _Org. Biomol. Chem._ 6,
1535–1539 (2008). CAS PubMed Google Scholar * Stephan, D. W. & Erker, G. Frustrated Lewis pairs: metal-free hydrogen activation and more. _Angew. Chem. Int. Ed._ 49, 46–76 (2010). CAS
Google Scholar * Stephan, D. W. et al. Metal-free catalytic hydrogenation of polar substrates by frustrated Lewis pairs. _Inorg. Chem._ 50, 12338–12348 (2011). CAS PubMed Google Scholar
* Stephan, D. W. ‘Frustrated Lewis pair’ hydrogenations. _Org. Biomol. Chem._ 10, 5740–5746 (2012). CAS PubMed Google Scholar * Sumerin, V. et al. Highly active metal-free catalysts for
hydrogenation of unsaturated nitrogen-containing compounds. _Adv. Synth. Catal._ 353, 2093–2110 (2011). CAS Google Scholar * Eros, G. et al. Expanding the scope of metal-free catalytic
hydrogenation through frustrated Lewis pair design. _Angew. Chem. Int. Ed._ 49, 6559–6563 (2010). CAS Google Scholar * Xu, B-H. et al. Reaction of frustrated Lewis pairs with conjugated
ynones-selective hydrogenation of the carbon–carbon triple bond. _Angew. Chem. Int. Ed._ 50, 7183–7186 (2011). CAS Google Scholar * Mahdi, T., Heiden, Z. M., Grimme, S. & Stephan, D.
W. Metal-free aromatic hydrogenation: aniline to cyclohexyl-amine derivatives. _J. Am. Chem. Soc._ 134, 4088–4091 (2012). CAS PubMed Google Scholar * Greb, L. et al. Metal-free catalytic
olefin hydrogenation: low-temperature H2 activation by frustrated Lewis pairs. _Angew. Chem. In. Ed._ 51, 10164–10168 (2012). CAS Google Scholar * Köster, R. Neue präparative Möglichkeiten
in der Bor- und Silicium-Chemie. _Angew. Chem._ 68, 383 (1956). Google Scholar * Köster, R., Bruno, G. & Binger, P. Borverbindungen, V Hydrierung von Bortrialkylen und -triarylen.
_Justus Liebigs Ann. Chem._ 644, 1–22 (1961). Google Scholar * DeWitt, E. J., Ramp, F. L. & Trapasso, L. E. Homogeneous hydrogenation catalyzed by boranes. _J. Am. Chem. Soc._ 83, 4672
(1961). CAS Google Scholar * Ramp, F. L., DeWitt, E. J. & Trapasso, L. E. Homogeneous hydrogenation catalyzed by boranes. _J. Org. Chem._ 27, 4368–4372 (1962). CAS Google Scholar *
Yalpani, M. & Köster, R. Partial hydrogenation: from anthracene to coronene. _Chem. Ber._ 123, 719–724 (1990). CAS Google Scholar * Köster, R., Schüßler, W. & Yalpani, M. Reduktion
kondensierter Arene mitBH-Boranen, I Reaktionen von Naphthalin, Anthracen und Phenanthren mit Tetraalkyldiboranen (6). _Chem. Ber._ 122, 677–686 (1989). Google Scholar * Yalpani, M.,
Lunow, T. & Köster, R. Reduction of polycyclic arenes by-boranes, II. Borane catalyzed hydrogenation of naphthalenes to tetralins. _Chem. Ber._ 122, 687–693 (1989). CAS Google Scholar
* Haenel, M. W., Narangerel, J., Richter, U-B. & Rufińska, A. The first liquefaction of high-rank bituminous coals by preceding hydrogenation with homogeneous borane or iodine catalysts.
_Angew. Chem._ 45, 1061–1066 (2006). CAS Google Scholar * Siau, W-Y., Zhang, Y. & Zhao, Y. Stereoselective synthesis of _Z_-alkenes. _Top. Curr. Chem._ 327, 33–58 (2012). CAS PubMed
Google Scholar * Jain, S. C. et al. Polyene pheromone components from an arctiid moth (_Utetheisa ornatrix_): characterization and synthesis. _J. Org. Chem._ 48, 2266–2270 (1983). CAS
Google Scholar * Fürstner, A., Guth, O., Rumbo, A. & Seidel, G. Ring closing alkyne metathesis. Comparative investigation of two different catalyst systems and application to the
stereoselective synthesis of olfactory lactones, azamacrolides, and the macrocyclic perimeter of the marine alkaloid nakadomarin A. _J. Am. Chem. Soc._ 121, 11108–11113 (1999). Google
Scholar * Ghosh, A. K., Wang, Y. & Kim, J. T. Total synthesis of microtubule-stabilizing agent (−)-laulimalide. _J. Org. Chem._ 66, 8973–8982 (2001). CAS PubMed Google Scholar *
Fürstner, A. & Davies, P. W. Alkyne metathesis. _Chem. Commun._ 2307–2320 (2005). * Caggiano, T. J., Siegel, S., King, A. O. & Shinkai, I. _Encyclopedia of Reagents for Organic
Synthesis_ Vol. 6 (ed. Paquette, L. A.) 3694–3869; 3861–3865; 3966–3867 (Wiley, 1995). Google Scholar * Schrock, R. R. & Osborn, J. A. Catalytic hydrogenation using cationic rhodium
complexes. II. The selective hydrogenation of alkynes to _cis_ olefins. _J. Am. Chem. Soc._ 98, 2143–2147 (1976). CAS Google Scholar * Sodeoka, M. & Shibasaki, M. New functions of
(arene)tricarbonylchromium(0) complexes as hydrogenation catalysts: stereospecific semihydrogenation of alkynes and highly chemoselective hydrogenation of αβ-unsaturated carbonyl compounds.
_J. Org. Chem._ 50, 1147–1149 (1985). CAS Google Scholar * Van Laren, M. W. & Elsevier, C. J. Selective homogeneous palladium(0)-catalyzed hydrogenation of alkynes to (_Z_)-alkenes.
_Angew. Chem. Int. Ed._ 38, 3715–3717 (1999). CAS Google Scholar * Radkowski, K., Sundararaju, B. & Fürstner, A. A functional-group-tolerant catalytic _trans_ hydrogenation of alkynes.
_Angew. Chem. Int. Ed._ 52, 355–360 (2013). CAS Google Scholar * Parks, D. J., von H. Spence, R. E. & Piers, W. E. Bis(pentafluorophenyl)borane: synthesis, properties, and
hydroboration chemistry of a highly electrophilic borane reagent. _Angew. Chem. Int. Ed._ 34, 809–811 (1995). CAS Google Scholar * Parks, D. J., Piers, W. E. & Yap, G. P. A. Synthesis,
properties, and hydroboration activity of the highly electrophilic borane bis(pentafluorophenyl)borane, HB(C6F5)2 . _Organometallics_ 17, 5492–5503 (1998). CAS Google Scholar * Parks, D.
& Piers, W. Hydroboration of vinyl silanes with bis-(pentafluoro phenyl)borane: ground state β-silicon effects. _Tetrahedron_ 54, 15469–15488 (1998). CAS Google Scholar * Jiang, C.,
Blacque, O. & Berke, H. Metal-free hydrogen activation by the frustrated Lewis pairs of ClB(C6F5)2 and HB(C6F5)2 and bulky Lewis bases. _Organometallics_ 28, 5233–5239 (2009). CAS
Google Scholar * Jiang, C., Blacque, O., Fox, T. & Berke, H. Reversible, metal-free hydrogen activation by frustrated Lewis pairs. _Dalton Trans._ 40, 1091–1097 (2011). PubMed Google
Scholar * Chernichenko, K., Nieger, M., Leskelä, M. & Repo, T. Hydrogen activation by 2-boryl-_N_,_N_-dialkylanilines: a revision of Piers' ansa-aminoborane. _Dalton Trans._ 41,
9029–9032 (2012). CAS PubMed Google Scholar * Chase, P. A. & Stephan, D. W. Hydrogen and amine activation by a frustrated Lewis pair of a bulky _N_-heterocyclic carbene and B(C6F5)3 .
_Angew. Chem. Int. Ed._ 47, 7433–7437 (2008). CAS Google Scholar * Robertson, A. P. M. et al. Experimental and theoretical studies of the potential interconversion of the amine-borane
_i_Pr2NH·BH(C6F5)2 and the aminoborane _i_Pr2N=B(C6F5)2 involving hydrogen loss and uptake. _Eur. J. Inorg. Chem._ 2011, 5279–5287 (2011). CAS Google Scholar * Erdmann, M. et al.
Functional group chemistry at intramolecular frustrated Lewis pairs: substituent exchange at the Lewis acid site with 9-BBN. _Dalton Trans._ 42, 709–718, (2013). CAS PubMed Google Scholar
* Brown, H. C. _Hydroboration_ (W. A. Benjamin, 1962). Google Scholar * Rokob, T. A., Hamza, A. & Pápai, I. Rationalizing the reactivity of frustrated Lewis pairs: thermodynamics of
H2 activation and the role of acid–base properties. _J. Am. Chem. Soc._ 131, 10701–10710 (2009). CAS PubMed Google Scholar * Dureen, M. A., Brown, C. C. & Stephan, D. W. Deprotonation
and addition reactions of frustrated Lewis pairs with alkynes. _Organometallics_ 29, 6594–6607 (2010). CAS Google Scholar * Dureen, M. A. & Stephan, D. W. Terminal alkyne activation
by frustrated and classical Lewis acid/phosphine pairs. _J. Am. Chem. Soc._ 131, 8396–8397 (2009). CAS PubMed Google Scholar * Jiang, C., Blacque, O. & Berke, H. Activation of
terminal alkynes by frustrated Lewis pairs. _Organometallics_ 29, 125–133 (2010). CAS Google Scholar * Moemming, C. M. et al. Formation of cyclic allenes and cumulenes by cooperative
addition of frustrated Lewis pairs to conjugated enynes and diynes. _Angew. Chem. Int. Ed._ 49, 2414–2417 (2010). CAS Google Scholar * Voss, T. et al. Frustrated Lewis pair behavior of
intermolecular amine/B(C6F5)3 pairs. _Organometallics_ 31, 2367–2378 (2012). CAS Google Scholar * Winkelhaus, D., Neumann, B., Stammler, H-G. & Mitzel, N. W. Intramolecular Lewis
acid–base pairs based on 4-ethynyl-2,6-lutidine. _Dalton Trans._ 41, 9143–9150 (2012). CAS PubMed Google Scholar * Sumerin, V. et al. Amine-borane mediated metal-free hydrogen activation
and catalytic hydrogenation. _Top. Curr. Chem._ 332, 111–155 (2013). CAS PubMed Google Scholar * Chai, J-D. & Head-Gordon, M. Long-range corrected hybrid density functionals with
damped atom–atom dispersion corrections. _Phys. Chem. Chem. Phys._ 10, 6615–6620 (2008). CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors acknowledge financial support
from the Academy of Finland (139550) and the Hungarian Research Foundation (OTKA, grant K-81927) and COST action CM0905 (Organocatalysis). The authors also thank A. Reznichenko for
discussions and corrections during the preparation of the manuscript, M. Lindqvist for corrections and S. Heikkinen for help with NMR measurements. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Chemistry, Laboratory of Inorganic Chemistry, University of Helsinki, PO Box 55, FIN-00014, Finland Konstantin Chernichenko, Martin Nieger, Markku Leskelä &
Timo Repo * Institute of Organic Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences, PO Box 17, H-1525, Budapest, Hungary Ádám Madarász & Imre Pápai Authors *
Konstantin Chernichenko View author publications You can also search for this author inPubMed Google Scholar * Ádám Madarász View author publications You can also search for this author
inPubMed Google Scholar * Imre Pápai View author publications You can also search for this author inPubMed Google Scholar * Martin Nieger View author publications You can also search for
this author inPubMed Google Scholar * Markku Leskelä View author publications You can also search for this author inPubMed Google Scholar * Timo Repo View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS K.C. and T.R. conceived and K.C. carried out the experiments. A.M. and I.P. designed and performed the DFT studies. All authors
discussed and co-wrote the paper. CORRESPONDING AUTHORS Correspondence to Imre Pápai or Timo Repo. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial
interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary information (PDF 6215 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Chernichenko, K., Madarász, Á., Pápai, I. _et al._ A frustrated-Lewis-pair approach to catalytic reduction of alkynes to _cis_-alkenes. _Nature Chem_ 5, 718–723 (2013).
https://doi.org/10.1038/nchem.1693 Download citation * Received: 22 February 2013 * Accepted: 13 May 2013 * Published: 07 July 2013 * Issue Date: August 2013 * DOI:
https://doi.org/10.1038/nchem.1693 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative