Play all audios:
ABSTRACT The significant degradation that fossilized biomolecules may experience during burial makes it challenging to assess the biogenicity of organic microstructures in ancient rocks.
Here we investigate the molecular signatures of 1.88 Ga Gunflint organic microfossils as a function of their diagenetic history. Synchrotron-based XANES data collected _in situ_ on
individual microfossils, at the submicrometre scale, are compared with data collected on modern microorganisms. Despite diagenetic temperatures of ∼150–170 °C deduced from Raman data, the
molecular signatures of some Gunflint organic microfossils have been exceptionally well preserved. Remarkably, amide groups derived from protein compounds can still be detected. We also
demonstrate that an additional increase of diagenetic temperature of only 50 °C and the nanoscale association with carbonate minerals have significantly altered the molecular signatures of
Gunflint organic microfossils from other localities. Altogether, the present study provides key insights for eventually decoding the earliest fossil record. SIMILAR CONTENT BEING VIEWED BY
OTHERS INHERITED GEOCHEMICAL DIVERSITY OF 3.4 GA ORGANIC FILMS FROM THE BUCK REEF CHERT, SOUTH AFRICA Article Open access 04 January 2021 PRESERVATION OF EARLY TONIAN MACROALGAL FOSSILS FROM
THE DOLORES CREEK FORMATION, YUKON Article Open access 13 April 2022 CHARACTERISATION OF IRON OXIDE ENCRUSTED MICROBIAL FOSSILS Article Open access 18 June 2020 INTRODUCTION Although a
number of studies have reported the morphological preservation of Precambrian organic microfossils, the search for the earliest traces of life on Earth has been and is still fraught with
controversies1,2,3,4,5,6,7. Difficulties not only pertain to the inevitable degradation of biosignatures that occurred during the geological history of their host rocks8,9 but also to the
possibility that abiotic chemical pathways yield organic microstructures exhibiting morphological and geochemical signatures very similar to biogenic ones10,11 as well as to the potential
secondary/late contamination of the samples12,13. Better understanding the impact of fossilization processes on biogenic molecular signatures is a major scientific challenge. Recent studies
have investigated the influence of key parameters such as temperature and mineral matrix during experimental degradation of organics14,15,16,17. These studies have provided new information
towards a generalized model of organic molecule degradation processes occurring during diagenesis and metamorphism. That said, few studies have relied on _in situ_ measurements to discuss
the molecular composition of Precambrian organic microfossils18,19,20,21. The present study focuses on organic microfossils hosted in 1.88 Ga Gunflint cherts (that is, silica-rich rocks).
The Gunflint Formation constitutes a cornerstone in life’s history: it shortly predates the earliest widely accepted evidence for fossil eukaryotes22,23,24. The Gunflint Formation is the
middle unit of the Animikie group of Northwestern Ontario25,26,27. This 120-m-thick formation is conformably overlain by the Rove Formation and extends northeast–southwest for ∼175 km from
Thunder Bay (Ontario, Canada) to northern Minnesota (USA)25,26,27. The Gunflint cherts host what are considered among the best morphologically preserved Precambrian organic
microfossils4,5,6. Although a high number of taxa have been reported (such as _Kakabekia_ spp., _Eoastrion_ spp. or _Eosphaera_ spp. for instance), the dominant components of these
microbiota are segmented tubular filaments (_Gunflintia_ spp.) and coccoid-like microstructures (_Huroniospora_ spp.)27,28,29,30. Although different levels of preservation have been
recognized29, Gunflint organic microfossils usually serve as references for evaluating the biogenicity of older organic microstructures found in Archean rocks4,5,6. The exceptional
morphological preservation of Gunflint organic microfossils has been related to the very-low-grade metamorphism experienced by this formation27: burial temperatures of ∼150 °C have been
estimated for the Gunflint Formation based on its mineral assemblage25 and microquartz oxygen isotopic composition26,31. Later on, hydrothermal circulation of oxygenated fluids resulting
from the Penokean Orogeny (≈ 40 million years after the deposition of the Gunflint Formation27,32) and from the Duluth intrusion (emplaced during the 1.1-Ga Mesoproterozoic continental
rifting33) may have locally increased temperatures25,26 without having induced any recrystallization of the microquartz matrix34,35. The 1.88-Ga Gunflint Formation thus constitutes a
‘natural laboratory’ very well suited to investigating the extent of the molecular preservation of organic microfossils submitted to different diagenetic and low-grade metamorphic
conditions. Here we report _in situ_ structural and chemical investigations of organic microfossils from five Gunflint cherts that experienced different diagenetic temperatures. These five
Gunflint cherts have been selected from the precambrian paleobiology research group (PPRG) collection36: Kakabeka Falls (PPRG 1,298), Schreiber Beach (PPRG 1,289), Triple Junction (PPRG
1,297), Mink Mountain (PPRG 1,374) and Discovery Point (PPRG 1,285). Importantly, the present chemical investigations at the submicrometre scale have been performed on focused ion beam (FIB)
ultrathin sections extracted from specimens found within freshly fractured fragments of samples that have never been embedded in epoxy, thereby avoiding contamination during sample
preparation. Special caution has to be taken when adopting such sampling strategy, as it may confuse the precise recognition of organic microfossils versus blob-like detrital organics. As
the organic microstructures investigated in the present study fulfill criteria for syngenicity as well as exhibit spectroscopic signatures thoroughly consistent with partially degraded
biomolecules, these targets are hereinafter referred to as organic microfossils. Optical microscopy has been performed on thin sections to document the presence of organic microfossils
within the five Gunflint cherts investigated, whereas Raman microspectroscopy has been used to obtain information on the structure of their aromatic skeleton, to estimate the maximum
temperature that they experienced37,38,39. Scanning and transmission electron microscopies (SEM and TEM) have been performed to document organic/mineral relationships. In parallel,
synchrotron-based scanning transmission X-ray microscopy (STXM) coupled with X-ray absorption near edge structure (XANES) spectroscopy has been used to determine, at the submicrometre scale,
