Play all audios:
ABSTRACT In animal models, resident memory CD8+ T (Trm) cells assist in respiratory virus elimination but their importance in man has not been determined. Here, using experimental human
respiratory syncytial virus (RSV) infection, we investigate systemic and local virus-specific CD8+ T-cell responses in adult volunteers. Having defined the immunodominance hierarchy, we
analyse phenotype and function longitudinally in blood and by serial bronchoscopy. Despite rapid clinical recovery, we note surprisingly extensive lower airway inflammation with persistent
viral antigen and cellular infiltrates. Pulmonary virus-specific CD8+ T cells display a CD69+CD103+ Trm phenotype and accumulate to strikingly high frequencies into convalescence without
continued proliferation. While these have a more highly differentiated phenotype, they express fewer cytotoxicity markers than in blood. Nevertheless, their abundance before infection
correlates with reduced symptoms and viral load, implying that CD8+ Trm cells in the human lung can confer protection against severe respiratory viral disease when humoral immunity is
overcome. SIMILAR CONTENT BEING VIEWED BY OTHERS UNIQUE PROPERTIES OF TISSUE-RESIDENT MEMORY T CELLS IN THE LUNGS: IMPLICATIONS FOR COVID-19 AND OTHER RESPIRATORY DISEASES Article 09
December 2022 INFLAMMATORY CONDITIONS SHAPE PHENOTYPIC AND FUNCTIONAL CHARACTERISTICS OF LUNG-RESIDENT MEMORY T CELLS IN MICE Article Open access 16 April 2025 PERIPHERAL AND LUNG RESIDENT
MEMORY T CELL RESPONSES AGAINST SARS-COV-2 Article Open access 21 May 2021 INTRODUCTION CD8+ T cells are essential effectors that eliminate intracellular pathogens and confer protection
against symptomatic reinfection via immune memory1. In animal models of respiratory virus infections such as respiratory syncytial virus (RSV) and influenza, memory CD8+ T cells reduce viral
replication, prevent infection or decrease disease severity, and confer cross-protection against antigenically distinct strains2. However, while some vaccine candidates against RSV and
influenza may have the capacity to induce CD8+ T cells, they have not yet been shown clinically to improve protection3,4,5. RSV is globally the commonest cause of lower respiratory tract
infection in children, leading to an estimated 3.4 million hospitalizations each year6. It is also a major contributor to mortality in older and immunosuppressed adults7. Recurrent
symptomatic RSV infection occurs throughout life even with a healthy immune system and limited viral antigenic variation8. Therefore, characterizing immune responses required for robust
protection has been problematic and effective vaccines remain a major clinical need2. We recently showed that anti-RSV IgA in the nasal mucosa correlated strongly with protection from
infection, but that the high levels required for immunity are poorly maintained, allowing recurrent infection9. Despite this, most older children and young adults suffer only minor symptoms,
implying that when antibodies fail to prevent infection, cell-mediated immunity reduces disease severity. In mice, depletion of RSV-specific CD8+ T cells leads to prolonged viral
replication, while adoptive transfer of virus-specific memory cells enhances virus clearance10,11. However, the absence of T cells also leads to reduced symptom severity and transfer of
RSV-specific memory T cells worsens disease, indicating that harmful immunopathology can outweigh the benefits of cell-mediated viral clearance under certain circumstances12,13. In humans,
the role of CD8+ T cells remains less clear with evidence of their protective role mainly limited to observations of children with T-cell defects (who suffer more severe disease with
prolonged viral shedding)14. In influenza, correlations between memory T cells in the blood and reduced severity of disease on subsequent infection have been shown15,16, but no such evidence
exists in RSV and the extent to which T cells contribute to protection or pathology in this and other respiratory viral infections remains unknown. Respiratory viruses are usually confined
to the lung with systemic spread only in the worst cases17. Virus-specific CD8+ T cells in peripheral blood are therefore unlikely in most situations to be directly relevant to protection.
Instead, studies of a range of tissues have recently defined a subset of non-circulating memory T cells specialized to protect sites of pathogen entry18. These resident memory T (Trm) cells
are not only poised for rapid killing on virus re-encounter but may also exhibit innate-like sensing functions19. In mouse models of influenza, CD4+ and CD8+ Trm cells in the lung confer
greater protection than spleen-derived cells20,21. However, restrictions on sampling of human lungs mean that little is known about these Trm cells except that they are abundant in
non-inflamed lung from tumour excisions or donated organ tissue22,23. We investigated the CD8+ T-cell response to experimental RSV infection in 49 healthy adult volunteers, around half of
who also underwent serial bronchoscopy. While controlled for variations in viral inoculum and co-morbidities, this cohort nevertheless represented a genotypically diverse antigen-experienced
population that allowed characterization of the breadth of virus-specific CD8+ T-cell responses and identification of novel immunodominant and subdominant epitopes. Analysis using major
histocompatibility complex (MHC)-peptide tetramers revealed highly contrasted kinetics, phenotypes and functionality of RSV-specific CD8+ T cells in the lower respiratory tract compared with
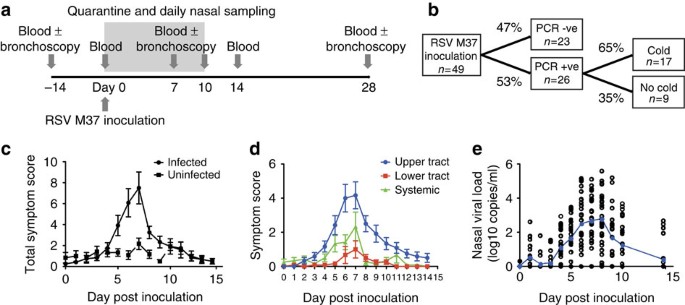
blood, the diversity of which allowed us to infer a specialised role in protection against RSV disease. RESULTS EXPERIMENTAL RSV INFECTION CAUSES UPPER TRACT DISEASE We enrolled 49 healthy
adults aged 18–50 years (median 20.5 years; Supplementary Table 1). Two weeks and immediately before inoculation, they underwent blood and nasal sampling (Fig. 1a). All individuals were then
inoculated with 104 plaque-forming units of RSV A Memphis 37 (RSV M37) by intranasal drops as previously described9, followed by sequential blood and nasal samples up to 6 months later.
Twenty-six subjects (53%) developed PCR+ infection (Fig. 1b). Of these, 17 (65%) suffered a ‘common cold’ according to standardized criteria (see Methods), while 9 (35%) reported minimal or
no symptoms. Symptoms in infected individuals peaked around day 7 post inoculation (Fig. 1c). While almost all symptomatic individuals complained of upper respiratory symptoms, lower
respiratory tract and systemic symptoms were unusual (Fig. 1d). In infected individuals, viral load peaked at days 7–8 post infection with a mean of 2.76 log10 copies per ml (±s.e.m. 0.322;
Fig. 1e), correlating with self-reported symptoms (Supplementary Fig. 1). RSV CAUSES INFLAMMATION IN THE LOWER RESPIRATORY TRACT Twenty-four volunteers additionally underwent serial
bronchoscopy (Supplementary Table 2). Thus, endobronchial biopsies, bronchial brushings and bronchoalveolar lavage (BAL) were obtained 14 days before inoculation and 7 or 10 and 28 days
after. Of these, 50% developed RSV infection. In view of the predominantly upper respiratory disease, we were surprised to find strikingly extensive macroscopic inflammation involving the
lower respiratory tract of infected individuals (Fig. 2a; Supplementary Table 3). Lower airways involvement was supported by quantitative PCR (qPCR) of bronchial brushings, which detected
RSV in all day 7 samples and 4/5 infected subjects at day 10, again peaking around 7 days (mean 2.22 log10 ml−1±s.e.m. 0.416; Fig. 2b). RSV could also be measured in BAL in 5/7 individuals
at day 7 at lower copy numbers (peak 1.48 log10 ml−1±s.e.m. 0.412). Interestingly, virus was detected in bronchial brushings of an additional four subjects in whom no virus was found in
nasal lavage. These were found at low-to-moderate copy numbers (0.31 log10 ml−1 at day 7, and 0.69, 0.17 and 1.76 log10 ml−1 at day 10) and were reproducible on repeat assay. To confirm the
unexpected presence of RSV in the lower airway, endobronchial biopsies were analysed by immunohistochemistry (Fig. 2c). While RSV antigen was not found pre-inoculation, all individuals with
PCR-detectable virus in the nose had at least moderate antigen staining at both acute and convalescent time points. Of these, 3/11 had extensive RSV antigen in bronchial biopsies at day 7–10
and 3/10 at day 28 (Supplementary Table 4). In contrast, PCR− subjects had, at most, staining of isolated epithelial cells, suggesting the presence of RSV antigen but no onward spread. RSV
antigen was associated with infiltration of CD8+ T cells (Fig. 2d). In those with PCR+ bronchial brushings, CD8+ T cells increased significantly in both the epithelial and subepithelial
layers at acute and convalescent time points (Fig. 2e). In contrast, while CD8+ T cells did increase transiently in the biopsies of PCR− individuals, these were less consistently seen and
did not remain significantly elevated at day 28. Thus, experimental infection of adults with RSV can lead to virus replication in the lower airway, which causes marked inflammation
associated with infiltration of CD8+ T cells. RSV VIRAL LOAD DRIVES CD8+ T-CELL PROLIFERATION To further investigate the kinetics of the response, CD8+ T cells induced by RSV were tracked by
flow cytometry using markers of proliferation (Ki-67) and activation (CD38; Fig. 3a). From day 7 post infection, activated CD8+ T cells in blood expanded peaking around day 10 (mean
2.1%±s.e.m. 0.297 of CD8+ lymphocytes), after which Ki-67+CD38+ CD8+ T cells rapidly returned to baseline frequencies (Fig. 3b). No significant proliferative response was seen in PCR−
individuals, indicating that antigen challenge without viral replication was not sufficient to induce CD8+ T-cell activation (Fig. 3c). Similarly, frequencies of activated CD8+ T cells
increased in BAL (Fig. 3d). However, the average frequency was significantly higher than in blood (_P_=0.0098 Wilcoxon matched pairs test; Fig. 3e). The appearance of activated CD8+ T cells
coincided with the fall in viral load (Fig. 1e). Antigen availability and inflammation both contribute to T-cell activation, and indeed, cumulative viral load and symptoms (by trapezoidal
area under the curve) both correlated with the peak frequency of activated CD8+ T cells (Fig. 3f,g), suggesting that increased virus burden and/or inflammatory changes in the airway drive
T-cell activation, which in turn leads to viral clearance. HLA-ASSOCIATED IMMUNODOMINANCE HIERARCHIES AGAINST RSV Antigenic targets of RSV-specific CD8+ T cells have not been systematically
investigated and only a few isolated epitopes have been identified24,25. To define the breadth of these responses, we generated a peptide library from RSV M37 predicted to bind the most
common human leukocyte antigen (HLA)-A and HLA-B alleles using the Immune Epitope Database consensus prediction tools26. The top 1% of predicted MHC-binding peptides for each RSV protein was
pooled and peptide pools used to screen peripheral blood mononuclear cells (PBMCs) from subjects at day 10 post infection by interferon-γ (IFN-γ) enzyme-linked immunospot (ELISpot). Pools
containing peptides from M, N, P, NS1 and NS2 (all internal proteins) induced IFN-γ production (Fig. 4a). Pools that stimulated responses were subsequently deconvoluted to individual
epitopes (Fig. 4b; Supplementary Table 5). In the context of A*01:01, two epitopes (previously described YLEKESIYY [YLE]25 and the novel VTDNKGAFKY [VTD]) that elicited dominant responses
were identified in the M protein and one subdominant epitope (LSDSTMTNY [LSD]) in the NS1 protein (Supplementary Table 5). A 10-mer differing from LSD in one additional amino acid also
induced responses but had a 100-fold lower MHC-binding affinity. YLE and VTD were conserved with the prototypic RSV A2 strain. Only a single A*02:01-restricted epitope (FLVNYEMKL [FLV]) was
identified, derived from the NS2 and conserved with RSV A2. Finally, with B*07:02, four epitopes were defined: NPKASLLSL (NPK, described previously25) and QVMLRWGVL (QVM) from N protein
(previously shown as part of a longer polypeptide to induce IFN-γ in some individuals27); IPAYRTTNY (IPA) from L; and KPNIRTTLL (KPN) from G (not conserved with RSV A2). VTD, LSD and FLV
have previously been predicted but not confirmed as epitopes on the basis of HLA-binding affinity alone28. Indeed, all but three epitopes exhibited moderate to good measured MHC
peptide-binding affinity (<500 nM). Importantly, each HLA allele presented epitopes from different RSV proteins with no overlap in the specificities of CD8+ T cells targeted against them.
In subjects with sufficient PBMCs, RSV-specific CD8+ T-cell responses were analysed before, during and after infection using IFN-γ ELISpot. Before infection, epitope-specific cells were
rare, with a median (interquartile range (IQR)) of 45 (6–61) spot-forming units (SFU) per million PBMCs (A*01:01); 28 (13–52) SFU per million PBMCs (B*07:02); and <5 (<5–11) SFU per
million PBMCs (A*02:01; Fig. 4c,d; Supplementary Table 6). At the peak of the CD8+ T-cell response, these had increased by ∼10-fold to 194 (114–452) SFU per million PBMCs for A*01:01; 231
(138–577) SFU per million PBMCs for B*07:02; and 28 (18–52) SFU per million PBMCs for A*02:01. The extent of expansion between the three HLA-restricted responses was similar (Fig. 4d;
Supplementary Fig. 2). By day 28, these populations had contracted but remained enlarged compared with baseline in most individuals. Throughout the course of infection, the immunodominance
hierarchy was maintained both between individuals and within subjects who expressed two or three of these HLA alleles. Thus, A*01:01-restricted responses were consistently greater than those
against B*07:02-restricted epitopes and those against the A*02:01-restricted FLV epitope were ∼10-fold lower. However, the FLV response was more prominent in individuals without the A*01:01
or B*07:02 alleles, suggesting the effect of immunodomination. Thus, we identified several new RSV epitopes (both immunodominant and subdominant) for three common HLA alleles with potential
as vaccine targets. The immunodominance hierarchies indicated marked quantitative differences in responses determined by HLA type and the influence of HLA alleles on each other when
co-expressed. RSV-SPECIFIC CD8+ T CELLS ACCUMULATE IN CONVALESCENT AIRWAYS MHC peptide tetramers were constructed using three immunodominant epitopes (A1-M-YLE, A2-NS2-FLV and B7-N-NPK) to
investigate RSV-specific CD8+ T cells in further detail by flow cytometry in blood and BAL (Fig. 5a). In blood at baseline, tetramer+ cells were found at low frequencies ranging from
undetectable to 0.03% of CD8+ lymphocytes (Fig. 5b,d). After day 7 post infection, these expanded coincident with the fall in viral load (Fig. 1e). They peaked at day 10 post infection with
up to a mean 18-fold increase, after which epitope-specific populations contracted rapidly. While tetramer+ cells still remained elevated in most individuals 28 days post infection, their
numbers were not maintained long term, so that by 6 months post infection there was no statistical difference compared with their preinfection levels. Analysis of matched BAL samples from
the same infected subjects, however, revealed an unexpected pattern of CD8+ T-cell kinetics that diverged markedly from those in blood (Fig. 5c). While in this compartment, tetramer+ cells
also started increasing in frequency after 7 days, at both day 10 and day 28 they were significantly more frequent than in blood (_P_=0.0078 and 0.0002, respectively, Wilcoxon matched pairs
test), with a sevenfold higher mean frequency of A1-M-YLE+ cells in BAL than in blood at day 10 (Fig. 5d,e). Furthermore, the frequency of tetramer+ cells continued to rise in BAL into the
convalescent period despite the absence of symptoms or virus detection by qPCR, while contraction of these populations had occurred in blood. Therefore, at day 28 post infection, the mean
frequency of A1-M-YLE+ cells was 114-fold greater in BAL than in blood. PHENOTYPICALLY DISTINCT RSV-SPECIFIC CD8+ T CELLS IN AIRWAYS In view of the strikingly divergent kinetics in BAL and
blood, we went on to analyse the phenotypic differences between RSV-specific CD8+ T cells in the two compartments. In BAL at rest, almost all (80–90%) tetramer+ cells displayed the canonical
CD69+CD103+ Trm phenotype (Fig. 6a). In contrast, before infection, no tetramer+ cells in blood expressed either marker. However, during infection, a proportion of tetramer+ cells in blood
upregulated CD103, peaking at day 10 then decreasing with resolution of infection. In BAL, the CD103 single-positive population also increased in frequency at day 10, transiently making up a
greater proportion of tetramer+ cells, which then returned to a predominantly CD69+CD103+ phenotype during convalescence. Analysis of CD45RA and CCR7 allowed further categorization of
memory subsets and indicated that the majority of tetramer+ cells exhibited effector memory (Tem) or effector memory re-expressing CD45RA (Temra) phenotypes, with predominantly Tem cells in
BAL (Fig. 6b). In blood, infection led to a significant increase in T-effector cells (expressing the CD45RA−/CCR7+ phenotype) at the expense of Temra cells, a change that was not seen in the
airway. This occurred via proliferation starting between day 3 and day 7 post infection, paralleling the polyclonal CD8+ T-cell response and peaking at around day 10, when ∼85% (IQR 70–92%)
of epitope-specific CD8+ T cells in blood showed activation and proliferation by CD38 and Ki-67 expression (Fig. 6c). This was also seen in tetramer+ BAL cells, although a proportion of
these remained quiescent. However, by day 28, despite the continued accumulation of tetramer+ cells in the BAL, there was no evidence of on-going activation or proliferation. In blood, these
cells upregulated perforin and granzyme B, which also peaked around day 10 with around 33% (median, IQR 17–46%) expressing cytotoxicity molecules (Fig. 7a). However, few tetramer+ BAL cells
expressed perforin. Furthermore, only a minority upregulated granzyme B, which again peaked around day 10. During infection, the co-stimulatory molecules CD27 and CD28 were both
downregulated in a proportion of RSV-specific CD8+ T cells in the blood and in the majority of individuals had not returned to baseline frequencies by day 28 (Fig. 7b). In contrast, even
before infection, a large proportion of RSV-specific CD8+ T cells had already downregulated CD28 and CD27+CD28+ double-positive cells were in the minority. Furthermore, there were no
significant changes in their expression following infection. Finally, following activation, RSV-specific CD8+ T cells in blood upregulated CCR5 and downregulated CD62L, allowing homing to
sites of inflammation and away from lymphoid tissues (Fig. 7c; Supplementary Fig. 3). In BAL, there was a trend towards tetramer+ Trm cells upregulating CCR5 and there was no CD62L
expression at any time. These data suggest that RSV infection induces a transient increase of virus-specific CD8+ T cells in the blood, with a phenotype characteristic of cytotoxic effector
cells, some of which changes persist into the convalescent period. In contrast, Trm cells in the airway alter less markedly following infection, with a large proportion showing no
proliferative response and overall reduced expression of cytotoxicity molecules. LIMITED FUNCTIONALITY OF RSV-SPECIFIC CD8+ MEMORY T CELLS In studies of influenza, virus-specific CD8+ memory
T-cell frequencies in the blood have been shown to correlate inversely with symptom severity during subsequent infection15,16. This has not been demonstrated with other viruses, and indeed
we found no significant difference in baseline frequencies of RSV-specific CD8+ T cells in peripheral blood between those subsequently deemed infected and those who remained uninfected
(Supplementary Fig. 4a). In addition, there was no statistical relationship between the magnitude of pre-existing HLA-restricted responses and the severity of disease, expressed throughout
as cumulative viral load or symptom score to smooth out day-to-day variability in spite of the close correlation between peak and cumulative values (Supplementary Fig. 4b). Analysis of CD8+
T cells induced by highly protective systemic vaccines, such as yellow fever, have displayed polyfunctionality that may correlate with their efficacy29. To investigate whether the
functionality of RSV-specific CD8+ T cells might be contributing to lack of protection, cytokine production was analysed by flow cytometry (Fig. 8a). Overall, the frequencies of
cytokine-producing CD8+ T cells following short-term peptide stimulation were modest with average median frequencies of 0.035 (against YLE) and 0.056% (against NPK). These were similar to
those seen against the immunodominant influenza epitope M1-GIL in A*02:01-expressing individuals from this cohort. However, most cytokine-expressing RSV-specific cells produced only a single
cytokine (IFN-γ, tumour necrosis factor or interleukin-2) with an average of only 2.63% (±s.e.m. 0.432) producing three (Fig. 8b,c; Supplementary Fig. 5). By comparison, influenza-specific
CD8+ T cells were more polyfunctional, with 39% producing two cytokines and a significantly higher proportion (15.1%±s.e.m. 2.30) producing three (_P_=0.0079 by Mann–Whitney test). Thus,
RSV-specific memory CD8+ T cells in blood have reduced functionality compared with those against influenza, potentially contributing to reduced protective capacity. CD8+ TRM CELLS IN AIRWAYS
CORRELATE WITH REDUCED DISEASE These findings mirror our previous study that showed RSV-specific nasal IgA correlating strongly with protection from PCR-confirmed infection with greater
predictive power than serum IgG in this regard9. However, where infection did occur, high nasal IgA titres did not reduce disease severity (Supplementary Fig. 6). We hypothesized that local
cell-mediated immunity could provide the next layer of defence by acting to directly eliminate cell-associated virus and thus reduce symptoms. To test this, we analysed the frequencies of
tetramer+ CD8+ T cells against immunodominant epitopes (representative of the RSV-specific CD8+ T-cell population before infection) and their relationship with infection risk and disease
severity. At baseline, RSV-specific CD8+ Trm cells were already enriched in BAL with significantly higher frequencies compared with blood irrespective of specificity (Fig. 9a). However, in
contrast to mucosal IgA, their frequency had no impact on the likelihood of PCR-confirmed RSV infection (Fig. 9b). Instead, the higher the frequency of RSV-specific CD8+ T cells in baseline
BAL, the lower the cumulative symptom score (Spearman’s _r_=−0.691, _P_=0.0142; Fig. 9c) and the lower the cumulative viral load (Spearman’s _r_=−0.668, _P_=0.0317; Fig. 9d) in those who
subsequently developed PCR+ infection. These correlations held true on correlation with lower respiratory tract symptoms alone (Fig. 9e, Spearman’s _r_=0.634, _P_=0.02) but less so with
upper tract or systemic symptoms (Supplementary Fig. 7). Similarly, higher frequencies of pre-existing CD8+ Trm cells correlated with lower bronchial viral load (Spearman’s _r_=0.57,
_P_=0.042; Fig. 9f), suggesting that these cells might have a direct role in viral clearance. In summary, while memory CD8+ T cells in blood do not correlate with protection against RSV,
higher frequencies of CD8+ Trm cells in the airway are associated with improved viral control and reduced symptom severity, suggesting that these are likely to have a role in ameliorating
disease if present in sufficiently high numbers. DISCUSSION Studies in animal models have increasingly highlighted the distinctiveness of local immunity and the importance of this
compartmentalization to protective immune responses. Here we present the first interventional study to track virus-specific cell-mediated immunity in the human airway. Longitudinal analysis
of the CD8+ T-cell response showed the extent to which these cells in the human airway diverge from those in the blood during RSV infection in almost every respect and provides strong
evidence for the role that human Trm cells play in protection. RSV caused surprisingly extensive inflammation in most infected individuals despite minimal or no lower respiratory tract
symptoms. Compared with rhinovirus (where neither macroscopic inflammation nor cellular infiltrates are significantly seen on bronchoscopy in healthy adults30), RSV led to substantially
greater lower respiratory tract involvement. Infection caused CD8+ T-cell infiltration of the respiratory mucosa with an unexpected accumulation of very large numbers of RSV-specific cells
well into convalescence, distinct from blood in the same individuals. In both blood and airway, activation of CD8+ T cells occurred acutely, coinciding with falling viral load, suggesting
their involvement in virus elimination. Shortly after peak viral shedding, proliferation ceased, implying that viral replication was required to drive this response. In immunodeficient
children, the absence of T cells is associated with prolonged and more severe infection, while RSV disease risk in older adults is believed to be related to fewer IFN-γ-producing T
cells14,31. However, the low frequency, short-lived boosting and possibly limited functionality of RSV-specific memory CD8+ T cells in blood meant that poor correlation with protection was
seen. In contrast, epitope-specific CD8+ Trm cells in the airways with their significantly higher frequencies and localization near the site of infection did indeed correlate with reduced
disease severity. This strongly supports the hypothesis that virus-specific CD8+ Trm cells in the airway play a direct role in early clearance of respiratory viruses, while memory T cells in
blood correlate indirectly (if at all). Previous studies in man, including those inferring the protective role of CD4+ and CD8+ T cells from peripheral blood in influenza15,16, make the
assumption that there is a direct and proportional relationship between blood and mucosal immunity. Our findings show that this assumption is fundamentally incorrect and that these
compartmentalized populations must be examined directly. Recent phase-I clinical trials have shown that virus-vectored RSV vaccine candidates can induce T cells in blood32. Our data suggest
that their capacity to induce Trm cells following intranasal administration should be explored further. These findings might also explain why CD8+ T-cell-inducing influenza vaccines that
induce primarily systemic responses demonstrate suboptimal efficacy5,33. In mice, expression of the receptors CD69 and CD103 allows retention of CD8+ Trm in tissues34. Although they may not
represent the entire Trm population35, CD69+CD103+ CD8+ Trm cells have been shown to localize to the murine airway following respiratory priming with influenza and mediate cross-protection
against heterologous strains21. Much less is known in humans, where previous studies have been based solely on uninfected lung tissue removed during cancer surgery or non-living organ
donations and limited to cross-sectional analysis of resting T cells22,23. These estimates of frequency showed that Trm cells are abundant in the lung where, at rest, influenza-specific CD8+
Trm cells are more frequent than those for cytomegalovirus. Our longitudinal analyses advance these findings by showing _in vivo_ how Trm cells in the human airway arise following viral
infection and how they relate to disease severity. During acute infection, RSV-specific CD8+ Trm cells were transiently joined by a CD103+CD69− population that could represent a population
that migrate from blood to airway, although the ontogeny of Trm cells has yet to be fully elucidated36,37. Interestingly, this may be the reverse of CD8+ Trm cells generated against herpes
simplex virus 1 (HSV-1) in murine skin, where sequential induction of first CD69 and then CD103 has been observed, suggesting the impact of tissue type, pathogen and/or interspecies
differences38. RSV-specific Trm cells in the airway showed phenotypic changes suggestive of advanced differentiation, with downregulation of co-stimulatory markers, but also reduced
expression of cytotoxicity molecules. We therefore propose that the very high Trm cell frequencies seen during early convalescence can confer absolute protection against early symptomatic
reinfection but may be functionally regulated to prevent excessive damage to the delicate lung architecture. Inflammatory changes seen in the airway during convalescence may represent the
effect of large numbers of CD8+ Trm cells interacting with residual RSV antigen, but these caused no symptoms in healthy adults. In contrast, in murine systems where large numbers of
RSV-specific CD8+ T cells are present at the same time as high viral titres, life-threatening immunopathology can occur39. In our study, it was not deemed acceptable to subject volunteers to
a fourth bronchoscopy to determine the longevity of Trm cells, while other phenotypic and functional assessments were likely to be qualitatively similar to the preinfection time point.
Nevertheless, it can be inferred that in these volunteers, CD8+ Trm frequencies had waned significantly, since the end of their last natural RSV infection (though less precipitously than in
blood). This waning in the lung contrasts with long-lived Trm cells in other tissues and may represent another mechanism by which immunopathology is reduced40. Outstanding questions
including how Trm cells differentiate and the conditions required for persistence remain major hurdles for generating protective cell-mediated immunity in the mucosa. Both residual antigen
and cytokines such as TGF-β have been implicated in Trm cell maintenance. Blockade of antigen recognition in the lungs of influenza-infected mice leads to loss of Trm cells, while TGF-β is
expressed in respiratory mucosa and _in vitro_ can induce upregulation of CD103 (ref. 41). This is supported by our findings of persistent RSV antigen in the airway associated with
virus-specific CD8+ Trm cells even 28 days after inoculation. While there was no apparent disease associated with high frequencies of RSV-specific CD8+ Trm cells in our cohort, damaging
immunopathology caused by boosting them in other age groups and settings remains a risk. These data show that immune responses in blood can fundamentally misrepresent those at the site of
infection. Our _ex vivo_ analyses of the respiratory compartment show that adaptive immunity against respiratory viruses comprises multiple specialised and non-redundant protective
mechanisms distinct in time and spatial organization. In RSV, locally produced mucosal IgA constitutes an initial barrier to virus entry but does not significantly modulate disease severity
once the barrier is breached9. When that occurs, CD8+ Trm cells provide early recognition and virus elimination, thus reducing symptom severity and viral load. Only after these does systemic
immunity act to prevent widespread disease. We therefore propose that Trm cells represent one of several immune mechanisms that should be harnessed together for optimal vaccine-mediated
protection. METHODS ETHICS STATEMENT The study was approved by the UK National Ethics Service London—Fulham (study numbers 10/H0711/94 and 11/LO/1826). Written informed consent was obtained
from all volunteers. STUDY DESIGN Healthy, non-smoking adults aged 18–55 years were recruited between 2012 and 2013. All subjects were genotyped for HLA class-I loci using sequence-based
typing. A total of 49 subjects were inoculated with 104 plaque-forming units of RSV Memphis 37 (Meridian Lifesciences, Memphis, USA). Following the challenge, participants were quarantined
for 10 days. Cold symptoms were assessed using symptoms diaries completed daily by participants. Reported symptoms with a score of 0 (absent), 2 (moderate) or 3 (severe) included sneezing,
nasal discharge, nasal obstruction or sore throat (upper respiratory tract), headache, malaise, fever (systemic) and cough, wheeze, shortness of breath (lower respiratory tract symptoms).
Cold was confirmed if two out of the following three conditions were fulfilled: nasal discharge lasting ≥3 days, subjective feeling of cold reported or cumulative 14-day symptom score ≥14.
Blood samples were obtained at baseline and time points post inoculation as indicated in the text. Nasal lavage samples were collected daily and used for viral load quantification by qPCR as
previously described9,42. Briefly, total RNA was isolated using the QIAamp Viral RNA kit (Qiagen) according to the manufacturer’s instructions. Reverse transcription of 13 μl of total
isolated RNA was achieved using the High Capacity RNA-to-cDNA kit (Applied Biosystems) according to the manufacturer’s instructions. qPCR with reverse trancription reactions for RSV N gene
were achieved using primers shown in Supplementary Table 7 and the TaqMan Universal Master Mix II (Applied Biosystems) and 7500 Fast Real-Time PCR System (Applied Biosystems). Absolute
quantification was calculated using a plasmid DNA standard curve. As shown previously, infection status as determined by qPCR positivity accorded 100% with multiplex qualitative PCR and
plaque assay. Bronchoscopists were blinded to the infection status of the volunteers. Bronchoscopies were undertaken at 14 days before inoculation and 7 or 10 days and 28 days post
infection. Macroscopic appearance of the airways was reported as ‘normal’, ‘erythematous’ or ‘erythematous with contact bleeding’. Bronchial brushings and biopsies were obtained at each time
point. Bronchial epithelial cells were washed with RPMI/10% fetal calf serum (FCS; R10) and stored in TRIzol reagent (Invitrogen, Grand Island, USA) for viral load quantification. BAL was
obtained by instillation of up to 120 ml of normal saline, filtered and centrifuged before flow cytometric analysis. IMMUNOHISTOCHEMISTRY Endobronchial biopsies were fixed immediately in 4%
paraformaldehyde and paraffin embedded. RSV was stained using polyvalent mouse anti-RSV antibody (NCL-RSV3, Leica Biosystems, UK) at 1:50 dilution. CD8+ T cells were identified by staining
with mouse anti-CD8 (M0707, Dako) at 1:100 dilution using the EnVision peroxidase staining method (Dako, Denmark) as previously described30. Briefly, 5-μm sections were stained according to
the manufacturer’s instructions and an irrelevant mouse IgG1 kappa antibody (MOPC21) was used as negative control for staining specificity of mouse monoclonal antibodies. RSV-infected A549
cells were used as positive staining controls. Quantification was achieved as previously described with operators blinded to sample timing and infection status30. Briefly, slides were coded
to avoid observer bias and areas of epithelium and subepithelium assessed using a Leitz Dialux 20 light microscope, Apple Macintosh computer and Image 1.5 software. Total epithelial and
subepithelial areas of two to three bronchial biopsies were counted for each bronchoscopy. Cell counts were expressed as the number of cut cell profiles with visible nucleus per mm2 of
subepithelium and per 0.1 mm2 of epithlium. The coefficient of variation for repeat counts of positive cells by a single observer ranged from 5 to 6%. PBMC ISOLATION PBMC isolation was
performed by density centrifugation using Histopaque 1077 (Sigma Aldrich, USA) according to the manufacturer’s protocol. Cells were either used immediately or cryopreserved in FCS (Gibco
Life Technologies, USA) with 10% dimethyl sulfoxide (DMSO) in liquid nitrogen for future assays. _EX VIVO_ INTERFERON-Γ ELISPOT ASSAY T-cell epitope mapping was performed by INF-γ ELISpot
using 2 × 105 PBMCs in triplicate stimulated with peptide pools (10 μg ml−1 of each peptide) for initial screening followed by deconvolution with single peptides (10 μg ml−1). Plates were
incubated for ≥17 h at 37 °C and subsequently developed (Mabtech, Sweden). SFU were counted using AID ELISpot software (Autoimmun Diagnostika GmbH, Germany) and analysed with positive wells
containing ≥20 spots per 106 cells and a _P_ value of ≤0.05 using a Student’s _t_-test in at least two experiments. FLOW CYTOMETRY HLA class-I (A*01:01 and B*07:02) tetramers were a gift
from Rafi Ahmed (Emory University, Atlanta, USA). Custom-made dextramers (A*02:01) were purchased from Immudex (Copenhagen, Denmark). Whole blood or BAL cell samples were stained by adding 2
μl of tetramer and incubating for 10 min at room temperature before staining with surface antibodies for 20 min. Dextramer staining was performed in the same way on freshly isolated PBMCs
or BAL cells. Surface staining of whole blood was followed by erythrocytes lysis with BD Lysis buffer. Cells were then fixed with 1% paraformaldehyde solution or prepared for intracellular
staining by fixing and permeabilization with BD Fixation/Permeabilization kit. The following surface antibodies (including clone and catalogue number) were used in the study: CD3 PE-CF594
(UCHT1, BD#562280), CD4 APC-H7 (SK3, BD#641398), CD38 PE Cy7 (HB7, BD#335825), Ki-67 FITC (B56, BD#556026), Perforin FITC (δG9, BD#556577), Granzyme B V450 (GB11, BD#561151), CD45RA FITC
(HI100, BD#555488), CCR7 PE (150503, BD#560765), CD27 V450 (M-T271, BD#560448), CD28 PE Cy7 (CD28.2, BD#560684), CCR5 V450 (2D7/CCR5, BD#562121), CD62L PE (DREG-56, BD#555544; all BD
Biosciences); CD8 PerCP Cy 5.5 (RPA-T8, #45-0088-42), CD69 FITC (FN50, 11-0699-42) and CD103 PE Cy7 (B-Ly7, #25-1038-42; all eBioscience). Dilutions at which antibodies were used are shown
in Supplementary Table 8. FlowJo software (FlowJo LLC, USA) was used for data analysis. _IN VITRO_ PEPTIDE STIMULATION AND INTRACELLULAR CYTOKINE STAINING Intracellular cytokine staining was
used to confirm epitopes identified by ELISpot. Thawed PBMCs were rested overnight and stimulated the next day for 6 h in culture medium (RPMI 1640, 10% FCS, 2 mM glutamine, 100 IU ml−1
penicillin/streptomycin) using selected peptides (10 μg ml−1) plus anti-CD28 and anti-CD49. After 2 h, Brefeldin A (1 μl/ml) was added and after 6 h cells were washed and stained with
live/dead marker (Invitrogen, USA); IFN-γ APC (B27, #554702), TNF PE Cy7 (MAb11, #557647), interleukin-2 FITC (5344.111, #340448; all BD Biosciences); and relevant surface markers. Dilutions
at which antibodies were used are shown in Supplementary Table 8. The frequencies of expression profiles for single, double and triple cytokine producers were calculated using the Boolean
combination gating option in FlowJo software (FlowJo LLC, USA). RSV MEMPHIS 37 SEQUENCE TRANSLATION AND _IN SILICO_ EPITOPE PREDICTION The genomic sequence of RSV Memphis 37 was translated
into proteins from all six possible open-reading frames and the resulting amino-acid sequences compared against 11 RSV reference protein sequences obtained from RefSeq using blastx. Hits
were filtered by removing those with an e-value lower than 1 × 10−6. These were then verified by manual inspection. The capacity of all 9- and 10-mer peptides derived from RSV M37 to bind
HLA-A*01:01, A*02:01 and B*07:02 was predicted with the Stabilized Matrix Method using the command-line version of the Immune Epitope Database consensus prediction tool26. Peptides were
selected if they scored in the top 1% of predictions for each length or if they were the top two peptides for the protein (to ensure even small proteins were represented for each allele).
Peptides were synthesized by A&A Labs LLC (San Diego, CA, USA) as crude material, and resuspended at 20 mg ml−1 in 100% DMSO (v/v). MHC PEPTIDE-BINDING ASSAYS Quantitative measurements
of peptide binding to HLA class-I molecules were made by inhibition of binding of radiolabelled standard peptides. MHC molecules were purified by affinity chromatography from the
Epstein–Barr virus-transformed homozygous cell line JY, and assays performed, as described previously43. Briefly, peptides were tested at six different concentrations covering a 100,000-fold
dose range in three or more independent assays. Lyophilized test peptides were solubilized in water, PBS (pH 7.2) or 100% DMSO and serially diluted in 0.05% (v/v) NP-40/PBS. Each peptide
dose (5 μl) was loaded into the reaction vessel (Costar 96-well polypropylene round-bottom plate). For positive (no peptide) and negative (no MHC) controls, wells were loaded with NP-40/PBS
alone. The MHC/labelled peptide reaction mix was prepared with PBS, purified MHC of the desired allele, protease inhibitor cocktail, β2 microglobulin and radiolabelled peptide. Reaction mix
(10 μl) was added to all but the negative control well and the plate sealed and incubated for 2 days in the dark at room temperature. The concentration of peptide yielding 50% inhibition of
the binding of the radiolabelled probe peptide (half-maximal inhibitory concentration (IC50)) was calculated. Under the conditions used, where [radiolabelled probe]<[MHC] and IC50≥[MHC],
the measured IC50 values are reasonable approximations of the true _K_d values44,45. STATISTICAL ANALYSIS On the basis of published data25, we calculated that a minimum sample size of 16 in
each arm (infected versus uninfected) would be sufficient to find a difference of 1.3% of epitope-specific CD8+ T cells with 80% power using a two-sided unpaired _t_-test with 5%
significance level (where the variability in each group was 1.4%). Samples were only excluded where insufficient cells or material remained. Statistical analysis was performed using Graphpad
Prism and R software. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Jozwik, A. _et al._ RSV-specific airway resident memory CD8+ T cells and differential disease severity after
experimental human infection. _Nat. Commun._ 6:10224 doi: 10.1038/ncomms10224 (2015). CHANGE HISTORY * _ 09 MARCH 2016 A correction has been published and is appended to both the HTML and
PDF versions of this paper. The error has been fixed in the paper. _ REFERENCES * Chiu, C. & Openshaw, P. J. Antiviral B cell and T cell immunity in the lungs. _Nat. Immunol._ 16, 18–26
(2015) . Article CAS Google Scholar * Openshaw, P. J. & Chiu, C. Protective and dysregulated T cell immunity in RSV infection. _Curr. Opin. Virol._ 3, 468–474 (2013) . Article CAS
Google Scholar * Guvenel, A. K., Chiu, C. & Openshaw, P. J. Current concepts and progress in RSV vaccine development. _Expert Rev. Vaccines_ 13, 333–344 (2014) . Article CAS Google
Scholar * He, X. S. et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. _J. Virol._ 80, 11756–11766 (2006) . Article CAS
Google Scholar * Lillie, P. J. et al. Preliminary assessment of the efficacy of a t-cell–based influenza vaccine, MVA-NP+M1, in humans. _Clin. Infect. Dis._ 55, 19–25 (2012) . Article CAS
Google Scholar * Nair, H. et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. _Lancet_
375, 1545–1555 (2010) . Article Google Scholar * Falsey, A. & Walsh, E. Viral pneumonia in older adults. _Clin. Infect. Dis._ 42, 518–524 (2006) . Article Google Scholar * Hall, C.,
Walsh, E., Long, C. & Schnabel, K. Immunity to and frequency of reinfection with respiratory syncytial virus. _J. Infect. Dis._ 163, 693–698 (1991) . Article CAS Google Scholar *
Habibi, M. S. et al. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. _Am. J. Respir. Crit. Care
Med._ 191, 1040–1049 (2015) . Article CAS Google Scholar * Cannon, M., Stott, E., Taylor, G. & Askonas, B. Clearance of persistent respiratory syncytial virus infections in
immunodeficient mice following transfer of primed T cells. _Immunology_ 62, 133–138 (1987) . CAS PubMed PubMed Central Google Scholar * Graham, B. S., Bunton, L. A., Wright, P. F. &
Karzon, D. T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. _J. Clin. Invest._ 88, 1026–1033 (1991) .
Article CAS Google Scholar * Cannon, M. J., Openshaw, P. J. & Askonas, B. A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial
virus. _J. Exp. Med._ 168, 1163–1168 (1988) . Article CAS Google Scholar * Alwan, W. H., Record, F. M. & Openshaw, P. J. CD4+ T cells clear virus but augment disease in mice infected
with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. _Clin. Exp. Immunol._ 88, 527–536 (1992) . Article CAS Google Scholar * Hall, C. B. et al. Respiratory
syncytial viral infection in children with compromised immune function. _N. Engl. J. Med._ 315, 77–81 (1986) . Article CAS Google Scholar * Wilkinson, T. M. et al. Preexisting
influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. _Nat. Med._ 18, 274–280 (2012) . Article CAS Google Scholar * Sridhar, S. et al.
Cellular immune correlates of protection against symptomatic pandemic influenza. _Nat. Med._ 19, 1305–1312 (2013) . Article CAS Google Scholar * Eisenhut, M. Extrapulmonary manifestations
of severe respiratory syncytial virus infection—a systematic review. _Crit. Care_ 10, R107 (2006) . Article Google Scholar * Park, C. O. & Kupper, T. S. The emerging role of resident
memory T cells in protective immunity and inflammatory disease. _Nat. Med._ 21, 688–697 (2015) . Article CAS Google Scholar * Schenkel, J. M., Fraser, K. A., Vezys, V. & Masopust, D.
Sensing and alarm function of resident memory CD8+ T cells. _Nat. Immunol._ 14, 509–513 (2013) . Article CAS Google Scholar * Teijaro, J. R. et al. Cutting edge: tissue-retentive lung
memory CD4 T cells mediate optimal protection to respiratory virus infection. _J. Immunol._ 187, 5510–5514 (2011) . Article CAS Google Scholar * Wu, T. et al. Lung-resident memory CD8 T
cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. _J. Leukoc. Biol._ 95, 215–224 (2013) . Article Google Scholar * Purwar, R. et al. Resident
memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. _PLoS ONE_ 6, e16245 (2011) . Article ADS CAS Google Scholar * Turner, D. L. et al. Lung
niches for the generation and maintenance of tissue-resident memory T cells. _Mucosal Immunol._ 7, 501–510 (2013) . Article Google Scholar * Goulder, P., Lechner, F., Klenerman, P.,
McIntosh, K. & Walker, B. Characterization of a novel respiratory syncytial virus-specific human cytotoxic T-lymphocyte epitope. _J. Virol._ 74, 7694–7697 (2000) . Article CAS Google
Scholar * Heidema, J. et al. Human CD8(+) T cell responses against five newly identified respiratory syncytial virus-derived epitopes. _J. Gen. Virol._ 85, 2365–2374 (2004) . Article CAS
Google Scholar * Kim, Y. et al. Immune epitope database analysis resource. _Nucleic Acids Res._ 40, W525–W530 (2012) . Article CAS Google Scholar * Venter, M., Rock, M., Puren, A. J.,
Tiemessen, C. T. & Crowe, J. E. Respiratory syncytial virus nucleoprotein-specific cytotoxic T-cell epitopes in a South African population of diverse HLA types are conserved in
circulating field strains. _J. Virol._ 77, 7319–7329 (2003) . Article CAS Google Scholar * Rock, M. T. et al. Identification of potential human respiratory syncytial virus and
metapneumovirus T cell epitopes using computational prediction and MHC binding assays. _J. Immunol. Methods_ 374, 13–17 (2011) . Article CAS Google Scholar * Akondy, R. S. et al. The
yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. _J. Immunol._ 183, 7919–7930 (2009) . Article CAS Google Scholar * Zhu, J. et al. AIrway
inflammation and illness severity in response to experimental rhinovirus infection in asthma. _Chest_ 145, 1219–1229 (2014) . Article CAS Google Scholar * Bree, G. J. et al. Respiratory
syncytial virus—specific CD8+ memory T cell responses in elderly persons. _J. Infect. Dis._ 191, 1710–1718 (2005) . Article Google Scholar * Green, C. A. et al. Chimpanzee adenovirus– and
MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. _Sci. Transl. Med._ 7, 300ra126 (2015) . Article ADS Google Scholar * Francis, J. N. et al. A novel
peptide-based pan-influenza A vaccine: a double blind, randomised clinical trial of immunogenicity and safety. _Vaccine_ 33, 396–402 (2015) . Article CAS Google Scholar * Skon, C. N. et
al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. _Nat. Immunol._ 14, 1285–1293 (2013) . Article CAS Google Scholar *
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. _Immunity_ 41, 886–897 (2014) . Article CAS Google Scholar * Gaide, O. et al. Common clonal origin of central and
resident memory T cells following skin immunization. _Nat. Med._ 21, 647–653 (2015) . Article CAS Google Scholar * Sheridan, B. S. & Lefrancois, L. Regional and mucosal memory T
cells. _Nat. Immunol._ 12, 485–491 (2011) . Article CAS Google Scholar * Mackay, L. K. et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. _Nat.
Immunol._ 14, 1294–1301 (2013) . Article CAS Google Scholar * Varga, S., Wang, X., Welsh, R. & Braciale, T. Immunopathology in RSV infection is mediated by a discrete oligoclonal
subset of antigen-specific CD4(+) T cells. _Immunity_ 15, 637–646 (2001) . Article CAS Google Scholar * Chang, J. & Braciale, T. J. Respiratory syncytial virus infection suppresses
lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. _Nat. Med._ 8, 54–60 (2002) . Article CAS Google Scholar * Lee, Y.-T. et al. Environmental
and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. _J. Virol._ 85, 4085–4094 (2011) . Article CAS Google Scholar
* Goritzka, M. et al. Alveolar macrophage–derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. _J. Exp. Med._ 212, 699–714 (2015) .
Article CAS Google Scholar * Sidney, J. et al. in _Current Protocols in Immunology_ eds Coligan J. E., Bierer B. E., Margulies D. H., Shevach E. M., Strober W. John Wiley & Sons, Inc.
(2013) . * Cheng, Y. & Prusoff, W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic
reaction. _Biochem. Pharmacol._ 22, 3099–3108 (1973) . Article CAS Google Scholar * Gulukota, K., Sidney, J., Sette, A. & DeLisi, C. Two complementary methods for predicting peptides
binding major histocompatibility complex molecules. _J. Mol. Biol._ 267, 1258–1267 (1997) . Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank the study participants
for their commitment and tolerance. This work presents independent research funded by the Medical Research Council (G0902266) and the Wellcome Trust (087805/Z/08/Z). Further support was
provided by the NIHR Clinical Research Facility and Biomedical Research Centre at Imperial College Healthcare NHS Trust. The views expressed are those of the authors and not necessarily
those of the Medical Research Council, the Wellcome Trust, the NHS, the NIHR or the Department of Health. A1-M-YLE and B7-N-NPK tetramers were kindly donated by Rafi Ahmed (Emory University,
Atlanta, USA). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * National Heart and Lung Institute, Imperial College London, London, W2 1PG, UK Agnieszka Jozwik, Maximillian S. Habibi, Allan
Paras, Jie Zhu, Aleks Guvenel, Jaideep Dhariwal, Mark Almond, Ernie H. C. Wong, Annemarie Sykes, Jerico Del Rosario, Maria-Belen Trujillo-Torralbo, Patrick Mallia, Onn Min Kon, Sebastian L.
Johnston, Peter J. Openshaw & Christopher Chiu * Division of Vaccine Discovery, Centre for Infectious Disease, La Jolla Institute of Allergy and Immunology, 9420 Athena Circle, La Jolla,
92037, California, USA Matthew Maybeno, John Sidney, Bjoern Peters & Alessandro Sette Authors * Agnieszka Jozwik View author publications You can also search for this author inPubMed
Google Scholar * Maximillian S. Habibi View author publications You can also search for this author inPubMed Google Scholar * Allan Paras View author publications You can also search for
this author inPubMed Google Scholar * Jie Zhu View author publications You can also search for this author inPubMed Google Scholar * Aleks Guvenel View author publications You can also
search for this author inPubMed Google Scholar * Jaideep Dhariwal View author publications You can also search for this author inPubMed Google Scholar * Mark Almond View author publications
You can also search for this author inPubMed Google Scholar * Ernie H. C. Wong View author publications You can also search for this author inPubMed Google Scholar * Annemarie Sykes View
author publications You can also search for this author inPubMed Google Scholar * Matthew Maybeno View author publications You can also search for this author inPubMed Google Scholar *
Jerico Del Rosario View author publications You can also search for this author inPubMed Google Scholar * Maria-Belen Trujillo-Torralbo View author publications You can also search for this
author inPubMed Google Scholar * Patrick Mallia View author publications You can also search for this author inPubMed Google Scholar * John Sidney View author publications You can also
search for this author inPubMed Google Scholar * Bjoern Peters View author publications You can also search for this author inPubMed Google Scholar * Onn Min Kon View author publications You
can also search for this author inPubMed Google Scholar * Alessandro Sette View author publications You can also search for this author inPubMed Google Scholar * Sebastian L. Johnston View
author publications You can also search for this author inPubMed Google Scholar * Peter J. Openshaw View author publications You can also search for this author inPubMed Google Scholar *
Christopher Chiu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.C., P.J.O., S.L.J., A.S. and B.P. designed and conceived the study;
C.C., M.S.H. and A.P. carried out the experimental infection study; C.C., A.P., J.D., M.A., E.H.C.W., A.S., J.D.R., M.-B.T.-T., P.M. and O.M.K. conducted the bronchoscopies; A.J., C.C.,
A.G., J.Z., M.M. and J.S. performed the laboratory experiments; C.C., A.J. and P.J.O. wrote the manuscript; all authors reviewed the manuscript before submission. CORRESPONDING AUTHOR
Correspondence to Christopher Chiu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION
Supplementary Figures 1-7 and Supplementary Tables 1-8 (PDF 497 kb) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the
Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jozwik, A., Habibi, M., Paras, A. _et al._ RSV-specific airway resident memory CD8+
T cells and differential disease severity after experimental human infection. _Nat Commun_ 6, 10224 (2015). https://doi.org/10.1038/ncomms10224 Download citation * Received: 26 October 2015
* Accepted: 16 November 2015 * Published: 21 December 2015 * DOI: https://doi.org/10.1038/ncomms10224 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative