Play all audios:
Two-dimensional materials have been an ideal material platform for constructing flexible ultrathin-film supercapacitors, offering great advantages of flexibility, ultra-thinness and even
transparency. Exploring new two-dimensional pseudocapacitive materials with high electrochemical activity is needed to achieve flexible ultrathin-film supercapacitors with higher energy
densities. Here we report an inorganic graphene analogue, α1-vanadyl phosphate ultrathin nanosheets with less than six atomic layers, as a promising material to construct a flexible
ultrathin-film pseudocapacitor in all-solid-state. The material exhibits a high potential plateau of ~ 1.0 V in aqueous solutions, approaching the electrochemical potential window of water
(1.23 V). The as-established flexible supercapacitor achieves a high redox potential (1.0 V) and a high areal capacitance of 8,360.5 μF cm−2, leading to a high energy density of 1.7 mWh cm−2
and a power density of 5.2 mW cm−2.
A rapidly growing demand for portable consumer electronics, such as flexible displays, mobile phones and notebook computers, has greatly promoted the development of flexible energy devices
in all-solid-state1,2,3,4,5,6,7. As the future-generation energy storage device, the flexible thin-film supercapacitor in all-solid-state offers the synergic benefits of flexibility,
thinness and transparency2,8,9,10,11,12. Among the different types of supercapacitors, pseudocapacitors have the advantage of a highly electroactive surface of the electrode materials owing
to fast redox reaction, and exhibit much higher energy density compared with electrical double-layer capacitors as well as higher power density compared with lithium-ion batteries13,14,
holding great promise for realizing high-performance flexible ultrathin-film supercapacitor in all-solid-state (FUSA) with pseudocapacitive behaviour15,16,17. Pursuing two-dimensional (2D)
graphene-like materials with pseudocapacitive characteristics represents a promising direction to accomplish the flexible ultrathin-film pseudocapacitor in all-solid-state (FUPA) with higher
energy density, and potentially excellent mechanical flexibility18,19,20. Moreover, obtaining the graphene-like pseudocapacitive materials not only requires the intrinsic layered structures
that can be exfoliated into 2D ultrathin nanosheets, but also needs to possess a high electrochemical activity and high redox potential that can lead to higher energy density (E~ V2).
However, the development of graphene-like pseudocapacitive materials is still at its early stage, especially for the FUPAs with high power and energy densities. For example, the reversible
redox Faradaic reactions occurred on the surface of layered Ni(OH)2 and Co3O4 exhibit the potential windows of only about 0.5 and 0.4 V, respectively13,20, both of which are still much lower
than the limited electrochemical window of the aqueous solution (1.23 V)21. Therefore, the discovery of new graphene-like pseudocapacitive materials with enhanced electrochemical
performance is much needed for constructing FUSA with higher power and energy densities, vital for satisfying the practical applications.
Vanadyl phosphate (VOPO4) has seen tremendous advances for layered materials with higher electrochemical performance22,23,24. Due to the enhanced inionicity of (V–O) bonds when (PO4)3− anion
is introduced, V4+/V5+ redox couple of VOPO4 possesses the higher potential than that for simple vanadium oxide25. Layered VOPO4 has a high redox potential of 1.0 V versus normal hydrogen
electrode (NHE), and thus it is promising to establish the pseudocapacitors with improved energy density (Supplementary Fig. S1)26,27. Furthermore, dehydrated vanadyl phosphate (VOPO4 2H2O)
has the characteristic layered structure, in which the sheets of VOPO4 form from vertex-sharing VO6 octahedra linking to phosphate PO4 tetrahedra. Between each VOPO4 layer, one water
molecule coordinates with a vanadium atom through an oxygen atom and the other links adjacent layers together through weak hydrogen bonds (Supplementary Fig. S2)28. The presence of weak
hydrogen bonds in VOPO4·2H2O provides the feasible clue to the exfoliation of layered VOPO4·2H2O into VOPO4 ultrathin nanosheets while maintaining the integrity of the in-plane structure.
With the synergic advantages of high redox potential and the layered structure, the exfoliated VOPO4 ultrathin nanosheets could be a promising new 2D graphene-like material with greatly
enhanced electrochemical properties. Although VOPO4 shows great potential for applications in flexible supercapacitors, its graphene-like material has long been unexplored in the past years,
let alone the realization of the corresponding energy storage devices.
Here we report a simple 2-propanol-assisted ultrasonication method to effectively exfoliate bulk VOPO4·2H2O into VOPO4 ultrathin nanosheets, with a thickness of less than six atomic layers,
as a new graphene-like material. To fully explore the electrochemical performance of VOPO4, a VOPO4/graphene hybrid film was layer-by-layer assembled to achieve both high planar conductivity
and superior electrochemical performance. The FUPA based on the VOPO4/graphene hybrid thin film demonstrated a high output voltage, a large specific capacitance and a high energy density,
opening up opportunities for exploring new quasi-2D materials for flexible energy device with higher energy density.
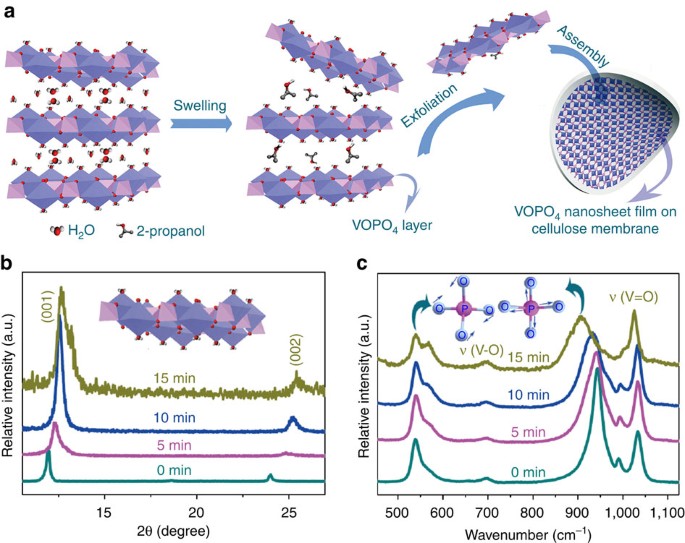
The VOPO4 ultrathin nanosheets were achieved by exfoliation of bulk VOPO4·2H2O through a simple ultrasonication method in 2-propanol with a short reaction time of 15 min. For bulk
VOPO4·2H2O, VOPO4 layers are linked together by hydrogen bonds from the interaction between H2O molecular and VOPO4 layers. As one kind of weak intermolecular force, hydrogen bond is very
sensitive to the applied external force29. For example, the applied strong ultrasonication provides the powerful force to trigger the breaking of hydrogen bonds. In our case, the exfoliation
process from bulk VOPO4 2H2O to VOPO4 graphene-like material was conducted in 2-propanol solution, in that 2-propanol as a secondary alcohol is more suitable than primary alcohols as the
dispersant due to the lower reactivity of hydroxyl group with the VOPO4 layer matrix (Supplementary Fig. S3). The interaction force of hydrogen bonds between 2-propanol and H2O further
promoted the H2O molecules extracted from the interlayer space of bulk VOPO4·2H2O. (Fig. 1a). The obtained VOPO4 ultrathin nanosheets were homogeneously dispersed in solution with high
stability for over several months.
(a) Schematic illustration for 2-propanol-assisted exfoliation process from bulk VOPO4·2H2O to graphene-like VOPO4 nanosheets. During the process of ultrasonication in 2-propanol solution,
the VOPO4 layers were swelled and interlayered H2O molecules effused, resulting in the VOPO4 ultrathin nanosheets. (b,c) XRD and Raman spectra of samples for the elongated ultrasonication
time of 0, 5, 10 and 15 min, respectively. The insets in c are the symmetric bending (left) and stretching (right) modes of O–P–O, respectively.
The structural transformation process during the exfoliation could be revealed by the characterizations of X-ray powder diffraction (XRD) patterns and Raman spectra, which performed on the
transferrable VOPO4 thin films. The XRD characterization of vacuum-filtration assembled films of samples at different sonication time is shown in Fig. 1b. The XRD pattern at the initial
sonication process can be readily indexed into the tetragonal VOPO4·2H2O with the standard JCPDS card No.84-0111 (VOPO4·2H2O, space group P4/nmm, a=6.202 Å, b=6.202 Å, c=7.410 Å). As the
strong ultrasonication proceeds, the (001) peak became relatively stronger compared with other XRD peaks, revealing that the applied sonication waves triggered the structural arrangements
and enhanced the c axis orientation for the formation process of high-quality 2D nanosheets. Furthermore, the (001) peak of VOPO4·2H2O gradually shifted from lower 2θ (11.74°) to a higher
one (13.5°) during the sonication process, indicating the decrease of the interlayer spacing distance from VOPO4·2H2O to the final VOPO4 ultrathin nanosheets as a result of the escape of
molecular H2O from VOPO4 interlayers.
In addition, Raman spectra shown in Fig. 1c further verified structural evolutions during the exfoliation process. The bands at 937 cm−1 were assigned to the symmetric O–P–O stretching
modes30,31. With the elongated exfoliation time, the red shift of Raman peaks of O–P–O stretching mode was more noticeable. Obviously, there was a strong microstructural correlation between
symmetry of O–P–O stretching modes with the hydrogen bonding among the interlayered H2O molecular. With the breaking of hydrogen bonds from the oxygen atoms of the P–O bond in VOPO4, the
mitigation of the steric hindrance would facilitate the occurrence of the O–P–O stretching modes with lower energy. However, the peaks related to the symmetric bending vibrations of O–P–O,
V–O and V=O stretching mode demonstrated little shift with no obvious peak position evolution (Supplementary Fig. S4). The Raman results confirmed that the exfoliated VOPO4 nanosheets
maintained the integrity of the in-plane VOPO4 structure without obvious structural deformations.
To unravel the microscopic outlook and structural crystallinity of exfoliated 2D VOPO4 nanosheets, microstructural characterizations were performed. Scanning electron microscopy (SEM) images
of the precursor VOPO4·2H2O and graphene-like VOPO4 nanosheets were taken and compared as shown in Fig. 2 and Supplementary Fig. S5. The bulk VOPO4·2H2O exhibited the typical layered
structure, of which the layers were tightly stacked. In contrast, SEM image of the exfoliated products after 15 min ultransonication (Fig. 2b) shows the morphology of ultrathin nanosheets
with a typical size ranging from 400 nm to several micrometres. Transmission electron microscopy (TEM) image of the exfoliated VOPO4 nanosheet in Fig. 2c reveals a free-standing, sheet-like
morphology with a lateral size of ~ 1 μm, and the nearly transparent feature implies the ultrathin thickness of exfoliated nanosheets. Atomic force microscopy image in Fig. 2d was taken to
further evaluate the thickness of the VOPO4 nanosheet. The measured height was ~ 4.07 nm, denoting that the nanosheet was comprised of 5–6 single layers, given that the c parameter of the
VOPO4·2H2O is 7.410 Å. The corresponding HR-TEM image and fast Fourier transform pattern are shown in Fig. 2e, demonstrating that the exfoliated sheets were single crystalline with [001]
preferential orientation. The interplanar distance of 0.31 nm fits well with the plane distance of d200 and d202, respectively. The orientation angle values 90° of these two planes of (200)
and (020) in HR-TEM image and fast Fourier transform pattern was consistent with those calculated from tetragonal crystallographic parameters of VOPO4·2H2O. These characterization results
showed that VOPO4·2H2O was successfully exfoliated into ultrathin VOPO4 nanosheets that exhibited good crystallinity and high c axis orientation, providing strong basis for further assembly
of VOPO4 nanosheets into large-area practical energy storage devices.
(a) Field emission scanning electron microscopy image of bulk VOPO4·2H2O precursors, where the stacked layers can be clearly seen. Scale bar, 200 nm. (b) The SEM image of exfoliated VOPO4
ultrathin nanosheets with warped edges exhibiting the ultrathin features. Scale bar, 200 nm. (c) TEM image of a typical ultrathin nanosheet. Scale bar, 500 nm. (d) Atomic force microscopy
image of a typical nanosheet with a thickness of 4.07 nm, corresponding to less than six atomic layers. Scale bar, 100 nm. (e) High-resolution TEM (HR-TEM) image of a typical nanosheets
showing the lattice fringes of (200) and (020) planes, and the inset is the corresponding fast Fourier transform patterns of the same area in the HR-TEM image. Scale bar, 2 nm.
The bulk VOPO4 is well known as a high-performance electrochemical material with a high redox voltage under aqueous solution of 1.0 V versus NHE, approaching the electrochemical window of
water (1.23 V)21, which entails the great fascination for the construction of high-efficiency energy storage devices. (Supplementary Fig. S1) In our work, VOPO4 ultrathin nanosheets with