the carbon and nitrogen speciation of Gunflint organic microfossils40,41. Based on the comparison with data collected on modern cyanobacteria (_Gloeobacter violaceus_) and modern
micro-algae (_Euglena gracilis_), the present contribution shows that the molecular signatures of some organic microfossils from the 1.88-Ga Gunflint cherts have been exceptionally preserved
(amide groups likely derived from protein compounds are detected), despite having experienced diagenetic temperatures of about 150–170 °C. The present study also demonstrates that slight
increases of temperature and the mineral matrix can significantly alter the molecular signatures of biogenic remains. RESULTS CHERT MINERALOGY AND MICROFOSSIL MORPHOLOGY The Gunflint cherts
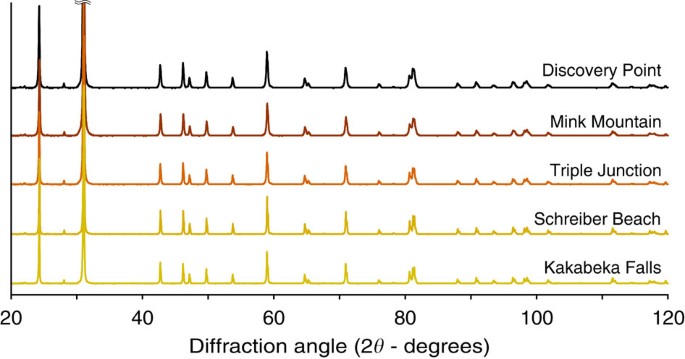
investigated contain mainly microquartz as indicated by X-ray diffraction (quartz _α_ with minor amount of quartz _β_; Fig. 1). The low concentration of minor phases (iron oxides and
carbonates) prevents their detection. _In situ_ secondary ion mass spectrometry measurements revealed that Schreiber Beach, Discovery Point and Mink Mountain microquartz have the same
silicon isotopic composition (_δ_30Si=+1.75±0.35‰). This suggests a unique seawater silica source for Gunflint microquartz and confirms its pristine nature, that is, no hydrothermal silica
is observed in the samples investigated34,35. The photomicrographs of thin sections of the Gunflint cherts investigated in the present study evidence the important diversity of
morphologically preserved organic microfossils within these samples, together with the presence of organic masses with no particular morphology (Fig. 2). Consistently with previous
observations27,28,29,30, the Gunflint microbiota appears dominated by more or less permineralized spheroidal microfossils of about 10–20 μm in diameter and exhibiting more or less thick
walls (Fig. 2). Raman mapping experiments confirm the organic nature of these microfossils and FIB–SEM imaging evidence that although some spheroidal microfossils have been fully
permineralized by silica, others can be filled by organics and micrometric mineral phases (Fig. 3). MICROSTRUCTURE OF ORGANIC CARBON The Raman spectra collected on Schreiber Beach organic
microfossils in the present study appear roughly similar to previous reports1,3. These spectra are typical of disordered carbons, with a composite G band (G+D2), a composite D band (D1+D4)
and a D3 band in between42,43. Of note, there is no consensus on the exact physical or chemical significance of these so-called ‘defect’ bands44. All the Gunflint cherts investigated are
quite homogeneous in terms of carbon structure, that is, in a given chert, all organic microfossils display a similar Raman spectrum (Fig. 4). Yet, slight differences can be observed from
one chert to another (Fig. 4). In particular, the composite D band of Triple Junction, Mink Mountain and Discovery Point organic microfossils is more asymmetrical than the one of Kakabeka
Falls and Schreiber Beach organic microfossils, probably due to a stronger contribution of the D1 band and a lower contribution of the D4 band (Fig. 4). Their composite G band is also
slightly shifted, probably because of a stronger contribution of the G band and a lower contribution of the D2 band (Fig. 4). The Raman spectrum of the Discovery Point organic microfossils
displays an additional peak at ∼1,090 cm−1 attributed to the presence of iron-rich calcium carbonates such as ankerites45,46. The shape of the Raman signal collected on carbonaceous
materials is directly related to their microstructure and has been shown to be related to the maximum temperature that they experienced37,38,39. The comparison of the Raman signatures of the
Gunflint organic microfossils with a recent temperature calibration study39 indicates that Kakabeka Falls and Schreiber Beach have both experienced ∼150–170 °C during diagenesis, whereas
Triple Junction, Mink Mountain and Discovery Point organic microfossils have been exposed to temperatures of ∼210–230 °C. The temperatures estimated here for the Kakabeka Falls and Schreiber
Beach microfossils are in good agreement with previous estimations based the mineral assemblage25,27 and microquartz oxygen isotopic composition26,31 of Gunflint cherts. SUBMICROMETRIC
ORGANIC AND MINERAL RELATIONSHIPS FIB ultrathin sections have been extracted from the walls of organic microfossils found using SEM within freshly fractured fragments of cherts for further
characterization using TEM and STXM. Again, special caution has been taken for such sampling strategy, as it may confuse the precise recognition of organic microfossils versus blob-like
detrital organics. TEM observations highlight textural differences among the organic microfossils investigated: although the Schreiber Beach organic microfossils appear almost free of
porosity at the nanoscale, the Mink Mountain organic microfossils exhibit nanoporosity (Fig. 5). TEM–energy dispersive X-ray spectrometry (EDXS) measurements show that Schreiber Beach
organic microfossils contain a significantly higher amount of nitrogen and sulfur than those from Mink Mountain (Fig. 5). In addition, TEM observations confirm the presence of iron oxides
around Mink Mountain organic microfossils (Fig. 5), in agreement with previous observations27, and of calcium carbonates within Discovery Point organic microfossils (Fig. 6). Although iron
oxides possibly precipitated during postdepositional circulation of oxygenated fluids27, carbonates may have precipitated either before or as a result of the degradation of organics.
NITROGEN-TO-CARBON ATOMIC RATIO The nitrogen-to-carbon atomic ratio (N/C) of the Gunflint organic microfossils investigated have been estimated from their X-ray absorption spectra47. Organic
microfossils from Kakabeka Falls and Schreiber Beach have a higher N/C (0.24 and 0.21±0.01, respectively) than the ones from Triple Junction and Mink Mountain (0.09 (0.05 after correction,
see below) and 0.03±0.01, respectively) (Fig. 7). Despite more significant molecular degradation (see below), Discovery Point organic microfossils exhibit a quite high N/C (0.15±0.01). For
comparison, a value of 0.17±0.01 is obtained from the X-ray absorption spectrum of modern micro-algae, whereas a value of 0.24±0.01 is estimated for modern cyanobacteria (Fig. 7). The
absorption signal at the calcium _L_-edge (340–360 eV) shows that all the Gunflint organic microfossils, except the ones from Mink Mountain, contain variable amounts of calcium. In addition,
Kakabeka Falls and Triple Junction organic microfossils appear associated with calcium and/or potassium nitrates (Ca(NO3)2 and KNO3) as indicated by their nitrogen speciation (see below).
If all the calcium in the Triple Junction organic microfossils occurred as calcium nitrates, the inorganic nitrogen contribution corresponding to nitrates could be directly derived from the
calcium content estimated from the intensity of the absorption at the calcium _L_-edge. Subtracting this inorganic nitrogen quantity from the total nitrogen contribution would lead to an
organic N/C of≈0.05±0.01 for Triple Junction organic microfossils, that is, to a value comparable to the N/C of Mink Mountain organic microfossils. As the calcium and potassium contents of
Kakabeka Falls organic microfossils are very low, such correction would not significantly modify the estimated organic N/C. CARBON SPECIATION AND MOLECULAR SIGNATURES After normalization to
the total carbon quantity, Kakabeka Falls and Schreiber Beach organic microfossils exhibit C-XANES spectra sharing similar features with spectra of modern cyanobacteria and modern
micro-algae (Fig. 8), that is, an intense peak at 288.2 eV attributed to the presence of amide functional groups, a second peak at 289.4 eV attributed to the presence of hydroxyl functional
groups and a third one, less intense, centred at 285.1 eV and attributed to the presence of aromatic and/or olefinic functional groups15,48. Compared with modern microorganisms, Kakabeka
Falls and Schreiber Beach organic microfossils display an additional absorption feature in their C-XANES spectra centred at 286.7 eV and likely to be attributed to the presence of carbonyl
or phenolic functional groups49,50. The C-XANES spectra of Triple Junction and Mink Mountain organic microfossils are quite different (Fig. 8). The absorption feature attributed to the
presence of aromatic and/or olefinic groups is broader, more intense and shifted to 284.9 eV, probably due to a higher content of olefins51 and/or of heteroaromatic moieties such as
benzoquinones48. The absorption peaks at 286.7, 288.2 and 289.5 eV do not contribute as significantly to the signal. Instead, two other features are observed: a peak centred at 286.4 eV,
attributed to the presence of unsaturated bond between carbon and heteroatoms such as nitrogen, sulfur and/or oxygen, and a peak centred at 288.6 eV attributed to the presence of carboxylic
functional groups49,50. The C-XANES spectrum of Discovery Point organic microfossils exhibits absorption peaks at the same energies as those of Triple Junction and Mink Mountain (Fig. 8).
Yet, the aromatic absorption feature (centred at 284.9 eV) is broader and more intense, and an additional sharp and intense peak at 290.3 eV indicates the nanoscale association between
organics and carbonates45,52,53, probably calcium carbonates as indicated by EDXS data (Fig. 6). NITROGEN SPECIATION AND MOLECULAR SIGNATURES Similar to C-XANES data, the N-XANES spectra
have been normalized to the total nitrogen quantity to facilitate their comparison. Kakabeka Falls, Schreiber Beach and Triple Junction organic microfossils exhibit a broad absorption
feature at 401.4 eV, possibly indicating the presence of amide, imine and nitrile groups, as well as a well-defined peak at 399.8 eV and a shoulder around 398.8 eV, which may denote the
presence of a significant amount of nitrogen within aromatic moieties54,55 (Fig. 9). Similar absorption features can be observed in the N-XANES spectrum of modern cyanobacteria and modern
micro-algae, and are usually attributed to the presence of amide (peak at 401.4 eV), imine, nitrile and/or aromatic nitrogen54 (Fig. 9). Two additional absorption features are observed at
401.7 and 405.4 eV in the N-XANES spectra of Kakabeka Falls and Triple Junction organic microfossils (Fig. 9). These peaks can be attributed to the presence of calcium and/or potassium
nitrates54. These microfossils thus contain both organic and inorganic nitrogen. The only absorption peak that can be observed in the N-XANES spectrum of Mink Mountain organic microfossils
occurs at 402.2 eV and may be attributed to the presence of pyrrole54,55 (Fig. 9). The lower signal-to-noise ratio is due to the lower nitrogen content of these microfossils. In contrast,
despite a high N/C ratio, the N-XANES spectrum of Discovery Point organic microfossils does not display any absorption peak (Fig. 9). DISCUSSION The 1.88-Ga Gunflint cherts belong to the
most famous Precambrian organic microfossil-rich formation4,5,6. Previous studies based on TEM-based electron energy loss spectroscopy (EELS) experiments have not reported the presence of
nitrogen-bearing functional groups within Gunflint organic microfossils2,3,18, probably because of the low spectral resolution of this technique and potentially because of electron radiation
damage (the radiation dose received by organics has been shown to be typically 100–1,000 times higher in TEM-based EELS than in STXM-based XANES spectroscopy56). The rare STXM-based C-XANES
data reported in the literature on Gunflint samples have not been collected _in situ_ but on powdered samples2,3, which prevented the demonstration of the syngenicity of the measured
organics. Taking advantage of the unique capabilities of STXM-based XANES spectroscopy at the carbon and nitrogen K edges to perform _in situ_ experiments at the submicrometre scale, the
present study shows that, in addition to the fine-scale morphologies, the molecular biosignatures of some Gunflint organic microfossils have been exceptionally preserved. In fact, despite
the 1.88-Gyr-long geological history that they experienced, Kakabeka Falls and Schreiber Beach organic microfossils exhibit C- and N-XANES spectra sharing strong similarities to those of
modern cyanobacteria and modern micro-algae. Despite a higher content of aromatic compounds compared to modern microorganisms, these microfossils exhibit a quite high content of oxygen-based
functional groups (carbonyl, phenolic, carboxylic and hydroxyl groups). In addition, these microfossils still contain amide functional groups (absorption feature at 288.2 eV), which were
likely to be involved in the proteinaceous compounds synthetized by the once living organisms15,52,53. Kakabeka Falls and Schreiber Beach organic microfossils exhibit quite high N/C values.
These values may be secondary, that is, may result from diagenetic processes. In fact, inorganic nitrogen in fluids can be incorporated within kerogen molecular structures during diagenesis
at temperatures as low as 100 °C57. Yet, although higher than those of modern micro-algae, the N/C of Kakabeka Falls and Schreiber Beach organic microfossils are comparable to those of
modern cyanobacteria. It thus can be assumed that the Gunflint organic microfossils initially exhibited high N/C values as do modern cyanobacteria and some modern marine microorganisms
showing N/C as high as 0.25–0.30 (refs 58, 59). The high N/C of Kakabeka Falls and Schreiber Beach organic microfossils may thus result from their exceptional preservation. The molecular
signatures of Triple Junction, Mink Mountain and Discovery Point organic microfossils have not been that well preserved. In fact, the Raman and XANES data reported here show that these
organic microfossils are more ‘mature’ than the ones from Kakabeka Falls and Schreiber Beach, that is, they exhibit a higher aromaticity and contain less sulfur-, nitrogen- and oxygen-rich
moieties49,50. In particular, these organic microfossils do not seem to contain amide, hydroxyl nor carbonyl functional groups. One explanation could be that the organic microfossils
investigated here were initially chemically different and/or experienced variable decay60. In other words, they may have been originally composed of different organics, which may have
followed different reaction pathways during diagenesis. Yet, morphologies, bulk mineralogy and silicon isotopic compositions suggest comparable depositional and burial histories. Another
possibility would be that they have experienced different oxidation conditions: oxic conditions have been shown to be detrimental to the preservation of biosignatures14 and the association
of Kakabeka Falls, Triple Junction and Mink Mountain organic microfossils with nitrates and iron oxides suggests that oxygenated fluids have circulated27. Yet, Schreiber Beach and Kakabeka
Falls organic microfossils exhibit very similar molecular signatures, even though only the latter are associated with nitrates. Furthermore, even though nitrates are associated to Kakabeka
Falls and Triple Junction organic microfossils, their molecular signatures are very different. The circulation of oxygenated fluids thus cannot be seen as the main process having an impact
on the molecular degradation of the investigated Gunflint organic microfossils. As the differences reported here are very similar to those resulting from thermal maturation49,50, the
simplest explanation remains that they result from the different burial temperature conditions experienced by the organic microfossils investigated. Interestingly, with increasing maturity,
a shift from 285.1 to 284.9 eV is observed for the aromatic/olefinic peak whereas the phenolic/carbonyl peak shifts from 286.7 to 286.4 eV. These shifts may result from the incorporation of
heteroatoms in newly condensed aromatic units and from condensation reactions between amino acids and phenolic groups, respectively49,50. In any case, the present contribution demonstrates
that a slight increase of diagenetic temperatures may be responsible for the significant degradation of fossilized molecular signatures. Regardless of their high N/C, to which inorganic
nitrogen may contribute, the molecular signature of Discovery Point organic microfossils appears less pristine, that is, more degraded, than those of Triple Junction and Mink Mountain
organic microfossils (Figs 8 and 9), even though they experienced similar diagenetic temperatures (that is, 210–230 °C; Fig. 4). The possibility that Discovery Point organic microfossils
were initially different from the other investigated Gunflint cherts cannot be entirely ruled out, even though nothing supports it (see above). In contrast, as illustrated by the similar
XANES signatures of modern micro-algae (_E. gracilis_) and modern cyanobacteria (_G. violaceus_), prokaryotes such as the ones fossilized within the investigated Gunflint cherts probably
originally exhibited very similar XANES signatures. The presence of nitrates might explain the better preservation of Triple Junction organic microfossils compared with those of Discovery
Point. Yet, Kakabeka Falls and Triple Junction organic microfossils are also associated with nitrates but exhibit C-XANES spectra very similar to the nitrate-free Schreiber Beach and Mink
Mountain organic microfossils, respectively (Figs 8 and 9). The postdepositional circulation of oxygenated fluids, which led to the precipitation of iron oxides27, may have also been
responsible for the molecular degradation of organic microfossils. Yet, Triple Junction and Mink Mountain organic microfossils exhibit very similar C-XANES spectra (Fig. 6), even though only
the latter are associated with iron oxides. Thus, the degree of molecular preservation of Gunflint organic microfossils is not correlated to the presence of nitrates and iron oxides.
Alternatively and more probably, the higher maturity of Discovery Point organic microfossils could be related to the nanoscale association between organics and carbonates revealed by TEM,
Raman and XANES analyses (Figs 3, 4 and 6). Among the Gunflint organic microfossils investigated, only the ones from Discovery Point exhibit such nanoscale association. It has previously
been reported that the presence of calcium carbonates during high-temperature organic maturation processes may favour the formation of turbostratic (graphitic) carbons37,61, through the
formation of calcium hydroxide and calcium carbide, especially under nitrogen-rich atmosphere62,63. This reaction pathway has been observed at high temperature; thus, such a scenario remains
highly speculative in the present case. In any case, although dedicated and thorough experimental investigations appear required, the present study illustrates the potential impact of
mineral phases on the preservation/degradation of fossilized molecular signatures. Altogether, the present contribution shows that the molecular signatures of the organic microfossils from
the 1.88-Ga Gunflint cherts have been preserved, although they experienced temperatures of about 150–170 °C. Such preservation can be qualified as exceptional, as amide groups derived from
protein compounds can still be detected. Amide groups are indeed generally lost during the very first stages of burial, either consumed by heterotroph organisms or thermally degraded at low
temperature (<< 100 °C)64. Although a number of taphonomic processes may be invoked, it can be assumed that this exceptional molecular preservation result from the early silicification
of Gunflint microbiota. Indeed, even though Kakabeka Falls and Schreiber Beach organic microfossils have experienced diagenetic temperatures similar to overmature clay-rich gas shales,
their molecular signatures are significantly better preserved than those corresponding to overmature kerogens65. The present study thus illustrates that Precambrian cherts having experienced
relatively low-grade metamorphism may contain (at least partially) chemically preserved remains of ancient life. As slight increases of temperature can strongly modify the molecular
signatures of biogenic remains, documenting the carbon and nitrogen speciation of organic microfossils and estimating the temperature that they experienced should be done in parallel. We
believe that the analytical strategy adopted here, combining Raman and STXM-based XANES spectroscopies, is an illustration of the technical and conceptual advances of Precambrian
palaeontology that will eventually lead to the elucidation of a large part of the mysteries that still shroud the early fossil record. METHODS X-RAY DIFFRACTION The bulk mineralogical
composition of the Gunflint cherts investigated has been determined using the X-ray diffractometer (Panalytical X'pert Pro) operating at IMPMC (Paris, France) with 40 kV and 40 mA Co Kα
radiation. Sample analyses have been carried out on finely ground powders deposited on a silicon sample holder, in the 20–120° 2_θ_ angle range, with a step size of 0.016° (2_θ_) for a
total counting time per sample of about 6 h. X-ray diffraction patterns have been analysed using the Eva software (Bruker) for background subtraction and peak finding. SECONDARY ION MASS
SPECTROMETRY Silicon isotopic compositions (_δ_30Si) of microquartz have been classically measured on the Cameca 1270 ion microprobe operating at UCLA (CA, USA). The measured _δ_30Si values
have been corrected using in house quartz standards (QZCRWU and chert Miocene) and are reported here as per mil deviations from the international standard NBS28. Samples have been sputtered
with a 25 μm size Cs+ primary beam of 30 nA intensity using a 10-kV acceleration voltage. The mass resolving power was set at _M_/Δ_M_∼4,000. Faraday cups have been used to measure
simultaneously 28Si− and 30Si− ions. Field diaphragm and magnetic field have been automatically centred during the analyses. A pre-sputtering of 60 s and 40 × 5-s-long cycles of acquisition
have led to a counting statistic of <0.1‰ and an external reproducibility of ∼0.3‰ (1_σ_). SCANNING ELECTRON MICROSCOPY SEM has been used to locate the organic microstructures within the
silica matrix of the investigated Gunflint cherts for subsequent _in situ_ extraction using FIB milling. To minimize contamination that may come from sample preparation, freshly fractured
fragments of samples have been directly observed after having been mounted on aluminum stubs without any additional preparation, except gold coating. SEM observations have been performed on
an SEM–field emission gun ultra 55 Zeiss (IMPMC, Paris, France) at a 15-kV accelerating voltage and a working distance of 7.5 mm. SEM images have been collected with both secondary electron
(SE2) and angle-selective backscattered detectors. Elemental maps have been collected using EDXS. RAMAN MICROSPECTROSCOPY AND RAMAN MAPPING Raman data have been obtained with a Renishaw
INVIA microspectrometer (IMPMC, Paris, France). Raman microspectroscopy measurements have been directly performed on freshly fractured samples at constant room temperature using the 514.5-nm
wavelength of a 50-mW Modulaser Argon laser (green laser) focused on the sample through a Leica DM LM microscope with a long working distance × 100 objective (numerical aperture=0.75). This
configuration yields a horizontal resolution of ≈1 μm for a laser power delivered at the sample surface always below 1 mW to prevent irreversible laser-induced thermal damage43. A
circularly polarized laser using a quarter wavelength plate allows limiting polarization effects. Light is dispersed by a grating with 1,800 lines per mm and the signal is analysed with a
RENCAM CCD (charge-coupled device) detector. Ten to fifteen spectra have been collected for each sample. Dynamic Raman hyperspectral mapping has been performed using an equivalent
spectrometer relying on the synchronization of CCD measurements with _x_,_y_ motorized stage displacements66. At each point, a correlation index between the measured spectrum and a reference
spectrum of organics is calculated. Points displaying high indexes are assigned a colour, from red to yellow/white. FIB MILLING AND FIB-SEM IMAGING FIB ultrathin sections have been
extracted from the walls of organic microfossils using an FEI Strata DB 235 (IEMN, Lille, France). This extraction procedure maintains textural integrity, even in the case of loosely
consolidated materials, and prevents shrinkage and deformation of microscale to nanoscale pores, even in the case of highly sensitive materials67. Milling at low Ga-ion currents has allowed
preventing common artefacts such as local gallium implantation, mixing of components, creation of vacancies or interstitials, creation of amorphous layers, local composition changes or
redeposition of the sputtered material on the sample surface and significant changes in the speciation of complex carbon-based polymers40,68. FIB-SEM imaging (backscattered electrons) of FIB
milled trenches have been performed using a Zeiss Crossbeam Auriga FIB-SEM (IPGP, Paris, France). TRANSMISSION ELECTRON MICROSCOPY TEM analyses have been performed on FIB sections to
document the textural nature of the investigated Gunflint organic microfossils and identify the mineral phases with which organics are closely associated at the nanoscale. TEM observations
have been performed with a JEOL 2100 field emission gun microscope (IMPMC, Paris, France) operating at 200 kV. Scanning TEM Z-contrast imaging has been performed using the high-angle annular
dark field mode. High-resolution TEM images have been collected using the bright-field mode, allowing us to resolve the crystalline planes (of the order of 0.1 nm) of the different phases.
X-RAY ABSORPTION SPECTROSCOPY XANES data have been collected on the STXM 10ID-1 beamline (SM beamline)69 at the Canadian Light Source. The 10ID-1 beamline works in the soft X-ray energy
range (130–2,500 eV) and is based on an elliptically polarized undulator. The Canadian Light Source storage ring is operated at 2.9 GeV and between 250 and 150 mA current. The microscope
chamber is first pumped down to 100 mTorr after sample insertion and back-filled with He gas. A 100-nm-thick titanium filter is used to remove the contribution of second-order light. Energy
calibration is done using the well-resolved 3p Rydberg peak of gaseous CO2 at 294.96 eV for the C K-edge and using the 1 s→π* photoabsorption resonance of gaseous N2 at 400.8 eV for the N
K-edge. X-ray absorption spectroscopy has been performed by collecting image stacks with a spatial resolution of 15 nm, that is, by rastering selected areas of samples in the x–y directions
at energy increments of 1 eV over the 270–450 eV energy range using the low-energy grating of the 10ID-1 SM beamline. Additional image stacks have been collected at energy increments of 0.1
eV over the carbon (270–340 eV) and the nitrogen (390–450 eV) absorption ranges, to resolve the fine structures near the C and N K-edges (XANES spectroscopy). Stack measurements have been
performed with a dwell time of ≤1 ms per pixel to prevent irradiation damage. Alignment of images of stacks and extraction of XANES spectra have been done using the aXis2000 software
(ver2.1n). Spectral peak positions, intensities and widths have been determined using the Athena software package70. The C- and N-XANES spectra shown in the present contribution correspond
to homogeneous organic-rich areas of several hundreds of square nanometres. DATA AVAILABILITY The data that support the findings of this study are available from the corresponding author
(S.B.) upon request. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Alleon, J. _et al._ Molecular preservation of 1.88 Ga Gunflint organic microfossils as a function of temperature and
mineralogy. _Nat. Commun._ 7:11977 doi: 10.1038/ncomms11977 (2016). CHANGE HISTORY * _ 14 AUGUST 2017 A correction has been published and is appended to both the HTML and PDF versions of
this paper. The error has not been fixed in the paper. _ REFERENCES * Schopf, J. W., Kudryavtsev, A. B., Agresti, D. G., Wdowiak, T. J. & Czaja, A. D. Laser-Raman imagery of Earth’s
earliest fossils. _Nature_ 416, 73–76 (2002). Article CAS ADS PubMed Google Scholar * De Gregorio, B. T., Sharp, T. G., Flynn, G. J., Wirick, S. & Hervig, R. L. Biogenic origin for
Earth’s oldest putative microfossils. _Geology_ 37, 631–634 (2009). Article CAS ADS Google Scholar * De Gregorio, B. T., Sharp, T. G., Rushdi, A. I. & Simoneit, B. R. T. in _Earliest
Life on Earth: Habitats, Environments and Methods of Detection_ (ISBN 978-90-481-8793-5) 239–289 (2011). * Schopf, J. W. & Kudryavtsev, A. B. Biogenicity of Earth’s earliest fossils: a
resolution of the controversy. _Gondwana Res._ 22, 761–771 (2012). Article ADS Google Scholar * Wacey, D. et al. Taphonomy of very ancient microfossils from the ∼3400 Ma Strelley Pool
Formation and ∼1900 Ma Gunflint Formation: new insights using a focused ion beam. _Precambian Res._ 220, 234–250 (2012). Article ADS Google Scholar * Brasier, M. D., Antcliffe, J.,
Saunders, M. & Wacey, D. Changing the picture of Earth’s earliest fossils (3.5-1.9 Ga) with new approaches and new discoveries. _Proc. Natl Acad. Sci. USA_ 112, 4859–4864 (2015). Article
CAS ADS PubMed PubMed Central Google Scholar * Wacey, D., Saunders, M., Kong, C., Brasier, A. & Brasier, M. 3.46 Ga Apex chert ‘microfossils’ reinterpreted as mineral artefacts
produced during phyllosilicate exfoliation. _Gondwana Res._ doi:10.1016/j.gr.2015.07.010 (2016). * Bernard, S. & Papineau, D. Graphitic carbons and biosignatures. _Elements_ 10, 435–440
(2014). Article CAS Google Scholar * Briggs, D. E. G. & Summons, R. E. Ancient biomolecules: their origins, fossilization, and role in revealing the history of life. _Bioessays_ 36,
482–490 (2014). Article CAS PubMed Google Scholar * García-Ruiz, J. M. et al. Self-assembled silica-carbonate structures and detection of ancient microfossils. _Science_ 302, 1194–1197
(2003). Article ADS PubMed Google Scholar * McCollom, T. M. & Seewald, J. S. Carbon isotope composition of organic compounds produced by abiotic synthesis under hydrothermal
conditions. _Earth Planet. Sci. Lett._ 243, 74–84 (2006). Article CAS ADS Google Scholar * Rasmussen, B., Fletcher, I. R., Brocks, J. J. & Kilburn, M. R. Reassessing the first
appearance of eukaryotes and cyanobacteria. _Nature_ 455, 1101–1104 (2008). Article CAS ADS PubMed Google Scholar * Dobrzhinetskaya, L., Wirth, R. & Green, H. Diamonds in Earth’s
oldest zircons from Jack Hills conglomerate, Australia, are contamination. _Earth Planet. Sci. Lett._ 387, 212–218 (2014). Article CAS ADS Google Scholar * Schiffbauer, J. D. et al.
Thermally-induced structural and chemical alteration of organic-walled microfossils: an experimental approach to understanding fossil preservation in metasediments. _Geobiology_ 10, 402–423
(2012). Article CAS PubMed Google Scholar * Li, J. et al. Impact of biomineralization on the preservation of microorganisms during fossilization: an experimental perspective. _Earth
Planet. Sci. Lett._ 400, 113–122 (2014). Article CAS ADS Google Scholar * Picard, A., Kappler, A., Schmid, G., Quaroni, L. & Obst, M. Experimental diagenesis of organo-mineral
structures formed by microaerophilic Fe (II)-oxidizing bacteria. _Nat. Commun._ 6, 6277–6277 (2015). Article CAS ADS PubMed Google Scholar * Picard, A., Obst, M., Schmid, G., Zeitvogel,
F. & Kappler, A. Limited influence of Si on the preservation of Fe mineral-encrusted microbial cells during experimental diagenesis. _Geobiology_ 14, 272–293 (2015). Google Scholar *
Moreau, J. W. & Sharp, T. G. A transmission electron microscopy study of silica and kerogen biosignatures in ∼1.9 Ga Gunflint microfossils. _Astrobiology_ 4, 196–210 (2004). Article CAS
ADS PubMed Google Scholar * De Gregorio, B. T. & Sharp, T. G. The structure and distribution of carbon in 3.5 Ga Apex chert: implications for the biogenicity of Earth’s oldest
putative microfossils. _Am Mineral._ 91, 784–789 (2006). Article CAS ADS Google Scholar * Igisu, M. et al. Micro-FTIR spectroscopic signatures of bacterial lipids in Proterozoic
microfossils. _Precambian Res._ 173, 19–26 (2009). Article CAS ADS Google Scholar * Muscente, A., Michel, F. M., Dale, J. G. & Xiao, S. Assessing the veracity of Precambrian ‘sponge’
fossils using _in situ_ nanoscale analytical techniques. _Precambrian Res._ 263, 142–156 (2015). Article CAS ADS Google Scholar * Fralick, P., Davis, D. W. & Kissin, S. A. The age
of the Gunflint Formation, Ontario, Canada: single zircon U--Pb age determinations from reworked volcanic ash. _Can. J. Earth Sci._ 39, 1085–1091 (2002). Article CAS ADS Google Scholar *
Knoll, A. H., Javaux, E. J., Hewitt, D. & Cohen, P. Eukaryotic organisms in Proterozoic oceans. _Phil. Trans. R. Soc. B_ 361, 1023–1038 (2006). Article CAS PubMed PubMed Central
Google Scholar * Pang, K. et al. The nature and origin of nucleus-like intracellular inclusions in Paleoproterozoic eukaryote microfossils. _Geobiology_ 11, 499–510 (2013). CAS PubMed
Google Scholar * Floran, R. J. & Papike, J. J. Mineralogy and petrology of the Gunflint Iron Formation, Minnesota-Ontario: correlation of compositional and assemblage variations at low
to moderate grade. _J. Petrol._ 19, 215–288 (1978). Article CAS ADS Google Scholar * Winter, B. L. & Knauth, L. P. Stable isotope geochemistry of cherts and carbonates from the 2.0
Ga Gunflint Iron Formation: implications for the depositional setting, and the effects of diagenesis and metamorphism. _Precambrian Res._ 59, 283–313 (1992). Article CAS ADS Google
Scholar * Shapiro, R. S. & Konhauser, K. O. Hematite-coated microfossils: primary ecological fingerprint or taphonomic oddity of the Paleoproterozoic? _Geobiology_ 13, 209–224 (2015).
Article CAS PubMed Google Scholar * Barghoorn, E. S. & Tyler, S. A. Microorganisms from the Gunflint Chert: these structurally preserved Precambrian fossils from Ontario are the most
ancient organisms known. _Science_ 147, 563–575 (1965). Article CAS ADS PubMed Google Scholar * Awramik, S. M. & Barghoorn, E. S. The Gunflint microbiota. _Precambrian Res._ 5,
121–142 (1977). Article ADS Google Scholar * Strother, P. K. & Tobin, K. Observations on the genus _Huroniospora_ Barghoorn: implications for paleoecology of the gunflint microbiota.
_Precambrian Res._ 36, 323–333 (1987). Article ADS Google Scholar * Marin, J., Chaussidon, M. & Robert, F. Microscale oxygen isotope variations in 1.9 Ga Gunflint cherts: assessments
of diagenesis effects and implications for oceanic paleotemperature reconstructions. _Geochim. Cosmochim. Acta_ 74, 116–130 (2010). Article CAS ADS Google Scholar * Schulz, K. J. &
Cannon, W. F. The Penokean orogeny in the Lake Superior region. _Precambrian Res._ 157, 4–25 (2007). Article CAS ADS Google Scholar * Paces, J. B. & Miller, J. D. Precise U-Pb ages
of Duluth Complex and related mafic intrusions, northeastern Minnesota: geochronological insights to physical, petrogenetic, paleomagnetic, and tectonomagmatic processes associated with the
1.1 Ga midcontinent rift system. _J. Geophys. Res._ 98, 13997–14013 (1993). Article CAS ADS Google Scholar * Marin-Carbonne, J., Faure, F., Chaussidon, M., Jacob, D. & Robert, F. A
petrographic and isotopic criterion of the state of preservation of Precambrian cherts based on the characterization of the quartz veins. _Precambrian Res._ 231, 290–300 (2013). Article CAS
ADS Google Scholar * Marin-Carbonne, J., Chaussidon, M. & Robert, F. Micrometer-scale chemical and isotopic criteria (O and Si) on the origin and history of Precambrian cherts:
implications for paleo-temperature reconstructions. _Geochim. Cosmochim. Acta_ 92, 129–147 (2012). Article CAS ADS Google Scholar * Moore, T. & Schopf, J. in _Geographic and Geologic
Data for PPRG Rock Samples. The Proterozoic Biosphere—A Multidisciplinary Study_ 603–693Cambridge Univ. Press (1992). * Wopenka, B. & Pasteris, J. D. Structural characterization of
kerogens to granulite-facies graphite: applicability of Raman microprobe spectroscopy. _Am. Mineral._ 78, 533–557 (1993). CAS Google Scholar * Beyssac, O., Goffé, B., Chopin, C. &
Rouzaud, J. N. Raman spectra of carbonaceous material in metasediments: a new geothermometer. _J. Metamorph. Geol._ 20, 859–871 (2002). Article CAS ADS Google Scholar * Lahfid, A. et al.
Evolution of the Raman spectrum of carbonaceous material in low-grade metasediments of the Glarus Alps (Switzerland). _Terra Nova_ 22, 354–360 (2010). Article CAS ADS Google Scholar *
Bernard, S. et al. Ultrastructural and chemical study of modern and fossil sporoderms by scanning transmission X-ray microscopy (STXM). _Rev. Palaeobot. Palynol._ 156, 248–261 (2009).
Article Google Scholar * Bertrand, L. et al. Emerging approaches in synchrotron studies of materials from cultural and natural history collections. _Top. Curr. Chem._ 374, 1–39 (2016).
Article CAS Google Scholar * Ferrari, A. C. & Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. _Phys. Rev. B_ 61, 14095–14107 (2000). Article CAS
ADS Google Scholar * Beyssac, O. et al. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. _Spectrochim. Acta A_ 59, 2267–2276 (2003).
Article ADS Google Scholar * Beyssac, O. & Lazzeri, M. in _Applications of Raman Spectroscopy to Earth Sciences and Cultural Heritage. EMU Notes in Mineralogy_ Vol. 12 (eds Dubessy,
J., Caumon, M.-C. & Rull, F.) 415–454 (European Mineralogical Union and the Mineralogical Society of Great Britain and Ireland, 2012). * Bernard, S. et al. Exceptional preservation of
fossil plant spores in high-pressure metamorphic rocks. _Earth Planet. Sci. Lett._ 262, 257–272 (2007). Article CAS ADS Google Scholar * Boulard, E., Guyot, F. & Fiquet, G. The
influence on Fe content on Raman spectra and unit cell parameters of magnesite-siderite solid solutions. _Phys. Chem. Miner._ 39, 239–246 (2012). Article CAS ADS Google Scholar * Alleon,
J., Bernard, S., Remusat, L. & Robert, F. Estimation of nitrogen-to-carbon ratios of organics and carbon materials at the submicrometer scale. _Carbon_ 84, 290–298 (2015). Article CAS
Google Scholar * Solomon, D. et al. Carbon (1 s) NEXAFS spectroscopy of biogeochemically relevant reference organic compounds. _Soil Sci. Soc. Am. J._ 73, 1817–1830 (2009). Article CAS
ADS Google Scholar * Bernard, S. et al. Geochemical evolution of organic-rich shales with increasing maturity: a STXM and TEM study of the Posidonia Shale (Lower Toarcian, northern
Germany). _Mar. Petrol. Geol._ 31, 70–89 (2012). Article CAS Google Scholar * Bernard, S., Wirth, R., Schreiber, A., Schulz, H. M. & Horsfield, B. Formation of nanoporous pyrobitumen
residues during maturation of the Barnett Shale (Fort Worth Basin). _Int. J. Coal. Geol._ 103, 3–11 (2012). Article CAS Google Scholar * Cody, G. D. et al. Establishing a molecular
relationship between chondritic and cometary organic solids. _Proc. Natl Acad. Sci. USA_ 108, 19171–19176 (2011). Article CAS ADS PubMed PubMed Central Google Scholar * Benzerara, K.
et al. Nanoscale detection of organic signatures in carbonate microbialites. _Proc. Natl Acad. Sci. USA_ 103, 9440–9445 (2006). Article CAS ADS PubMed PubMed Central Google Scholar *
Couradeau, E. et al. An early-branching microbialite cyanobacterium forms intracellular carbonates. _Science_ 336, 459–462 (2012). Article CAS ADS PubMed Google Scholar * Leinweber, P.
et al. Nitrogen K-edge XANES-an overview of reference compounds used to identify unknown organic nitrogen in environmental samples. _J. Synchrotron Radiat._ 14, 500–511 (2007). Article CAS
PubMed Google Scholar * Cody, G. D. et al. Molecular signature of chitin-protein complex in Paleozoic arthropods. _Geology_ 39, 255–258 (2011). Article CAS ADS Google Scholar *
Hitchcock, A. P., Dynes, J. J., Johansson, G., Wang, J. & Botton, G. Comparison of NEXAFS microscopy and TEM-EELS for studies of soft matter. _Micron_ 39, 311–319 (2008). Article CAS
PubMed Google Scholar * Schimmelmann, A. & Lis, G. P. Nitrogen isotopic exchange during maturation of organic matter. _Org. Geochem._ 41, 63–70 (2010). Article CAS Google Scholar *
Geider, R. & La Roche, J. Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. _Eur. J. Phycol._ 37, 1–17 (2002). Article Google Scholar * Engel, A.
S., Porter, M. L., Stern, L. A., Quinlan, S. & Bennett, P. C. Bacterial diversity and ecosystem function of filamentous microbial mats from aphotic (cave) sulfidic springs dominated by
chemolithoautotrophic ‘_Epsilonproteobacteria’_. _FEMS Microbiol. Ecol._ 51, 31–53 (2004). Article CAS PubMed Google Scholar * Briggs, D. E. G. The role of decay and mineralization in
the preservation of soft-bodied fossils. _Annu. Rev. Earth Planet. Sci._ 31, 275–301 (2003). Article CAS ADS Google Scholar * Noda, T., Inagaki, M., Hirano, S.-I. & Amanuma, K.
Effect of coexisting minerals on graphitization of carbon. I. Heat treatments of carbon under 3 kbar in the presence of limestone. _Bull. Chem. Soc. Jpn._ 41, 1245–1248 (1968). Article CAS
Google Scholar * Hirano, S., Inagaki, M. & Saito, H. Cooperative accelerating effect of calcium carbonate and gaseous nitrogen on graphitization of carbon. _Carbon_ 17, 395–398
(1979). Article CAS Google Scholar * Tsubouchi, N., Xu, C. & Ohtsuka, Y. Carbon crystallization during high-temperature pyrolysis of coals and the enhancement by calcium. _Energ.
Fuels_ 17, 1119–1125 (2003). Article CAS Google Scholar * Vandenbroucke, M. & Largeau, C. Kerogen origin, evolution and structure. _Org. Geochem._ 38, 719–833 (2007). Article CAS
Google Scholar * Bernard, S. & Horsfield, B. Thermal maturation of gas shale systems. _Annu. Rev. Earth Planet. Sci._ 42, 635–651 (2014). Article CAS ADS Google Scholar * Bernard,
S., Beyssac, O. & Benzerara, K. Raman mapping using advanced line-scanning systems: geological applications. _Appl. Spectrosc._ 62, 1180–1188 (2008). Article CAS ADS PubMed Google
Scholar * Schiffbauer, J. D. & Xiao, S. Novel application of focused ion beam microscopy (FIB-EM) in preparation and analysis of microfossil ultrastructures: A new view of complexity in
early Eukaryotic organisms. _Palaios_ 24, 616–626 (2009). Article ADS Google Scholar * Bassim, N. D. et al. Minimizing damage during FIB sample preparation of soft materials. _J.
Micros._ 245, 288–301 (2012). Article CAS Google Scholar * Kaznatcheev, K. V. et al. Soft X-ray spectromicroscopy beamline at the CLS: commissioning results. _Nucl. Instr. Meth. Phys.
Res. A_ 582, 96–99 (2007). Article CAS ADS Google Scholar * Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. _J.
Synchrotron Radiat._ 12, 537–541 (2005). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We gratefully acknowledge support from the ERC (project PaleoNanoLife—PI:
F. Robert). Special thanks go to J. William Schopf for having provided the samples, Mélinée Deretz for administrative simplification, Imene Esteve (IMPMC) for her expert support of the SEM,
Stephan Borensztajn (IPGP) for the realization of FIB–SEM experiments, David Troadec (IEMN) for the preparation of FIB sections, Jean-Michel Guigner (IMPMC) for his expert support of the
TEM, and Jian Wang and Jay Dynes for their expert support of the STXM at the Canadian Light Source (CLS). The ion microprobe of UCLA is supported by an NSF instrumentation and facility
grant. The SEM facility of the IMPMC is supported by Region Ile de France grant SESAME Number I-07-593/R, INSU-CNRS, INP-CNRS and UPMC-Paris 6, and by the Agence Nationale de la Recherche
(ANR) grant number ANR-07-BLAN-0124-01. The FIB–SEM facility of the IPGP is supported by Region Ile de France grant SESAME Number 12015908 and by the IPGP multidisciplinary programme PARI.
The TEM facility of the IMPMC is supported by Region Ile de France grant SESAME 2000 E 1435. STXM-based X-ray absorption spectroscopy data were acquired at beamline 10ID-1 at the CLS, which
is supported by the NSERC, the CIHR, the NRC and the University of Saskatchewan. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institut de Minéralogie, de Physique des Matériaux et de
Cosmochimie (IMPMC), Sorbonne Universités - CNRS UMR 7590, Muséum National d’Histoire Naturelle, UPMC Univ Paris 06, IRD UMR 206, 61 rue Buffon, Paris, France, 75005 Julien Alleon, Sylvain
Bernard, Corentin Le Guillou, Sylvain Pont, Olivier Beyssac & François Robert * Univ Lyon, UJM Saint Etienne, Laboratoire Magma et Volcans, UBP, CNRS, IRD, 23 rue Dr Paul Michelon, St
Etienne, 42100, France Johanna Marin-Carbonne * Department of Earth, Planetary and Space Sciences, University of California–Los Angeles, 595 Charles Young Drive East, Los Angeles,
90095-1567, California, USA Kevin D. McKeegan Authors * Julien Alleon View author publications You can also search for this author inPubMed Google Scholar * Sylvain Bernard View author
publications You can also search for this author inPubMed Google Scholar * Corentin Le Guillou View author publications You can also search for this author inPubMed Google Scholar * Johanna
Marin-Carbonne View author publications You can also search for this author inPubMed Google Scholar * Sylvain Pont View author publications You can also search for this author inPubMed
Google Scholar * Olivier Beyssac View author publications You can also search for this author inPubMed Google Scholar * Kevin D. McKeegan View author publications You can also search for
this author inPubMed Google Scholar * François Robert View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.A., S.B., C.L.G., J.M.-C. and F.R.
conceived and designed the present research. J.A. and S.P performed the XRD experiments. J.M.-C. and K.D.McK. performed the SIMS experiments. J.A. and S.B. performed the SEM experiments.
J.A., S.B. and O.B. performed the Raman spectroscopy and Raman mapping experiments. C.L.G. performed the TEM experiments. J.A., S.B. and C.L.G. performed the STXM-based XANES experiments,
interpreted the data and wrote the present article. CORRESPONDING AUTHOR Correspondence to Sylvain Bernard. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial
interests. RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included
in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain
permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Alleon, J., Bernard, S., Le Guillou, C. _et al._ Molecular preservation of 1.88 Ga Gunflint organic microfossils as a function of temperature and mineralogy. _Nat Commun_
7, 11977 (2016). https://doi.org/10.1038/ncomms11977 Download citation * Received: 14 August 2015 * Accepted: 18 May 2016 * Published: 17 June 2016 * DOI: https://doi.org/10.1038/ncomms11977
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative