Play all audios:
ABSTRACT Wnt/β-catenin signalling directs fundamental processes during metazoan development and can be aberrantly activated in cancer. Wnt stimulation induces the recruitment of the scaffold
protein Axin from an inhibitory destruction complex to a stimulatory signalosome. Here we analyse the early effects of Wnt on Axin and find that the ADP-ribose polymerase Tankyrase
(Tnks)—known to target Axin for proteolysis—regulates Axin’s rapid transition following Wnt stimulation. We demonstrate that the pool of ADP-ribosylated Axin, which is degraded under basal
conditions, increases immediately following Wnt stimulation in both _Drosophila_ and human cells. ADP-ribosylation of Axin enhances its interaction with the Wnt co-receptor LRP6, an
essential step in signalosome assembly. We suggest that in addition to controlling Axin levels, Tnks-dependent ADP-ribosylation promotes the reprogramming of Axin following Wnt stimulation;
and propose that Tnks inhibition blocks Wnt signalling not only by increasing destruction complex activity, but also by impeding signalosome assembly. SIMILAR CONTENT BEING VIEWED BY OTHERS
TOR TARGETS AN RNA PROCESSING NETWORK TO REGULATE FACULTATIVE HETEROCHROMATIN, DEVELOPMENTAL GENE EXPRESSION AND CELL PROLIFERATION Article 11 February 2021 THE ROLE OF LYSINE
PALMITOYLATION/MYRISTOYLATION IN THE FUNCTION OF THE TEAD TRANSCRIPTION FACTORS Article Open access 23 March 2022 THE TRIP12 E3 LIGASE INDUCES SWI/SNF COMPONENT BRG1-Β-CATENIN INTERACTION TO
PROMOTE WNT SIGNALING Article Open access 05 June 2025 INTRODUCTION The Wnt/β-catenin signal transduction pathway directs fundamental processes during metazoan development and tissue
homeostasis, whereas deregulation of Wnt signalling underlies numerous congenital disorders and carcinomas1,2. Two multimeric protein complexes with opposing functions—the cytoplasmic
destruction complex and the plasma membrane-associated signalosome—control the stability of the transcriptional co-factor β-catenin to coordinate the state of Wnt pathway activation. In the
absence of Wnt stimulation, β-catenin is targeted for proteasomal degradation by the destruction complex, which includes the two tumour suppressors: Axin and Adenomatous polyposis coli
(APC), and two kinases: casein kinase 1α (CK1α) and glycogen synthase kinase 3 (GSK3)3,4,5,6. Engagement of Wnt with its transmembrane receptors, Frizzled and low-density lipoprotein
receptor-related protein 5/6 (herein LRP6), induces rapid LRP6 phosphorylation, recruitment of Axin to phospho-LRP6, and assembly of the signalosome, which includes two other Axin-associated
components, GSK3 and Dishevelled (Dvl)7,8,9,10,11,12,13,14. Signalosome assembly results in the inhibition of β-catenin proteolysis; consequently stabilized β-catenin promotes the
transcriptional regulation of Wnt pathway target genes. As a key component in both the destruction complex and the signalosome, Axin is tightly regulated. Under basal conditions, Axin is
maintained at very low levels, and serves as the concentration-limiting scaffold for assembly of the destruction complex15,16. Following Wnt exposure, the rapid association of phospho-Axin
with phospho-LRP6 (refs 7, 12, 14) triggers Axin dephosphorylation, inducing a conformational change that inhibits Axin’s interaction with both the destruction and signalosome
complexes14,17,18. Axin is subsequently degraded7,18,19,20,21,22,23,24; however, Axin proteolysis occurs several hours after Wnt exposure, and thus does not regulate Axin’s essential role
during the initial activation of the Wnt pathway. The mechanisms that rapidly reprogram Axin from inhibitory to stimulatory roles following Wnt exposure remain uncertain. In current models,
Wnt stimulation induces Axin’s dissociation from the destruction complex, thereby promoting its interaction with the signalosome1,5,6,13,14,18,22,25,26,27. As Wnt stimulation induces Axin
dephosphorylation3,18,24, decreased phosphorylation was postulated to facilitate the dissociation of Axin from the destruction complex17; however, recent work revealed that the interaction
of Axin with LRP6 precedes Axin dephosphorylation, and that dephosphorylation serves to inhibit, rather than enhance this interaction14. Furthermore, some findings have challenged prevailing
models, providing evidence that Axin’s interaction with the destruction complex is not diminished upon Wnt stimulation2,19. Thus, whereas the rapid switch in Axin function following Wnt
stimulation is essential for the activation of signalling, the underlying mechanisms remain uncertain. During investigation of this critical process, we have discovered an unanticipated role
for the ADP-ribose polymerase Tankyrase (Tnks) in the reprogramming of Axin activity following Wnt exposure. As Tnks-mediated ADP-ribosylation is known to target Axin for proteolysis28,
small molecule Tnks inhibitors have become lead candidates for development in the therapeutic targeting of Wnt-driven cancers29,30,31. Here we identify a novel mechanism through which Tnks
regulates Axin: by promoting Axin’s central role in rapid Wnt pathway activation. We find that Wnt stimulation modulates Axin levels biphasically in both _Drosophila_ and human cells.
Unexpectedly, Axin is rapidly stabilized following Wnt stimulation, before its ultimate proteolysis hours later. In an evolutionarily conserved process, the ADP-ribosylated pool of Axin is
preferentially increased immediately following Wnt exposure. ADP-ribosylation enhances Axin’s association with phospho-LRP6, providing a mechanistic basis for the rapid switch in Axin
function following Wnt stimulation. Our results thus indicate that Tnks inhibition not only increases basal Axin levels, but also impedes the Wnt-dependent interaction between Axin and LRP6,
suggesting a basis for the potency of Tnks inhibitors in Wnt-driven cancers. Thus, Tnks not only targets Axin for proteolysis independently of Wnt stimulation, but also promotes Axin’s
central role in Wnt pathway activation, which may be relevant to the context-dependent activation of Wnt signalling and the treatment of Wnt-driven cancers with Tnks inhibitors. RESULTS AN
_IN VIVO_ ASSAY FOR AXIN REGULATION FOLLOWING WG EXPOSURE The precise onset of Wingless (Wg) /Wnt expression in fourteen segmental stripes during early _Drosophila_ embryogenesis has
provided a powerful _in vivo_ model for the signalling events triggered by Wg (Supplementary Fig. 1)32,33. However, previous studies reported contradictory conclusions regarding Axin
regulation, with the primary response to Wg exposure described as either Axin proteolysis23, or Axin recruitment from cytosol to plasma membrane34. Due to technical limitations in these
studies, the analysis of Axin was restricted to several hours after the onset of Wg exposure. In addition, Axin was overexpressed at levels high enough to abrogate Wg signalling, which
disrupts physiological regulation. We reasoned that the conflicting conclusions in previous reports might have resulted from these experimental barriers. Therefore, we sought to develop a
system that would permit an _in vivo_ analysis of Axin at near-physiological levels immediately following Wg stimulation. Because endogenous levels of Axin in _Drosophila_ embryos are too
low to be visualized with Axin antibodies, we generated transgenes encoding Axin tagged with a V5 epitope (_Axin-V5)_, and used site-specific integration35,36 coupled with the UAS/Gal4
system37 to direct their expression at near-physiological levels. Expression of _Axin-V5_ during early embryogenesis was induced with a maternal _α-tubulin_ enhancer (_mat-Gal4_) that drives
ubiquitous transcription38. In _Axin_ null mutant embryos, Wg signalling is aberrantly activated throughout the ectoderm39, as revealed by the ectopic expression of both _engrailed_ and
_wg_ (Supplementary Fig. 2a–h). Expression of _Axin-V5_ in _Axin_ null mutants completely prevented this constitutive activation of the Wg pathway, and critically, did not disrupt normal
signalling (Supplementary Fig. 2i–n). Thus, Axin-V5 replaced the function of endogenous Axin, and was expressed at levels that were regulated physiologically. Further, in wild-type embryos
expressing _Axin-V5_, the segmentally striped pattern of Wg, the resultant stabilization of Armadillo (Arm)/β-catenin in Wg-responding cells40, the Wg-dependent expression of
_engrailed_41,42, and the specification of Wg-dependent cell fates, as revealed by the patterning of the larval cuticle43, were normal (Supplementary Fig. 3a–h). To compare the relative
levels of Axin-V5 and endogenous Axin, we examined embryonic lysates. Detection of endogenous Axin by immunoblotting of cell extracts had not been possible with existing Axin antibodies18.
Therefore, we generated a new Axin antibody that allowed sensitive detection of endogenous Axin, and confirmed its specificity using RNAi-mediated _Axin_ knockdown (Supplementary Fig. 3i).
We used this Axin antibody to immunoblot lysates from wild-type embryos expressing _Axin-V5_ (Supplementary Fig. 3j). Axin-V5 was distinguished from endogenous Axin by its slower migration
rate in electrophoresis, resulting from the V5 tag. Immunoblotting revealed that Axin-V5 was not overexpressed relative to the level of endogenous Axin; instead, Axin-V5 levels were lower
than the level of endogenous Axin both before and after the onset of Wg expression (Supplementary Fig. 3j–l). We conclude that Axin levels were increased by less than twofold following the
expression of Axin-V5. BIPHASIC REGULATION OF AXIN FOLLOWING WG AND WNT EXPOSURE Thus, expression of these _Axin_ transgenes in wild-type embryos enabled an _in vivo_ analysis of Axin at
near-physiological levels before, during, and after the onset of Wg stimulation. We found that during the first 3 h of embryogenesis, before and during the onset of Wg expression, Axin was
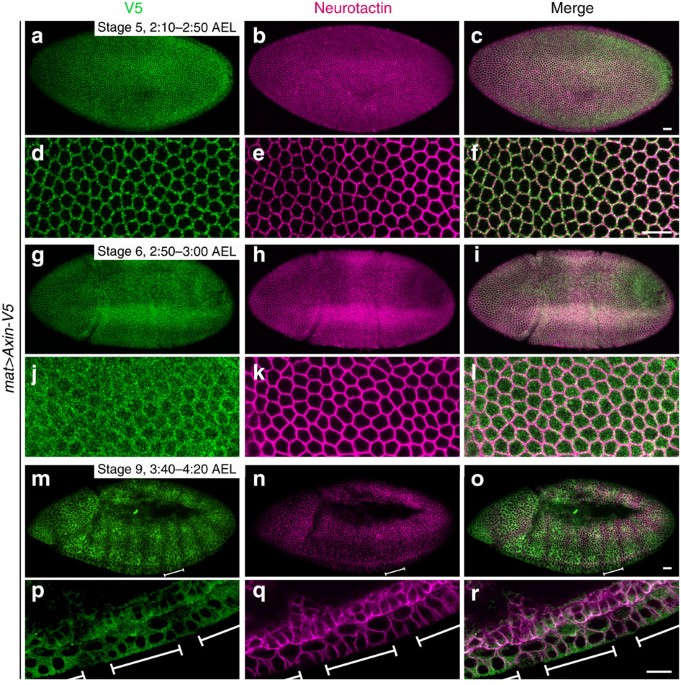
distributed uniformly throughout the ectoderm (Figs 1a–l and 2a–c). However, within 30 min after the onset of Wg expression in segmental stripes, a major change in Axin was observed,
starting in late stage 8 and persisting throughout stage 9. Axin was no longer distributed uniformly, but instead was present in wide segmental stripes of increased intensity (Fig. 1m–r;
Supplementary Fig. 4). The Axin fluorescence intensity in these stripes was two- to threefold higher than that in the interstripes (Supplementary Fig. 4). Moreover, the temporal appearance
of the Axin stripes was coincident with the stabilization of Arm/β-catenin in segmental stripes40 (Supplementary Figs 5d–f and 6a–c), suggesting that the Axin stripes were an early indicator
of the initial cellular response to Wg stimulation. To determine the relative position of the segmental stripes of Axin and Wg, we co-stained embryos expressing _Axin-V5_ with V5 and Wg
antibodies. Within 30 min after the onset of Wg expression in segmental stripes, all Axin stripes were centered over Wg stripes (Fig. 2d–f,m–r; Supplementary Fig. 5a–c). As Wg is not
required for embryonic segmentation43, we tested whether Wg was important for generating the segmental stripes of Axin. In contrast with wild-type embryos, no Axin stripes were observed in
_wg_ null mutants; instead, the Axin-V5 staining was uniform (Supplementary Fig. 6d–g). Thus, Wg is required for the differential regulation of Axin in the embryonic ectoderm. To rule out
the possibility that the segmental Axin stripes were caused artifactually by the V5 epitope tag, we generated transgenic flies expressing GFP-tagged Axin; _Axin-GFP_ was also expressed using
the _mat-Gal4_ driver. Before, during, and after the onset of Wg expression, the Axin-GFP and Axin-V5 patterns were identical, as were the kinetics of Wg-dependent changes in their
distribution (Supplementary Fig. 7). These data supported the hypothesis that within 30 min after Wg exposure, Axin is tightly regulated in Wg-responding cells, and that the rapid appearance
of Axin in stripes of increased intensity coincides with the initial activation of the pathway. To further test this hypothesis, we examined endogenous Axin levels in lysates from wild-type
embryos. Embryos were collected at developmental stages that just preceded (1.5 to 2.5 h of development) or followed (3.5 to 4.5 h of development) the onset of Wg expression. To monitor the
initial activation of the Wg pathway, we examined the accumulation of phosphorylated Arrow/LRP6 with an antibody directed against phosphothreonine1572 in human LRP6 (ref. 44), a residue
that is conserved in _Drosophila_ (Supplementary Fig. 8a). RNAi-mediated _arrow_ knockdown in S2R+ cells confirmed the specificity of this signal (Supplementary Fig. 8b,c). After the onset
of Wg stimulation, phospho-Arrow levels increased and remained elevated (Supplementary Fig. 9a). We found that Axin levels increased by approximately threefold after Wg stimulation
(Supplementary Fig. 9a,b; stage 9). The increase in Axin levels likely resulted from increased protein stability, as the _Axin_ gene is not a transcriptional target of the Wg pathway, and
instead _Axin_ transcripts are expressed ubiquitously39. These findings provided further evidence that Axin is unexpectedly stabilized following Wg stimulation, and supported the kinetics of
Axin stabilization observed in the embryos by immunostaining. In addition, we found that after the initial increase in Axin during the early response to Wg exposure, Axin levels
subsequently decreased (Supplementary Fig. 9a,b; stage 13, 9h 20 min to 10h 20 min of development), as reported previously for mammalian Axin18,19,22,23,24. To investigate the kinetics of
this process, we examined the _in vivo_ pattern of embryonic Axin stripes. We found that the Axin stripes persisted for 80 to 100 min following Wg exposure (Fig. 2d–f,m–r; Supplementary Figs
5a–c and 7m–o; late stage 8 to stage 9). After that time, Axin levels decreased, initially in cells exposed to the highest levels of Wg (Fig. 2g–i, 2 h following Wg exposure; mid-stage 10),
and subsequently, in their neighbours (Fig. 2j–l,s–x, 4 h following Wg exposure; Stage 11-13). Thus, when Axin was stabilized in the early phase following Wg exposure, Axin and Wg stripes
overlapped (Fig. 2d–f,m–r; Supplementary Figs 5a–c and 7m–o). By contrast, when Axin was destabilized in the delayed phase, Axin and Wg stripes were spatially juxtaposed (Fig. 2j–l,s–x), as
observed previously23. In summary, our unprecedented ability to visualize Axin before, during, and after the onset of Wg exposure revealed that Axin is regulated biphasically in response to
Wg stimulation _in vivo_, which reconciles the contradictory conclusions in previous reports23,34. To determine whether the biphasic regulation of Axin in response to Wg stimulation is
evolutionarily conserved, we examined Axin1 levels in cultured human cells exposed to Wnt (Supplementary Fig. 9c–f). Treatment of two cell lines, HEK293T or SW480, with Wnt3A protein
resulted in rapid pathway activation, with the accumulation of phospho-LRP6 within 15 to 30 min (Supplementary Fig. 9c,e). In both cell lines, the levels of endogenous Axin1 protein
increased within 15 to 30 min following Wnt3A treatment (Supplementary Fig. 9c–f). This increase in Axin1 likely resulted from increased protein stability, as the _Axin1_ gene is not a
transcriptional target of the Wnt pathway45,46, and the increased Axin1 protein level was observed within 15 min of Wnt exposure. By 2 h after Wnt treatment, Axin1 levels decreased
(Supplementary Fig. 9c–f), as reported previously18,24. These findings suggested that the initial stabilization and subsequent destabilization of Axin in response to Wnt exposure, and the
kinetics of this response, are evolutionarily conserved. TNKS PROMOTES AXIN STABILIZATION FOLLOWING WG EXPOSURE As Tnks is known to target Axin for proteolysis28, we sought to determine
whether Tnks regulates Axin before and/or following Wg exposure. The Tnks-dependent proteolysis of Axin requires direct interaction of Tnks with a small region in the Axin amino terminus,
the Tnks-binding domain (TBD; refs 28, 47, 48). To determine whether the evolutionarily conserved TBD facilitates the degradation of _Drosophila_ Axin, we generated an _Axin-V5_ transgene
with a 21 amino-acid deletion that eliminates the TBD (AxinΔTBD; Supplementary Fig. 10a,b). To enable their direct comparison, the _Axin-V5_ and _AxinΔTBD-V5_ transgenes were integrated at
the same genomic site (_attP33)_, and were expressed using the same _mat-Gal4_ driver. By comparison with Axin-V5, the levels of AxinΔTBD were increased by three- to fourfold (Supplementary
Fig. 10c–g), even before the onset of Wg expression (0–2 h of embryogenesis, up to stage 4; Supplementary Fig. 10c–e). We conclude that the TBD is important to control the basal levels of
_Drosophila_ Axin independently of Wg exposure. To determine whether the TBD has additional roles in Axin regulation following Wg exposure, we analysed embryos expressing _AxinΔTBD_ after
the onset of Wg expression. Unexpectedly, and in contrast with Axin-V5 (Fig. 3a–c), AxinΔTBD-V5 was not present in segmental stripes 30 min after Wg exposure; instead, AxinΔTBD levels were
increased uniformly in all ectodermal cells (Fig. 3g–i; Supplementary Fig. 11a–c; late stage 8 and stage 9). Thus, the early stripes of Axin that form in response to Wg exposure were
dependent on the TBD; deletion of the TBD resulted in the aberrant Axin stabilization in all cells. To determine whether the TBD is also required for the subsequent degradation of Axin
induced by Wg, we examined embryos at later developmental stages (Fig. 3j–l). By comparison with their neighbours, the levels of AxinΔTBD were decreased in Wg-responding cells 2 h after Wg
exposure, with kinetics similar to those observed for wild-type Axin (Fig. 3d–f,j–l; Supplementary Fig. 12i–k; mid-stage 10). These findings indicated that the TBD is not required for the
Wg-dependent proteolysis of Axin. Therefore, we hypothesized that Tnks facilitates the stabilization of Axin following Wg exposure, but is dispensable for the subsequent Axin degradation. To
test this hypothesis, we examined _Tnks_ null mutant embryos expressing _Axin-V5_. In contrast with wild-type embryos, no early stripes of Axin were present in _Tnks_ mutants; instead, Axin
levels were uniformly high throughout the embryonic ectoderm (Fig. 3m–o; Supplementary Fig. 11d–f). Two hours after Wg exposure, the levels of Axin decreased in Wg-responding cells in
_Tnks_ mutants, as we had observed in wild-type embryos (Fig. 3d–f,p–r). Thus, the same phenotype was present in embryos expressing _AxinΔTBD_ or in _Tnks_ null mutant embryos: the early
Axin stripes were absent, whereas the late Axin stripes were present. These data indicated that in the absence of Tnks, the initial Wg-dependent regulation of Axin did not occur, and Axin
was aberrantly stabilized in all cells, but that the subsequent Wg-dependent Axin proteolysis occurred with normal kinetics. To determine whether the regulation of Axin at the earliest times
after Wg exposure promotes activation of the Wg pathway, we analysed the expression of the transcription factor Engrailed. During early embryogenesis, the initial transcription of
_engrailed_ is not dependent on Wg; however, Wg signalling is required subsequently to maintain _engrailed_ expression41,42. The expression of _engrailed_ is among the earliest readouts for
the activation of Wg signalling. Upon expression of _Axin-V5_ in either wild-type or _Axin_ null mutant embryos, both the onset and maintenance of _engrailed_ expression in segmental stripes
were normal (Fig. 4a,b and Supplementary Fig. 2m; compare with Supplementary Fig. 2b). In contrast, upon expression of _AxinΔTBD-V5_ in either wild-type or _Axin_ null mutant embryos, the
initiation of _engrailed_ expression was normal, but the maintenance of _engrailed_ expression was markedly diminished, as revealed by the decreased width of Engrailed stripes (Fig. 4c,d;
Supplementary Fig. 12m), indicating defects in Wg signalling. Similarly, examination of larval cuticles following expression of _Axin-GFP_ revealed normal epidermal patterning, in which
regions of naked cuticle alternated between ventral segmental denticle belts (Supplementary Fig. 13a). In contrast, a segment polarity phenotype was observed upon expression of
_AxinΔTBD-GFP_ (Supplementary Fig. 13b), in which the ventral epidermis formed denticles in place of naked cuticle43. These findings suggested that Tnks promotes not only the initial
Wg-dependent regulation of Axin, but also the activation of Wg signalling. We tested this hypothesis by examining Wg pathway activation in _Tnks_ null mutant embryos expressing _Axin-V5_. In
_Tnks_ mutant embryos expressing _Axin-V5_, the initiation of _engrailed_ expression was normal (Fig. 4e; stage 8), but its maintenance was markedly disrupted (Fig. 4f; stage 9). Therefore,
the same defects in Wg pathway activation were observed in _Tnks_ mutant embryos expressing _Axin-V5_ and in wild-type embryos expressing _AxinΔTBD-V5_. Nonetheless, no defects in
_engrailed_ expression were observed in _Tnks_ mutants (Supplementary Fig. 14), and these mutants were viable under standard laboratory conditions49,50,51. These findings revealed that a
less than twofold increase in Axin levels by the expression of _Axin-V5_ (Supplementary Fig. 3l) uncovers the importance of Tnks in promoting Wg signalling during embryogenesis, and
suggested that the regulation of Axin by Tnks following Wg exposure can be functionally compensated until Axin reaches a critical threshold level. Altogether, these findings indicated that
Tnks promotes not only the stabilization of Axin after Wg exposure, but also the activation of Wg signalling. TNKS PROMOTES WG SIGNALLING THROUGH A NOVEL MECHANISM We sought to elucidate the
mechanism through which Tnks regulates Axin and the activation of Wg signalling. In the current model, Tnks promotes Axin degradation, limiting the activity of the destruction complex, and
thereby facilitating the activation of Wg signalling28. Alternatively, Tnks might also regulate the rapid transition of Axin following Wg stimulation. To distinguish between these two
possibilities, we tested whether an increase in the basal levels of Axin, comparable to the higher levels present in AxinΔTBD or in _Tnks_ mutants, was sufficient to inhibit Axin regulation
or Wg pathway activation. We generated transgenic flies in which the _Axin-V5_ transgene was integrated at a different site in the genome (_attP40_), which is known to result in higher
expression levels than the original site (_attP33_)35. Indeed, immunoblotting of lysates revealed that the basal levels of Axin in embryos expressing _Axin-V5_ integrated at the _attP40_
site were significantly higher than the Axin levels in embryos expressing either _Axin-V5_ or _AxinΔTBD_ integrated at the _attP33_ site (Fig. 5a,b). Similarly, comparison of fluorescence
intensity revealed that levels of _attP40 Axin-V5_ were higher than _attP33 Axin-V5, attP33 AxinΔTBD-V5_, or _Tnks_ null mutant embryos expressing _attP33 Axin-V5_ (Supplementary Fig. 15).
However, despite the increased levels of Axin present in embryos expressing _attP40 Axin-V5_, the early regulation of Axin in Wg-responding cells, as revealed by the position, width and
timing of appearance of the Axin stripes, was the same in embryos expressing either _attP40 Axin-V5_ or _attP33 Axin-V5_ (Fig. 5d-i, compare with Fig. 2d–f and Supplementary Fig. 6a–c).
Further, both the initiation and the maintenance of _engrailed_ expression were similar in embryos expressing either _attP33 Axin-V5_ or _attP40 Axin-V5_, as was the embryonic hatch rate
(Fig. 5c,j,k, compare with Fig. 4a,b). These results were in sharp contrast with the absence of early Axin stripes, the disrupted _engrailed_ expression, and the decreased embryonic hatch
rate in embryos expressing _AxinΔTBD_ or in _Tnks_ mutants expressing _attP33 Axin-V5_ (Figs 3g–i,m–o and 4c–f; Supplementary Fig. 12l–n; Fig. 5c). Altogether, these findings demonstrate
that the level to which Axin is increased in _Tnks_ mutant embryos expressing _Axin-V5_ is not sufficient to disrupt Axin regulation or to inhibit Wg signalling. Instead, these results
suggested that in addition to its known role in Axin proteolysis, Tnks also acts through a distinct mechanism to promote the initial regulation of Axin and the activation of signalling
following Wg exposure. WNT STIMULATION INCREASES THE ADP-RIBOSYLATED AXIN LEVEL Our unanticipated findings suggested that Tnks-mediated ADP-ribosylation of Axin facilitates rapid Axin
regulation and the activation of signalling following Wnt exposure. To test this hypothesis, we examined whether Wnt stimulation modulates the levels of ADP-ribosylated Axin. Detection of
ADP-ribosylated Axin has been challenging, as the levels are very low under basal conditions52, and ADP-ribosylation does not markedly alter the electrophoretic mobility of Axin28,52.
Therefore, to detect ADP-ribosylated Axin, we utilized a previously developed pull down assay based on the Trp–Trp–Glu (WWE) domain of the RING-type E3 ubiquitin ligase RNF146/Iduna coupled
to glutathione _S_-transferase (GST)52. Tnks and RNF146 are known to form a stable complex that targets substrates for ADP-ribosylation and subsequent ADP-ribose-dependent
ubiquitination52,53,54,55. The WWE domain of RNF146 interacts directly with the poly(ADP-ribose) in these Tnks substrates to promote their ubiquitination. As a control for specificity of the
GST-WWE assay, pull downs were simultaneously performed using a GST-WWER164A mutant control, in which an arginine to alanine substitution in the RNF146 WWE domain abolishes interaction with
poly(ADP-ribose)52. As an additional control for specificity, we generated a construct that encodes mouse Axin1 with a nine amino-acid deletion in the TBD, _Axin1ΔTBD_, which is predicted
to prevent the Tnks-dependent ADP-ribosylation of Axin1 (refs 28, 47, 48). GST-WWE pull downs were performed with lysates from HEK293T cells expressing either _Flag-Axin1_ or
_Flag-Axin1ΔTBD_, followed by immunoblotting with Flag antibody (Fig. 6a). Although the levels of Axin1 and Axin1ΔTBD in these lysates were comparable, GST-WWE pulled down Axin1, but not
Axin1ΔTBD. Further, neither Axin1 nor Axin1ΔTBD was pulled down by the GST-WWER164A control. Tnks-mediated ADP-ribosylation does not markedly alter the electrophoretic mobility of Axin28,52;
nonetheless, we detected small mobility shifts in the ADP-ribosylated Axin isolated by GST-WWE pull down (Supplementary Fig. 16a), suggesting that Axin is pulled down through its
ADP-ribosylation, rather than through association with another ADP-ribosylated protein. These findings confirmed the specificity of the GST-WWE pull-down assay for the detection of
ADP-ribosylated Axin. To determine whether Wnt stimulation induces changes in the level of endogenous ADP-ribosylated Axin, we performed GST-WWE pull downs on lysates from HEK293T cells
treated with Wnt3A protein. The accumulation of phospho-LRP6 was used to monitor activation of the Wnt pathway (Fig. 6b, input). Unexpectedly, within 30 min of Wnt3A exposure, the level of
ADP-ribosylated Axin1 increased by four- to fivefold (Fig. 6b,c). These results demonstrate that the pool of ADP-ribosylated Axin increases rapidly following Wnt exposure. To determine
whether the increase in the pool of ADP-ribosylated Axin in response to Wnt exposure is an evolutionarily conserved process, we used GST-WWE pull downs to analyse lysates from _Drosophila_
S2R+ cells treated with Wg conditioned medium (Fig. 6d). We analysed two downstream signalling events to monitor the temporal response of these cells to Wg exposure: the phosphorylation of
Arrow/LRP6 (refs 8, 56), and the stabilization of Arm/β-catenin40,57 (Supplementary Fig. 8d). Treatment of S2R+ cells with Wg conditioned medium resulted in increased levels of phospho-Arrow
within 10 min, which peaked at 40 to 50 min and remained elevated for more than 2 h. In addition, Arm/β-catenin was stabilized within 20 min of Wg exposure and remained elevated for 2 h
(Supplementary Fig. 8d), as reported previously57. Our new Axin antibody allowed sensitive detection of endogenous Axin, and revealed a mobility shift following Wg stimulation (Figs 6d and
7a, input), consistent with the Wnt-dependent dephosphorylation of Axin observed previously in mammalian cells14,17,18. GST-WWE pull down followed by immunoblotting with the Axin antibody
revealed that the levels of ADP-ribosylated Axin increased within one hour of stimulation with Wg. In contrast, Axin was not pulled down by the GST-WWER164A control, confirming specificity
of the signal (Fig. 6d). The increase in ADP-ribosylated Axin levels ranged from four- to sevenfold, indicating that, as observed in human cells, ADP-ribosylated Axin accumulated rapidly
following Wg stimulation in _Drosophila_ cells (Fig. 6d,e). To further test this hypothesis _in vivo_, we quantitated changes in the levels of ADP-ribosylated Axin following Wg stimulation
in lysates from staged _Drosophila_ embryos expressing _Axin-V5_ using GST-WWE pull downs. These studies revealed that the total levels of Axin-V5 increased by approximately threefold in
stage 9 embryos (after Wg stimulation) by comparison with stage 4–5 embryos (before Wg stimulation; Fig. 6f,g). Importantly, the level of ADP-ribosylated Axin-V5 increased by ∼13-fold after
Wg stimulation (Fig. 6f,h). As described above, small mobility shifts were detected in ADP-ribosylated Axin (Supplementary Fig. 16b). These results indicated that the pool of ADP-ribosylated
Axin is preferentially increased following Wg exposure. Therefore, we conclude that the rapid increase in ADP-ribosylated Axin is an evolutionarily conserved response to Wnt/Wg stimulation.
ADP-RIBOSYLATION ENHANCES THE INTERACTION OF AXIN WITH LRP6 On the basis of the rapid accumulation of ADP-ribosylated Axin in both _Drosophila_ and human cells following Wnt exposure, we
speculated that ADP-ribosylation of Axin might facilitate Axin’s interaction with phospho-LRP6 and the subsequent activation of the Wnt pathway. Indeed, we observed that in lysates of human
cells exposed to Wnt, not only Axin, but also phospho-LRP6 was pulled down by GST-WWE (Fig. 6b). Analogously, in lysates of _Drosophila_ cultured cells or embryos exposed to Wg,
phospho-Arrow was pulled down by GST-WWE, but not the GST-WWER164A control, indicating a requirement for ADP-ribosylation (Fig. 6d,f). Thus, we hypothesized that both phospho-LRP6 and
phospho-Arrow were pulled down through their association with ADP-ribosylated Axin following Wnt exposure. To test this hypothesis, we repeated the GST-WWE pull downs after depleting
_Drosophila_ Axin using RNAi-mediated knockdown in cultured _Drosophila_ cells (Fig. 7a). Treatment of S2R+ cells with _Axin_ dsRNA resulted in a marked reduction in Axin levels, to the
extent that Arm/β-catenin was aberrantly stabilized in the absence of Wg stimulation (Supplementary Fig. 3i). In the presence or absence of _Axin_ knockdown, the level of phospho-Arrow was
comparable (Fig. 7a, input); however, the pull down of phospho-Arrow by GST-WWE was completely dependent on Axin (Fig. 7a). Although we cannot exclude the possibility that phospho-LRP6/Arrow
is ADP-ribosylated in an Axin-dependent manner, these findings support the hypothesis that following Wg exposure, phospho-Arrow interacts with ADP-ribosylated Axin. To further test the
hypothesis that the ADP-ribosylation of Axin promotes its interaction with phospho-LRP6 following Wnt stimulation, we treated HEK293T cells expressing Flag-Axin1 with either a dimethyl
sulfoxide (DMSO) control or with the small molecular inhibitor XAV939 (ref. 28), to inhibit the catalytic activity of the two human Tnks proteins. We immunoprecipitated from lysates of these
cells with Flag antibody, followed by immunoblotting with phospho-LRP6 antibodies. Treatment with XAV939, but not the DMSO control, markedly decreased the Wnt-dependent interaction between
Axin1 and phospho-LRP6 within 30 min of Wnt3A exposure (Fig. 7b). To further test this hypothesis, we immunoprecipitated lysates of HEK293T cells expressing _Flag-Axin1_ or _Flag-Axin1ΔTBD_
with Flag antibody, followed by immunoblotting with phospho-LRP6 antibodies directed against either phospho-serineS1490 [S1490 (ref. 8)] or phosphothreonine1572 [T1572 (ref. 44)]. By 30 min
after treatment with Wnt3A, Axin1 interacted with phospho-LRP6, as revealed by both phospho-LRP6 antibodies (Fig. 7c,d)8,12. In contrast, deletion of the TBD in Axin1 diminished the
interaction of Axin with phospho-LRP6 (Fig. 7c,d). Analogously, deletion of the TBD in _Drosophila_ Axin decreased the Wg-dependent interaction between Axin and phospho-Arrow (Supplementary
Fig. 17a). Deletion of the TBD in human Axin1 had no detectable effect on Axin1’s interaction with GSK3β, β-catenin or Dvl (Supplementary Fig. 17b,c). Altogether, these results indicated
that the Tnks-dependent ADP-ribosylation of Axin promotes Axin’s rapid interaction with phospho-LRP6 following Wnt stimulation. Finally, we tested whether the poly(ADP-ribosyl) polymerase
(PARP) activity of Tnks promotes the initial activation of Wnt signalling. We generated HA-tagged _Tnks_ transgenes for expression in _Drosophila_ embryos: one transgene encoded wild-type
Tnks, whereas the other transgene was identical, except for a methionine to valine substitution (M1064V) in the catalytic PARP domain (Fig. 8a). The analogous mutation (M1054V) in the highly
conserved PARP domain of human Tnks2 is known to abolish its catalytic activity58. Both _Tnks_ transgenes were integrated at the same genomic site, and expressed using the _mat-Gal4_
driver. As noted above, in _Tnks_ null mutant embryos expressing _Axin-V5_, the Wg-dependent expression of _engrailed_ was disrupted (Fig. 4f). In contrast, _engrailed_ expression was fully
restored in _Tnks_ mutant embryos expressing the wild-type _Tnks_ transgene (Fig. 8b), but not the _Tnks__M1064V_ transgene (Fig. 8c). Together, these findings support the conclusion that
the ADP-ribosylation of Axin by Tnks promotes not only the interaction between Axin and phospho-LRP6 following Wg exposure, but also the initial activation of Wg signalling. DISCUSSION We
demonstrate that Wnt exposure induces biphasic regulation in the level of Axin, and a large increase in the level of ADP-ribosylated Axin immediately after stimulation. ADP-ribosylation
enhances the interaction of Axin with phospho-LRP6, and promotes the activation of Wnt signalling. Our findings lead to three major revisions of the current model for the role of Tnks in the
activation of the Wnt pathway. First, Tnks serves bifunctional roles under basal conditions and after stimulation, revealing a remarkable economy and coordination of pathway components.
Second, our results provide a mechanistic basis for the rapid reprogramming of Axin function in response to Wnt stimulation, and thereby reveal an unanticipated role for Tnks in this process
(Fig. 8d). These findings suggest that Wnt exposure either rapidly increases the ADP-ribosylation of Axin or inhibits the targeting of ADP-ribosylated Axin for proteasomal degradation,
through mechanisms yet to be elucidated. Finally, we demonstrate that pharmacologic inactivation of Tnks diminishes the interaction of Axin with LRP6, revealing a previously unknown
mechanism through which small molecule Tnks inhibitors disrupt Wnt signalling, distinct from their known role in stabilizing the destruction complex. In the absence of Wnt stimulation, the
concentration-limiting levels of Axin regulate its scaffold function in the destruction complex15,16. As components of the destruction complex participate in other signalling pathways, the
low levels of Axin were proposed to maintain modularity of the Wnt pathway16. Our new findings indicate that Axin levels are not only regulated in the absence of Wnt, but also regulated
biphasically following Wnt stimulation. This sequential modulation of Axin divides activation of the pathway into an early, fast phase and a delayed long-term phase. During embryogenesis,
the earliest expression of Wg triggers the rapid appearance of Axin in segmental stripes, which is a novel hallmark for the initial activation of the pathway. Our findings reveal that Wnt
exposure induces a rapid increase in the total level of Axin, and importantly, a preferential increase in the level of the ADP-ribosylated Axin. The early Axin stripes are absent in _Tnks_
null mutant embryos and are also absent when the Tnks binding domain in Axin is deleted. Therefore, we propose that Axin ADP-ribosylation contributes to Axin stabilization and to the rapid
response to Wg stimulation. We postulate that the initial increase in levels of ADP-ribosylated Axin jump-starts the response to Wnt stimulation by enhancing the Axin-LRP6 interaction,
whereas the subsequent decrease in Axin levels prolongs the duration of signalling by reducing destruction complex assembly. Thus, Wnt stimulation induces rapid increases in the levels of
not only cytoplasmic β-catenin, but also ADP-ribosylated Axin. Previous work that coupled mathematical modelling with experimental analysis revealed that several Wnt signalling systems were
responsive to the relative change in β-catenin levels, rather than their absolute value59. This dependence was proposed to impart robustness and resistance to noise and cellular variation.
Our data raise the possibility that a similar principle applies to changes in Axin levels on the Axin-LRP6 interaction, as the marked increase in ADP-ribosylated Axin levels following Wnt
stimulation is evolutionarily conserved. Thus, the relative change in levels of ADP-ribosylated Axin may promote signalling following Wnt exposure by facilitating the fold change in
β-catenin levels. Our findings have relevance for the context-specific _in vivo_ roles of Tnks in Wnt signalling suggested in previous studies. Tnks inhibition disrupts Wnt signalling in a
number of cultured cell lines28, but _in vivo_ studies in several model organisms suggested that the requirement for Tnks in promoting Wnt signalling is restricted to specific cell types or
developmental stages. In mice, functional redundancy exists between the two Tnks homologues60, such that _Tnks_ single mutants are viable and fertile, whereas double mutants display
embryonic lethality without overt Wnt-related phenotypes60 (see Discussion in ref. 61). However, a missense mutation in the TBD of Axin2 that is predicted to disrupt ADP-ribosylation
resulted in either activating or inhibiting effects on Wnt signalling that were dependent on developmental stage. Tnks inhibitors resulted in the same paradoxical effects, suggesting complex
roles in mouse embryonic development61. Analogously, treatment of fish with Tnks inhibitors resulted in no observed defects in Wnt-mediated processes during development28; however, the
regeneration of injured fins in adults, a process that requires Wnt signalling62, was disrupted28,63. Similarly, the finding that _Drosophila Tnks_ null mutants are viable49,50,51 was
unexpected, as Tnks is highly evolutionarily conserved, and no other Tnks homologues exist in fly genomes. Nonetheless, our studies reveal that a less than twofold increase in Axin levels
uncovers the importance of Tnks in promoting Wg signalling during embryogenesis. Therefore, we postulate that Tnks loss can be compensated during development unless Axin levels are
increased, but that the inhibition of Wg signalling resulting from Tnks inactivation cannot be attributed solely to increased Axin levels (Supplementary Fig. 3, Figs 4f and 5). Furthermore,
_Drosophila_ Tnks is essential for Wg target gene activation in the adult intestine, and exclusively within regions of the gradient where Wg is present at relatively low concentration49,64.
Thus, the context-specific roles of Tnks observed in different model organisms may reflect the mechanisms described herein, which reveal that the Wnt-induced association of Axin with LRP6
occurs even in the absence of Axin ADP-ribosylation, but is markedly enhanced in its presence. We postulate that by enhancing this interaction, Tnks-dependent ADP-ribosylation of Axin serves
to amplify the initial response to Wnt stimulation, and thus is essential in a subset of _in vivo_ contexts. The recent discovery that Tnks enhances signalling in Wnt-driven cancers has
raised the possibility that Tnks inhibitors will offer a promising new therapeutic option28,63. Indeed, preclinical studies have supported this possibility30. Tnks inhibitors were thought
previously to disrupt Wnt signalling solely by increasing the basal levels of Axin, and thus by increasing destruction complex activity. However, our findings indicate that the degree to
which the basal level of Axin increases following Tnks inactivation is not sufficient to disrupt Wnt signalling in some _in vivo_ contexts. Instead, our results reveal that Tnks inhibition
simultaneously disrupts signalling at two critical and functionally distinct steps: by promoting activity of the destruction complex and by diminishing an important step in signalosome
assembly: the Wnt-induced interaction between LRP6 and Axin. On the basis of these findings, we propose that the efficacy of Tnks inhibitors results from their combined action at both of
these steps, providing a rationale for their use in the treatment of a broad range of Wnt-driven cancers. Therefore, our results suggest that in contrast with the current focus on tumours in
which attenuation of the destruction complex aberrantly activates Wnt signalling (such as those lacking APC), the preclinical testing of Tnks inhibitors could be expanded to include cancers
that are dependent on pathway activation by Wnt stimulation. These include the colorectal, gastric, ovarian and pancreatic cancers that harbour inactivating mutations in _RNF43_ (refs 65,
66, 67, 68, 69, 70), a negative Wnt feedback regulator that promotes degradation of the Wnt co-receptors Frizzled and LRP6 (refs 70, 71, 72). METHODS _DROSOPHILA_ STOCKS AND GENETICS To
generate the _pUASTattB Axin-V5_ transgene, we isolated a KpnI/HindIII fragment from _pAc5.1-Daxin-3xHA_ (ref. 28) and a HindIII/XbaI fragment encoding a triple V5 epitope from _pBlue
SK-3xV5_ (ref. 73), and ligated these fragments into _pUASTattB_ (refs 36, 37), at the KpnI and XbaI sites. To generate the _pUASTattB-AxinΔTBD-V5_ transgene, residues D-12 through K-32 were
deleted by PCR-based mutagenesis of _pUASTattB-Axin-V5_ using the oligonucleotide: 5′-GGTATCTGCTACCCCTTCGGTCATATGTTTCCGGATTCC-3′. The resulting _AxinΔTBD-V5_ fragment was digested with KpnI
and XbaI, and inserted into the _pUASTattB vector_ at the KpnI and XbaI sites. Transgenic flies were generated using phiC31-based integration36 at the _attP33_ or _attP40_ sites on
chromosome 2 (ref. 35). _pUASTattB Axin-GFP_ was generated using the Gateway LR Clonase system (Invitrogen), with an entry plasmid containing an _Axin_ cDNA amplified by PCR from an ovarian
library74 and the _pTWG_ destination vector containing sequences encoding _GFP_ (Drosophila Genomics Resource Center). _Axin-GFP_ was amplified by PCR using primers that introduced KpnI and
XbaI restriction sites at the 5′ and 3′ ends, respectively. Following KpnI and XbaI digestion, the amplified product was cloned into _pUASTattB_. Transgenic flies were generated using
phiC31-based site-specific integration at the _attP33 site_. _Axin-GFP_ has an 18 amino-acid linker sequence between the carboxy terminus of Axin and the amino terminus of GFP:
KPSD/kgradpaflykvvssat/(GFP) (‘/’ represents the junction between Axin and GFP, with additional amino acids shown in lowercase). The _pUASTattB-AxinΔTBD-GFP_ transgene was generated by
PCR-based mutagenesis of _pUASTattB-Axin-GFP_, in which residues D-12 through K-32 were deleted using the oligonucleotide: 5′-GGAATCCGGAAACATATGACCGAAGGGGTAGCAGATACC-3′. The resulting
_AxinΔTBD-GFP_ fragment was digested with KpnI and XbaI and inserted into the _pUASTattB_ vector at the KpnI and XbaI sites. To generate the _pUASTattB Tnks-HA_ transgene, a PCR fragment
encoding Tnks was amplified from the cDNA _LD22548_ (Drosophila Genomics Research Center), with the addition of an XhoI restriction site to 5′ end using the primer
5′-ACTCTCGAGATGGCCAACAGCAGCCGAAGT-3′. A fragment encoding a double-HA epitope followed by a KpnI restriction site was added to the 3′ end of the _Tnks_ cDNA using the PCR primer
5′-CATAGTCCGGGACGTCATAGGGATAGCCCGCATAGTCAGGAACATC-3′. The _Tnks-HA_ fragment was digested with XhoI and KpnI, and inserted into the _pUASTattB_ vector at the XhoI and KpnI sites. _pUASTattB
Tnks__M1064V__-HA_ was generated by replacing the methionine at position 1,064 with valine by PCR-based mutagenesis of _pUAST-Tnks-2_ × _HA_ using the oligonucleotide
5′-ATTGGCGGCGTGTTTGGGGCT-3′, and cloned into the XhoI and KpnI restriction sites of _pUASTattB._ Transgenic flies were generated using phiC31-based integration at the _attP40_ site. To
obtain embryos expressing _Axin-V5_ driven by _mat-Gal4_: _mat-Gal4_ females were crossed to _UAS-Axin-V5_ males. F1 female progeny of the genotype _UAS-Axin-V5 / mat-Gal4_ were crossed to
_UAS-Axin-V5_ males. To obtain _Tnks_ null mutant embryos expressing _Axin-V5_ driven by _mat-Gal4_: _UAS-Axin-V5; Tnks__19_ _mutant_ males were crossed to _mat-Gal4; Tnks__19_ females. F1
female progeny of the genotype _UAS-Axin-V5/mat-Gal4; Tnks__19_ were crossed to _UAS-Axin-V5_; _Tnks__19_ males. To obtain _Tnks_ null mutant embryos expressing _Tnks-HA_ and _Axin-V5_
driven by _mat-Gal4_: _UAS-Tnks-HA_, _UAS-Axin-V5_; _Tnks__503_ mutant males were crossed to _mat-Gal4; Tnks__503_ females. F1 female progeny of the genotype _UAS-Tnks-HA_,
_UAS-Axin-V5/mat-Gal4_; _Tnks__503_ were crossed to _UAS-Tnks-HA_, _UAS-Axin-V5_; _Tnks__503_ males. To obtain Tnks null mutant embryos expressing _Tnks__M1064V__-HA_ and _Axin-V5_ driven by
_mat-Gal4: UAS-Tnks__M1064V__-HA, UAS-Axin-V5; Tnks__503_ mutant males were crossed to _mat-Gal4; Tnks__503_ females. F1 female progeny of the genotype _UAS-Tnks__M1064V__-HA,
UAS-Axin-V5/mat-Gal4; Tnks__503_ were crossed to _UAS-Tnks__M1064V__-HA, UAS-Axin-V5; Tnks__503_ males. ANTIBODIES The primary antibodies used for immunostaining were rabbit anti-V5
(1:1,000, Abcam, ab9116), rabbit anti-GFP (1:200, Invitrogen, A11122), mouse anti-Engrailed (1:100, concentrated 4D9; Developmental Studies Hybridoma Bank, DSHB), mouse anti-Wg (1:200, 4D4
concentrated antibody, DSHB), mouse anti-Arm (1:20, N2 7A1, DSHB) and mouse anti-Neurotactin (1:20, BP106, DSHB). Secondary antibodies were goat or donkey Alexa Fluor 488 or 555 conjugates
(1:400, Invitrogen, A21202, A11008, A31572, A31570). The primary antibodies used for immunoblotting were mouse anti-V5 (1:5,000, Invitrogen, 46-1157), guinea pig anti-Axin (1:1,000 (ref.
49)), mouse anti-Wg (1:100, 4D4, DSHB), rabbit anti-Kinesin Heavy Chain (1:10,000, Cytoskeleton, AKIN01), mouse anti-Arm (1:100, N2 7A1, DSHB), mouse anti-α-Tubulin (1:10,000, DM1A, Sigma,
T6199), rabbit anti-α-Tubulin (1:10,000, Sigma, SAB3501071), mouse anti-Flag M2 (1:1000, Sigma, F3165), rabbit anti-GSK3β (1:1,000, Cell Signaling, 9315), mouse anti-β-catenin (1:4,000, BD
Biosciences, 610154), rabbit anti-Axin1 (1:1,000, Cell Signaling, #2074), rabbit anti-LRP6 (1:1,000, Cell Signaling, 2560), rabbit anti-c-Myc (1:1,000, Santa Cruz Biotechnology, sc-40),
rabbit anti-phospho-LRP6 [Ser1490] (1:1,000, Cell Signaling8, 2568), rabbit anti-phospho-LRP6 [Thr1572] (1:1,000, Millipore44, 07-2187), and guinea pig anti-Arrow (1:1,000 (ref. 75)).
Secondary antibodies used for immunoblotting were goat anti-rabbit HRP conjugate (1:10,000, Biorad, 170-6515), goat anti-mouse HRP conjugate (1:10,000, Biorad, 170-6516) and goat anti-guinea
pig HRP conjugate (1:10,000, Jackson ImmunoResearch). GST-WWE PULL DOWNS For GST pull downs, GST-WWE and GST-WWER164A beads were generated as described previously52. Embryos, S2R+ or
HEK293T cells were treated as indicated, then washed once with cold 1 × PBS and lysed in lysis buffer (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1% NP-40, 10% glycerol, 1.5 mM EDTA [pH 8.0]) or
RIPA buffer (50 mM Tris-HCl (pH 8.5), 300 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS) supplemented with 1 μM of the poly(ADP-ribose) glycohydrolase inhibitor ADP-HDP (Enzo Life
Sciences), and protease and phosphatase inhibitor cocktail (1:100, Thermo Scientific). Lysates were incubated with GST-WWE or GST-WWER164A beads overnight at 4 °C. Following incubation,
beads were washed four times in wash buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP-40, 10% Glycerol, 1.5 mM EDTA [pH 8.0]) supplemented with 1 μM ADP-HPD and protease and phosphatase
inhibitor cocktail (1:100). Bound materials were eluted with 2 × sample buffer and resolved by SDS–PAGE, transferred to PVDF or nitrocellulose membranes and blotted with the indicated
antibodies. PLASMIDS Plasmids used for transfection of human cell lines were _pCS2+ Flag-mouse Axin1_ (ref. 8) and _pCS2+ Flag-mouse Axin1ΔTBD_. To generate the _pCS2+ Flag-mouse Axin1ΔTBD_
plasmid, residues P-26 through E-34 were deleted by PCR-based mutagenesis of _pCS2+ Flag-mouse Axin1_ using the oligonucleotide: 5′-GATGCCGGAGAACTGGTATCTACTGAT-3′. The resulting Flag-mouse
_AxinΔTBD_ fragment was digested with ClaI and BglII, and then inserted into the _pCS2+ vector_ at the ClaI and BglII sites. Plasmids used for transfection of _Drosophila_ cells were
_pAc5.1-Axin-V5_ and _pAc5.1-AxinΔTBD-V5_. To generate the _pAc5.1-Axin-V5_ and _pAc5.1-AxinΔTBD-V5_ plasmids: fragments encoding Axin-V5 and AxinΔTBD-V5 from _pUASTattB-Axin-V5_ and
_pUASTattB-AxinΔTBD-V5_, respectively, were digested using KpnI and XbaI. The resulting fragments were inserted into the _pAc5.1_ vector (Invitrogen) at the KpnI and XbaI sites. STUDY DESIGN
AND STATISTICAL ANAYSIS In all experiments reported in this study, Student’s _t_-test and ANOVA test were performed using Prism (GraphPad Software Inc., CA, USA) or the SAS version 9.4
(Statistical Analysis System Institute Inc., NC, USA). Student’s _t_-test was used to compare two groups for all data sets. ANOVA test was used for comparison of more than two groups. _P_
values and detailed test statistics are provided in the figure legends. No blinding was done and no particular randomization method was used. All experiments were performed at least three
times. Additional methods and fly stocks used are described in Supplementary Information. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Yang, E. _et al_. Wnt pathway activation by
ADP-ribosylation. _Nat. Commun._ 7:11430 doi: 10.1038/ncomms11430 (2016). REFERENCES * MacDonald, B. T., Tamai, K. & He, X. Wnt/beta-catenin signaling: components, mechanisms, and
diseases. _Dev. Cell._ 17, 9–26 (2009). Article CAS PubMed PubMed Central Google Scholar * Clevers, H. & Nusse, R. Wnt/beta-catenin signaling and disease. _Cell_ 149, 1192–1205
(2012). Article CAS PubMed Google Scholar * Ikeda, S. et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes
GSK-3beta-dependent phosphorylation of beta-catenin. _EMBO J._ 17, 1371–1384 (1998). Article CAS PubMed PubMed Central Google Scholar * Behrens, J. et al. Functional interaction of an
axin homolog, conductin, with beta-catenin, APC, and GSK3beta. _Science_ 280, 596–599 (1998). Article CAS ADS PubMed Google Scholar * Kimelman, D. & Xu, W. beta-catenin destruction
complex: insights and questions from a structural perspective. _Oncogene_ 25, 7482–7491 (2006). Article CAS PubMed Google Scholar * Stamos, J. L. & Weis, W. I. The beta-catenin
destruction complex. _Cold Spring Harb. Perspect. Biol._ 5, a007898 (2013). Article PubMed PubMed Central Google Scholar * Mao, J. et al. Low-density lipoprotein receptor-related
protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. _Mol. Cell._ 7, 801–809 (2001). Article CAS PubMed Google Scholar * Tamai, K. et al. A mechanism for Wnt
coreceptor activation. _Mol. Cell._ 13, 149–156 (2004). Article CAS PubMed Google Scholar * Bilic, J. et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6
phosphorylation. _Science_ 316, 1619–1622 (2007). Article CAS ADS PubMed Google Scholar * Zeng, X. et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation.
_Nature_ 438, 873–877 (2005). Article CAS ADS PubMed PubMed Central Google Scholar * Davidson, G. et al. Casein kinase 1gamma couples Wnt receptor activation to cytoplasmic signal
transduction. _Nature_ 438, 867–872 (2005). Article CAS ADS PubMed Google Scholar * Zeng, X. et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6
phosphorylation/activation via frizzled, dishevelled and axin functions. _Development_ 135, 367–375 (2008). Article CAS PubMed Google Scholar * Schwarz-Romond, T., Metcalfe, C. &
Bienz, M. Dynamic recruitment of axin by Dishevelled protein assemblies. _J. Cell. Sci._ 120, 2402–2412 (2007). Article CAS PubMed Google Scholar * Kim, S. E. et al. Wnt stabilization of
beta-catenin reveals principles for morphogen receptor-scaffold assemblies. _Science_ 340, 867–870 (2013). Article CAS ADS PubMed PubMed Central Google Scholar * Salic, A., Lee, E.,
Mayer, L. & Kirschner, M. W. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. _Mol. Cell._ 5, 523–532 (2000).
Article CAS PubMed Google Scholar * Lee, E., Salic, A., Kruger, R., Heinrich, R. & Kirschner, M. W. The roles of APC and Axin derived from experimental and theoretical analysis of
the Wnt pathway. _PLoS Biol._ 1, E10 (2003). Article PubMed PubMed Central Google Scholar * Luo, W. et al. Protein phosphatase 1 regulates assembly and function of the beta-catenin
degradation complex. _EMBO J._ 26, 1511–1521 (2007). Article CAS PubMed PubMed Central Google Scholar * Willert, K., Shibamoto, S. & Nusse, R. Wnt-induced dephosphorylation of axin
releases beta-catenin from the axin complex. _Genes Dev._ 13, 1768–1773 (1999). Article CAS PubMed PubMed Central Google Scholar * Li, V. S. et al. Wnt signaling through inhibition of
beta-catenin degradation in an intact Axin1 complex. _Cell_ 149, 1245–1256 (2012). Article CAS PubMed Google Scholar * Cselenyi, C. S. et al. LRP6 transduces a canonical Wnt signal
independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. _Proc. Natl Acad. Sci. USA_ 105, 8032–8037 (2008). Article CAS ADS PubMed PubMed Central
Google Scholar * Kofron, M. et al. Wnt11/beta-catenin signaling in both oocytes and early embryos acts through LRP6-mediated regulation of axin. _Development_ 134, 503–513 (2007). Article
CAS PubMed Google Scholar * Liu, X., Rubin, J. S. & Kimmel, A. R. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha
proteins. _Curr. Biol._ 15, 1989–1997 (2005). Article CAS PubMed Google Scholar * Tolwinski, N. S. et al. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of
Zw3/Gsk3beta activity. _Dev. Cell._ 4, 407–418 (2003). Article CAS PubMed Google Scholar * Yamamoto, H. et al. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen
synthase kinase-3beta regulates its stability. _J. Biol. Chem._ 274, 10681–10684 (1999). Article CAS PubMed Google Scholar * Valvezan, A. J., Zhang, F., Diehl, J. A. & Klein, P. S.
Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. _J. Biol. Chem._ 287, 3823–3832 (2012). Article CAS PubMed
Google Scholar * Malbon, C. C. & Wang, H. Y. Dishevelled: a mobile scaffold catalyzing development. _Curr. Top. Dev. Biol._ 72, 153–166 (2006). Article CAS PubMed Google Scholar *
Hernandez, A. R., Klein, A. M. & Kirschner, M. W. Kinetic Responses of beta-Catenin Specify the Sites of Wnt Control. _Science_ 338, 1337–1340 (2012). Article CAS ADS PubMed Google
Scholar * Huang, S. M. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. _Nature_ 461, 614–620 (2009). Article CAS ADS PubMed Google Scholar * Waaler, J. et
al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. _Cancer Res._ 72, 2822–2832 (2012).
Article CAS PubMed Google Scholar * Lau, T. et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. _Cancer Res._ 73, 3132–3144 (2013).
Article CAS PubMed Google Scholar * Liu, C. & He, X. Destruction of a destructor: a new avenue for cancer therapeutics targeting the Wnt pathway. _J. Mol. Cell Biol._ 2, 70–73
(2010). Article CAS PubMed Google Scholar * van den Heuvel, M., Nusse, R., Johnston, P. & Lawrence, P. A. Distribution of the wingless gene product in Drosophila embryos: a protein
involved in cell-cell communication. _Cell_ 59, 739–749 (1989). Article CAS PubMed Google Scholar * Baker, N. E. Molecular cloning of sequences from wingless, a segment polarity gene in
Drosophila: the spatial distribution of a transcript in embryos. _EMBO J._ 6, 1765–1773 (1987). Article CAS PubMed PubMed Central Google Scholar * Cliffe, A., Hamada, F. & Bienz, M.
A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. _Curr. Biol._ 13, 960–966 (2003). Article CAS PubMed Google Scholar * Markstein, M., Pitsouli,
C., Villalta, C., Celniker, S. E. & Perrimon, N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. _Nat. Genet._ 40, 476–483
(2008). Article CAS PubMed PubMed Central Google Scholar * Bischof, J., Maeda, R. K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using
germ-line-specific phiC31 integrases. _Proc. Natl Acad. Sci. USA_ 104, 3312–3317 (2007). Article CAS ADS PubMed PubMed Central Google Scholar * Brand, A. H. & Perrimon, N. Targeted
gene expression as a means of altering cell fates and generating dominant phenotypes. _Development_ 118, 401–415 (1993). CAS PubMed Google Scholar * Hacker, U. & Perrimon, N.
DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. _Genes Dev._ 12, 274–284 (1998). Article CAS PubMed PubMed
Central Google Scholar * Hamada, F. et al. Negative regulation of Wingless signaling by D-axin, a Drosophila homolog of axin. _Science_ 283, 1739–1742 (1999). Article CAS ADS PubMed
Google Scholar * Riggleman, B., Schedl, P. & Wieschaus, E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. _Cell_
63, 549–560 (1990). Article CAS PubMed Google Scholar * Bejsovec, A. & Martinez Arias, A. Roles of wingless in patterning the larval epidermis of Drosophila. _Development_ 113,
471–485 (1991). CAS PubMed Google Scholar * DiNardo, S., Sher, E., Heemskerk-Jongens, J., Kassis, J. A. & O'Farrell, P. H. Two-tiered regulation of spatially patterned engrailed
gene expression during Drosophila embryogenesis. _Nature_ 332, 604–609 (1988). Article CAS ADS PubMed PubMed Central Google Scholar * Nusslein-Volhard, C. & Wieschaus, E. Mutations
affecting segment number and polarity in Drosophila. _Nature_ 287, 795–801 (1980). Article CAS ADS PubMed Google Scholar * MacDonald, B. T., Yokota, C., Tamai, K., Zeng, X. & He,
X. Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. _J. Biol. Chem._ 283, 16115–16123 (2008). Article
CAS PubMed PubMed Central Google Scholar * Leung, J. Y. et al. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. _J.
Biol. Chem._ 277, 21657–21665 (2002). Article CAS PubMed Google Scholar * Jho, E. H. et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the
signaling pathway. _Mol. Cell. Biol._ 22, 1172–1183 (2002). Article CAS PubMed PubMed Central Google Scholar * Morrone, S., Cheng, Z., Moon, R. T., Cong, F. & Xu, W. Crystal
structure of a Tankyrase-Axin complex and its implications for Axin turnover and Tankyrase substrate recruitment. _Proc. Natl Acad. Sci. USA_ 109, 1500–1505 (2012). Article CAS ADS PubMed
PubMed Central Google Scholar * Guettler, S. et al. Structural basis and sequence rules for substrate recognition by Tankyrase explain the basis for cherubism disease. _Cell_ 147,
1340–1354 (2011). Article CAS PubMed Google Scholar * Wang, Z. et al. The ADP-ribose polymerase Tankyrase regulates adult intestinal stem cell proliferation during homeostasis in
Drosophila. _Development_ (in the press). * Wang, Z. et al. Wnt/Wingless pathway activation is promoted by a critical threshold of Axin maintained by tumor supressor Apc and ADP-ribose
polymerase Tankyrase. _Genetics_ 203, 1–13 (2016). Article Google Scholar * Feng, Y. et al. The Drosophila tankyrase regulates Wg signaling depending on the concentration of Daxin. _Cell
Signal._ 26, 1717–1724 (2014). Article CAS PubMed PubMed Central Google Scholar * Zhang, Y. et al. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and
Wnt signalling. _Nat. Cell. Biol._ 13, 623–629 (2011). Article CAS PubMed Google Scholar * DaRosa, P. A. et al. Allosteric activation of the RNF146 ubiquitin ligase by a
poly(ADP-ribosyl)ation signal. _Nature_ 517, 223–226 (2014). Article ADS PubMed PubMed Central Google Scholar * Callow, M. G. et al. Ubiquitin ligase RNF146 regulates tankyrase and Axin
to promote Wnt signaling. _PLoS ONE_ 6, e22595 (2011). Article CAS ADS PubMed PubMed Central Google Scholar * Wang, Z. et al. Recognition of the iso-ADP-ribose moiety in
poly(ADP-ribose) by WWE domains suggests a general mechanism for poly(ADP-ribosyl)ation-dependent ubiquitination. _Genes Dev._ 26, 235–240 (2012). Article PubMed PubMed Central Google
Scholar * Wehrli, M. et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. _Nature_ 407, 527–530 (2000). Article CAS ADS PubMed Google Scholar *
Yanagawa, S., Lee, J. S. & Ishimoto, A. Identification and characterization of a novel line of Drosophila Schneider S2 cells that respond to wingless signaling. _J. Biol. Chem._ 273,
32353–32359 (1998). Article CAS PubMed Google Scholar * Sbodio, J. I., Lodish, H. F. & Chi, N. W. Tankyrase-2 oligomerizes with tankyrase-1 and binds to both TRF1
(telomere-repeat-binding factor 1) and IRAP (insulin-responsive aminopeptidase). _Biochem. J._ 361, 451–459 (2002). Article CAS PubMed PubMed Central Google Scholar * Goentoro, L. &
Kirschner, M. W. Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. _Mol. Cell._ 36, 872–884 (2009). Article CAS PubMed PubMed Central Google
Scholar * Chiang, Y. J. et al. Tankyrase 1 and tankyrase 2 are essential but redundant for mouse embryonic development. _PLoS ONE_ 3, e2639 (2008). Article ADS PubMed PubMed Central
Google Scholar * Qian, L., Mahaffey, J. P., Alcorn, H. L. & Anderson, K. V. Tissue-specific roles of Axin2 in the inhibition and activation of Wnt signaling in the mouse embryo. _Proc.
Natl Acad. Sci. USA_ 108, 8692–8697 (2011). Article CAS ADS PubMed PubMed Central Google Scholar * Wehner, D. & Weidinger, G. Signaling networks organizing regenerative growth of
the zebrafish fin. _Trends Genet._ 31, 336–343 (2015). Article CAS PubMed Google Scholar * Chen, B. Z. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. _Nat. Chem. Biol._ 5, 100–107 (2009). Article CAS ADS PubMed PubMed Central Google Scholar * Tian, A., Benchabane, H., Wang, Z. & Ahmed, Y. Regulation of
stem cell proliferation and cell fate specification by wingless/Wnt signaling gradients enriched at adult intestinal compartment boundaries. _PLoS Genet._ 12, e1005822 (2016). Article
PubMed PubMed Central Google Scholar * Jiao, Y. et al. Whole-exome sequencing of pancreatic neoplasms with acinar differentiation. _J. Pathol._ 232, 428–435 (2014). Article CAS PubMed
PubMed Central Google Scholar * Ryland, G. L. et al. RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. _J. Pathol._ 229, 469–476 (2013). Article CAS PubMed
Google Scholar * Wang, K. et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. _Nat. Genet._ 46, 573–582 (2014). Article
CAS PubMed Google Scholar * Giannakis, M. et al. RNF43 is frequently mutated in colorectal and endometrial cancers. _Nat. Genet._ 46, 1264–1266 (2014). Article CAS PubMed PubMed
Central Google Scholar * Ivanov, I., Lo, K. C., Hawthorn, L., Cowell, J. K. & Ionov, Y. Identifying candidate colon cancer tumor suppressor genes using inhibition of nonsense-mediated
mRNA decay in colon cancer cells. _Oncogene_ 26, 2873–2884 (2007). Article CAS PubMed Google Scholar * Koo, B. K. et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces
endocytosis of Wnt receptors. _Nature_ 488, 665–669 (2012). Article CAS ADS PubMed Google Scholar * Hao, H. X. et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive
manner. _Nature_ 485, 195–200 (2012). Article CAS ADS PubMed Google Scholar * de Lau, W., Peng, W. C., Gros, P. & Clevers, H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt
signal strength. _Genes Dev._ 28, 305–316 (2014). Article CAS PubMed PubMed Central Google Scholar * Moqtaderi, Z. & Struhl, K. Expanding the repertoire of plasmids for PCR-mediated
epitope tagging in yeast. _Yeast_ 25, 287–292 (2008). Article CAS PubMed Google Scholar * Grosshans, J., Schnorrer, F. & Nusslein-Volhard, C. Oligomerisation of Tube and Pelle leads
to nuclear localisation of dorsal. _Mech. Dev._ 81, 127–138 (1999). Article CAS PubMed Google Scholar * Marois, E., Mahmoud, A. & Eaton, S. The endocytic pathway and formation of
the Wingless morphogen gradient. _Development_ 133, 307–317 (2006). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank J. Cho, G. Deshpande, S. Ogden and E.
Wieschaus for comments on the manuscript, K. Beckett, J. Byun, F. Cong, Y. Han, S. Kim, A. Lavanway, B. MacDonald and S. Ogden for technical advice, the investigators listed in Methods for
generously sharing reagents, and V. Marlar for technical assistance. This work was funded by grants from the NIH (RO1CA105038 to Y.A., R01GM081635 and R01GM103926 to E.L., P40OD018537 to the
Bloomington Drosophila Stock Center), the Emerald Foundation (to Y.A.), the Norris Cotton Cancer Center (to Y.A. and S.F.), the Academic Research Fund (MOE2014-T2-2-039 to NT) and the
National Science Foundation (DBI-1039423 for purchase of a Nikon A1RSi confocal microscope). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Genetics and the Norris Cotton Cancer
Center, Geisel School of Medicine at Dartmouth College, Hanover, 03755, New Hampshire, USA Eungi Yang, Ofelia Tacchelly-Benites, Zhenghan Wang, Michael P. Randall, Ai Tian, Hassina
Benchabane, Claudio Pikielny & Yashi Ahmed * Department of Pharmacology and Toxicology, Geisel School of Medicine at Dartmouth College, Hanover, 03755, New Hampshire, USA Sarah
Freemantle * Department of Biological Sciences, Yale-NUS College, National University of Singapore, Singapore, 138615, Singapore Nicholas S. Tolwinski * Department of Cell and Developmental
Biology, Vanderbilt Ingram Cancer Center, and Vanderbilt Institute of Chemical Biology, Nashville, 37232, Tennessee, USA Ethan Lee Authors * Eungi Yang View author publications You can also
search for this author inPubMed Google Scholar * Ofelia Tacchelly-Benites View author publications You can also search for this author inPubMed Google Scholar * Zhenghan Wang View author
publications You can also search for this author inPubMed Google Scholar * Michael P. Randall View author publications You can also search for this author inPubMed Google Scholar * Ai Tian
View author publications You can also search for this author inPubMed Google Scholar * Hassina Benchabane View author publications You can also search for this author inPubMed Google Scholar
* Sarah Freemantle View author publications You can also search for this author inPubMed Google Scholar * Claudio Pikielny View author publications You can also search for this author
inPubMed Google Scholar * Nicholas S. Tolwinski View author publications You can also search for this author inPubMed Google Scholar * Ethan Lee View author publications You can also search
for this author inPubMed Google Scholar * Yashi Ahmed View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS E.Y., O.T.-B., Z.W., A.T., H.B.,
S.F., C.P., N.S.T., E.L. and Y.A. conceived the study. E.Y., O.T.-B., Z.W. and Y.A. designed and performed the experiments. M.P.R. assisted in the experiments. C.P. provided input. E.Y.,
O.T.-B. and Y.A.wrote the paper. CORRESPONDING AUTHOR Correspondence to Yashi Ahmed. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures 1-18, Supplementary Methods and Supplementary References (PDF 1538 kb) RIGHTS AND PERMISSIONS This work is licensed
under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the
material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, E., Tacchelly-Benites, O.,
Wang, Z. _et al._ Wnt pathway activation by ADP-ribosylation. _Nat Commun_ 7, 11430 (2016). https://doi.org/10.1038/ncomms11430 Download citation * Received: 18 May 2015 * Accepted: 23 March
2016 * Published: 03 May 2016 * DOI: https://doi.org/10.1038/ncomms11430 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative