Play all audios:
ABSTRACT Moderate levels of reactive oxygen species (ROS) are now recognized as redox signalling molecules. However, thus far, only mitochondria and NADPH oxidases have been identified as
cellular sources of ROS in signalling. Here we identify a globin (GLB-12) that produces superoxide, a type of ROS, which serves as an essential signal for reproduction in _C. elegans_. We
find that GLB-12 has an important role in the regulation of multiple aspects in germline development, including germ cell apoptosis. We further describe how GLB-12 displays specific
molecular, biochemical and structural properties that allow this globin to act as a superoxide generator. In addition, both an intra- and extracellular superoxide dismutase act as key
partners of GLB-12 to create a transmembrane redox signal. Our results show that a globin can function as a driving factor in redox signalling, and how this signal is regulated at the
subcellular level by multiple control layers. SIMILAR CONTENT BEING VIEWED BY OTHERS A NUCLEIC ACID BINDING PROTEIN MAP OF GERMLINE REGULATION IN _CAENORHABDITIS ELEGANS_ Article Open access
11 August 2024 HOW GERM GRANULES PROMOTE GERM CELL FATE Article 18 June 2024 A GERMLINE-TO-SOMA SIGNAL TRIGGERS AN AGE-RELATED DECLINE OF MITOCHONDRIAL STRESS RESPONSE Article Open access
08 October 2024 INTRODUCTION Reactive oxygen species (ROS)-based redox signalling is involved at all levels of cellular organization, from cell differentiation to cell death1,2. In this type
of signalling, low levels of ROS act as biological messengers by inducing reversible oxidative modifications in downstream proteins and thereby influencing signalling pathways3,4. These ROS
are enzymatically generated by cells to serve this signalling function. However, in the majority of the reported redox-sensitive signalling pathways, it is often not known which proteins
are responsible for generating the redox signal and how cells spatially and temporally link ROS production to specific signalling pathways and so achieve desired cellular outcomes. Globins
are increasingly hypothesized to play a role in redox biology5. These proteins are characterized by a common tertiary structure and the presence of a haem group, whereby their function is
largely determined by how this haem group is incorporated in the surrounding globin fold. The best-characterized function of globins is in oxygen diffusion and transport, as exemplified by
vertebrate haemoglobin and myoglobin. The haem iron in these globins is pentacoordinated, leaving the sixth coordination site of the iron free for reversible binding of diatomic ligands,
such as O2. In a second type of globins, named hexacoordinated globins, all six positions of the haem iron are bound6. Since their initial discovery two decades ago, these hexacoordinated
globins are now recognized to be broadly present in plants and animals7. Because of the hexacoordinated nature of the haem iron, ligand binding/transfer becomes more complex8 or even
absent9. On the other hand, this hexacoordinated state of the iron seems to favour electron transfer9,10,11, indicating that redox reactions involving the haem iron could be a key element in
the physiological function of these globins. Therefore, increasing attention has been placed on the potential roles of hexacoordinated globins and globin-like proteins in signalling and
redox chemistry, such as protection against ROS and electron transfer to molecular partners7. However, even though a fairly detailed understanding on the structure and biochemistry of
hexacoordinated globins is now present, it has thus far been extremely difficult to understand what their possible physiological roles are. _C. elegans_ has thirty-three globin-like
proteins, which display different sizes, are differently located and are likely to be functionally diverse12. This makes _C. elegans_ an attractive model to study the potential roles of
globins and globin-like proteins in redox metabolism. A genome-wide RNAi screen in _C. elegans_ reported abnormal egg laying and embryonic lethality following RNAi for GLB-12 (ref. 13),
providing a clear phenotype for this so far uncharacterized globin. In this study, we analyse the role of GLB-12 in more detail and show that this protein is hexacoordinated, functions as a
redox signalling protein and has an essential role in the reproduction of _C. elegans_. RESULTS GLB-12 IS ESSENTIAL FOR REPRODUCTION To characterize the role of GLB-12 in reproduction, we
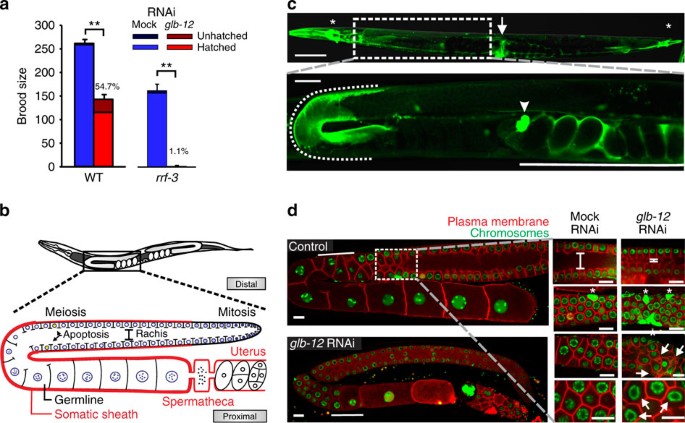
analysed the effect of reduced GLB-12 levels on the brood size of _C. elegans_. We observed that _glb-12_ RNAi reduced fecundity and increased embryonic lethality in wild-type (WT) worms and
caused sterility in the majority of worms in the RNAi-hypersensitive strain _rrf-3_ (Fig. 1a). Expression analysis showed that GLB-12 is present in distinct parts of the somatic
reproductive system (Fig. 1b,c; Supplementary Fig. 1a)—the distal gonadal sheath, the proximal part of the spermatheca, and the uterus—and in several head and tail neurons and the vulva.
GLB-12 does not appear to be directly present in the germline: integration of the GLB-12::GFP reporter, which is necessary to allow expression in the germline, showed no visible expression
in this tissue (Supplementary Fig. 1c). Further, GLB-12 appears to act directly from the somatic gonad to regulate reproduction: _glb-12_ RNAi only had an effect on reproduction in a
soma-specific, but not in germline- or neuronal-specific RNAi strains (Supplementary Fig. 1b), while _glb-12_ RNAi in the GLB-12 reporter strain reduced reporter levels in the somatic gonad
only (Supplementary Fig. 1c). To understand the impact of GLB-12 on gonad morphology, we used a strain visualizing the germline architecture by fluorescent markers (Fig. 1b,d). In the first
generation, _glb-12_ RNAi caused a range of defects in the adult germline (Fig. 1d): delayed meiotic progression, a considerably smaller rachis, increased apoptosis levels, and irregular
shaped compartments and nuclei. In the second generation, _glb-12_ RNAi led to abnormal germline development and failure to produce oocytes (Supplementary Fig. 1d). GLB-12 is thus essential
for germline development and regulation and appears to be involved in several aspects of reproduction. GLB-12 IS A HEXACOORDINATED GLOBIN WITH REDOX PROPERTIES To understand how GLB-12 could
influence reproduction on a molecular level, we analysed the biochemical and structural properties of the purified globin. Spectroscopic analysis, which allows to discriminate between
different globin forms, showed that reduced deoxy (Fe2+) GLB-12 displays a hexacoordinated haem iron (Fig. 2a; Supplementary Fig. 2a). Hexacoordinated globins are present in prokaryotes,
plants and animals, but their function is largely unclear7. They can potentially bind diatomic ligands such as O2, CN− and CO to their haem iron, thereby replacing the endogenous HisE7
ligand. However, even though GLB-12 was able to bind CN− and CO _in vitro_ (Fig. 2a), both reactions proceeded very slowly, requiring several minutes (reaction kinetics not shown). In
addition, upon exposure to air, GLB-12 is spontaneously oxidized to the ferric state (Fe3+) (Fig. 2a) and is thus incapable of binding O2 under physiological conditions. These results argue
against a role for GLB-12 in reversible ligand binding and/or transport, the best known function for globins. On the other hand, the spontaneous oxidation of air-exposed GLB-12 is in line
with the observation that hexacoordination appears to lower the redox potential and promotes electron transfer of the haem iron9,10,11. By using cyclic voltammetry to study the
electrochemical properties of GLB-12 in more detail, we indeed observed a relatively low reduction potential for the GLB-12 redox couple Fe2+/Fe3+ (−0.244 V versus saturated calomel
reference electrode (SCE)), which is largely stable within the physiological pH range (Fig. 2b; Supplementary Fig. 2b). To determine whether GLB-12 could transfer electrons to other
molecules, we included cytochrome c peroxidase (CCP) in this experimental setup as an electron acceptor and subsequent catalyst for H2O2 reduction. It is important to note that CCP by itself
shows no electrocatalytic activity under these conditions14. When H2O2 was added to GLB-12 and CCP, the reduction current increased and the oxidation peak disappeared, indicating very fast
GLB-12 oxidation (Fig. 2c). This shows that GLB-12 can donate electrons to CCP in a continuous manner, allowing the latter to reduce H2O2. Compared to other tested globins, GLB-12 showed a
10-fold higher electron transfer rate to CCP (Supplementary Fig. 2c). Combined, these results support a role for GLB-12 in electron transfer reactions. GLB-12 PRODUCES O2.− AS A SIGNALLING
MOLECULE Because GLB-12 can participate in electron transfer and has a reduction potential lower than the O2/O2.− couple15, it may directly interact with O2 to generate O2.−. To test this
hypothesis, we used an _in vitro_ enzymatic reduction system for haem proteins16 and included lucigenin, which emits light upon reaction with O2.− (Supplementary Fig. 2d). Addition of GLB-12
and glucose-6-phosphate, the driving force of this system, led to increased luminescence, showing that GLB-12 can convert O2 to O2.− (Fig. 2d; Supplementary Fig. 2d). O2.− levels are
proportional to GLB-12 concentration, indicative of a 1:1 stoichiometry (Supplementary Fig. 2e). We also validated this _in vitro_ method using haem proteins with a reduction potential
higher and lower than the O2/O2.− couple (Fig. 2e). Noteworthy, O2.− production rate of cytochrome P450 was 40-fold higher than that of GLB-12. We subsequently hypothesized that _in vivo_
O2.− produced by GLB-12 could act as a redox signalling molecule. Decreasing GLB-12 levels by RNAi would thus decrease this O2.− signal, and exposing worms to O2.− scavengers could
potentially even further reduce the O2.− signal. Using the antioxidants _N_-acetylcysteine (NAC) or ascorbic acid, scavengers of O2.− and other ROS, indeed further aggravated the reduction
in brood size by _glb-12_ RNAi (Fig. 2f), providing a first indication that GLB-12 generates O2.− as a signalling molecule. The crystal structure of the GLB-12 globin domain, refined to 1.65
Å resolution (Table 1), further supported our current findings. It confirmed haem hexacoordination, with strong Fe-N bonds with the distal His92 and the proximal His127 residues (Fig. 3a;
Supplementary Fig. 3a). Overall, the hexacoordinated GLB-12 globin domain is closely related to the 3D structures of human and murine neuroglobin10,17,18,19, human cytoglobin20,
non-symbiotic rice haemoglobin21, _C. elegans_ GLB-6 (ref. 9) and _Geobacter sulfurreducens_ globin-coupled sensor22, although GLB-12 possesses several unique structural features. The
proximal His127 is highly exposed to the solvent, which is the result of a hydrogen bond between the A-propionate of the haem to Ser126 OH. As a result, two water molecules are hosted at the
entrance of the proximal site (hydrogen bonded to Ser126 OG and to Gln130 NE2, respectively), and four additional water molecules and one acetate ion fall next to the propionate
(Supplementary Fig. 3a). This exposure to a polar aqueous environment would favour oxidation of the ferrous haem, which is consistent with the low GLB-12 redox potential reported. On the
distal site of the haem cavity, the D-propionate stabilizes the location of the E-helix N-terminal region through hydrogen bonding (Supplementary Fig. 3a). In addition, residues Phe89 and
Phe96 (surrounding the distal His92) are part of a wide cluster of aromatic/hydrophobic residues (Val54, Phe58, Leu59, Val62, Phe73, Phe152) (not highlighted). These could restrict E-helix
movements required to achieve a pentacoordinated haem and would thereby limit ligand binding. This explains the slow CN− and CO binding we observed. Additionally, this aromatic/hydrophobic
residue cluster creates a tunnel of about 50 Å3 in the globin domain, nestled among the B-, E-, and G-helices (Fig. 3a). Interestingly, this otherwise apolar tunnel shows a strong negative
charge distribution at the solvent exit (resulting from residues Asp48, Asp49, Asp52 and Glu165) (Fig. 3a,b). In the crystal structure the tunnel exit to the solvent appears rather narrow;
however, structural fluctuations may vary its diameter, providing a direct connection between the haem distal site and the solvent region. Given this, it is possible that the negative
charges located at the tunnel exit would help to remove small and negatively charged species such as O2.−, produced by redox reactions at the distal site, from the protein core. Further,
while the haem pocket in globins usually prevents haem iron oxidation by creating a hydrophobic environment, in GLB-12 three polar amino acids (Lys88, Arg95 and Arg129) are located at the
edge of the haem cavity (Fig. 3c), possibly to enhance solvent access to the haem and stimulate haem iron oxidation and O2.− production. Mutation of these three polar amino acids to the
hydrophobic leucine indeed reduced or prevented O2.− production (Fig. 3d), while other biochemical characteristics remained largely unaffected (Supplementary Fig. 3b–d). Furthermore,
transgene animals bearing these mutations in an RNAi-resistant _glb-12_ gene (Supplementary Fig. 3e,f) failed to show rescue of the reduced brood size following _glb-12_ RNAi (Fig. 3e). Also
the K88L mutant, even though it can still produce some O2.−, failed to rescue the reduction in brood size. Overall, these results show that GLB-12 possesses specific structural properties
associated with a role in redox biology and further support that GLB-12 produces O2.− as a signalling molecule. GLB-12 SHOWS A DISTINCT TISSUE AND SUBCELLULAR EXPRESSION Because O2.− is a
very short-lived signalling molecule, we reasoned that GLB-12 would show a very specific tissue and subcellular localization to achieve an exact juxtaposition with potential downstream
targets. Indeed, besides a very distinct expression pattern in the somatic gonad (Fig. 1c), the GLB-12 reporter was also found to be membrane-bound through a short N-terminal extension (Fig.
4a; Supplementary Fig. 4a). This extension harbours predicted sites for both myristoylation23 and palmitoylation24 (Fig. 4a), post-translational modifications that promote stable membrane
attachment25. Deletion of these sites indeed prevented plasma membrane localization (Fig. 4b; Supplementary Fig. 4b). Myristoylation and palmitoylation are specifically associated with
protein translocation to membrane rafts, which are dynamic membrane subdomains that compartmentalize cellular processes, including specific redox signalling events26. For GLB-12, we indeed
observed a clustered distribution, both in the somatic gonad and in the nervous system (Supplementary Fig. 4c,d). Finally, a cytoplasmic version of the RNAi-resistant _glb-12_ gene was not
capable of rescuing the decreased fecundity following _glb-12_ RNAi (Fig. 4c). Combined, these results support that the localization of GLB-12 serves as a spatial determinant for the O2.−
signal, both on a tissue and intracellular level. GLB-12 INTERACTS WITH AN INTRA- AND EXTRACELLULAR SOD _In vivo_, O2.− produced by GLB-12 may be converted into the more stable H2O2 by
superoxide dismutases (SODs), H2O2 being an accepted messenger in redox signalling1,27. To test this hypothesis, we reduced GLB-12 levels in mutants for the five _C. elegans sod_ genes
(_sod-1_ to _sod-5_) and found an aggravated effect on fecundity in the main cytoplasmic _sod-1_ mutant and, surprisingly, a reduced effect in the extracellular _sod-4_ mutant (Fig. 5a).
Also in the second cytoplasmic _sod-5_ mutant and, to a lesser extent, in the mitochondrial _sod-3_ mutant an aggravated effect was observed (Supplementary Fig. 5a). Further, the effect in a
_sod-1;sod-4_ and a _sod-4_ mutant are comparable, indicating that SOD-4 is epistatic to SOD-1 (Fig. 5a). Expression analysis of SOD-1 and both isoforms of SOD-4 showed that they are
present in the entire somatic gonad (Fig. 5b, Supplementary Fig. 5b), thus overlapping in expression with GLB-12. When these reporter constructs were expressed in the corresponding _sod_
mutant, the _glb-12_ RNAi effect on worm fecundity largely reverted to levels observed in the WT (Fig. 5c). Targeting a H2O2-specific GFP-probe, roGFP2-ORP1 (ref. 28), to the intracellular
location of GLB-12 showed that GLB-12 depletion indeed lowered H2O2 levels _in vivo_ in the somatic gonad, while overexpressing GLB-12 increased H2O2 levels (Fig. 5d, Supplementary Fig. 5c).
Although not statistically significant, H2O2 levels appeared to be further reduced in a _sod-1_ background following GLB-12 depletion, again indicating that the intracellular SOD-1 converts
GLB-12-produced O2.− to H2O2 (Fig. 5d,e). Surprisingly, intracellular H2O2 levels in a _sod-4_ background appeared to be less reduced compared to the WT following GLB-12 depletion (Fig.
5d), although this difference is again not statistically significant. The type of interaction between GLB-12 and SOD-4 is at this moment not clear; GLB-12-produced O2.− could potentially
penetrate the membrane through anion channels29 and be converted by the extracellular SOD-4, or O2.− could be generated extracellularly by an unknown source. To further test the hypothesis
that GLB-12/SOD-1 and SOD-4 work in parallel on both sides of the gonadal sheath cell membrane, we artificially targeted PRDX-2, an H2O2 scavenger, to both sides of the membrane (Fig. 5f,g;
Supplementary Fig. 5d,e); following _glb-12_ RNAi, the presence of PRDX-2 should then mimic the loss of SOD. This was indeed observed, with the strongest effect by the intracellular PRDX-2
(Fig. 5f). Taken together, these results strongly suggest that SOD-1 and SOD-4 modulate the downstream effects of GLB-12 in opposite ways. Endogenous occurring H2O2 scavengers like
peroxiredoxins and catalases could further influence the GLB-12-based redox signal. However, loss of relevant peroxiredoxins and catalases did not affect the _glb-12_ RNAi phenotype,
indicating that these H2O2 scavengers do not interact with GLB-12 (Supplementary Fig. 5f). Surprisingly however, when both SOD-1 and each of these H2O2 scavengers are lost, brood sizes
following _glb-12_ RNAi are comparable to the WT and not to the SOD-1 single mutant (Supplementary Fig. 5f). This may suggest that lack of H2O2 scavenging becomes important when the GLB-12
redox signal is severely diminished, that is, when GLB-12 levels are reduced and SOD-1 is absent, but further analysis is needed to test this hypothesis. Finally, GLB-12 overexpression in
the WT as well as in the _sod-1_ or _sod-4_ background did not result in an obvious effect on fecundity (Supplementary Fig. 5g), even though GLB-12 overexpression led to an increase in
peroxide levels (Fig. 5d). Given the complexity of GLB-12-based signalling, negative feedback regulation might potentially be an explanation for this absence of a phenotype. GLB-12 MODULATES
APOPTOSIS VIA THE P38/JNK MAPK PATHWAYS To identify potential downstream targets of GLB-12, we focused on its role in germline apoptosis, which is regulated by a well-characterized
signalling cascade30. In WT worms, _glb-12_ RNAi led to a doubling in germ cell corpses, while germline proliferation was not significantly affected under these conditions (Fig. 6a,b;
Supplementary Table 1). In a _ced-3_ mutant, in which germline apoptosis is absent, no corpses were present following both control and _glb-12_ RNAi (Fig. 6b). An increase in germ cell
corpses can be caused by an increase in germline apoptosis, or by a decrease in corpse engulfment and removal by the somatic gonad. Because _glb-12_ RNAi still caused increased germline
apoptosis in engulfment-defective mutants, did not affect the speed of corpse removal by the somatic gonad and had no visible effects on somatic gonad structure (Supplementary Fig. 6a–c), we
concluded that GLB-12 is directly involved in germline apoptosis. In loss-of-function mutants for the pro-apoptotic genes _cep-1_, _ced-13_ and _egl-1_, GLB-12 depletion still caused an
increase in germline apoptosis (Fig. 6b; Supplementary Fig. 6d), indicating that GLB-12 does not signal through these proteins. The p38/JNK MAPK pathways act independently of CEP-1, CED-13
and EGL-1 (Fig. 6b) and are responsible for stress-induced germ cell apoptosis31. _glb-12_ RNAi in loss-of-function mutants for these pathways no longer led to an increase in germ cell
apoptosis (Fig. 6c; Supplementary Fig. 6e), while in a _vhp-1_ mutant, an inhibiting phosphatase for these pathways32, _glb-12_ RNAi caused a larger increase in germ cell apoptosis. Further,
_glb-12_ RNAi in the WT also increased the relative amount of phosphorylated PMK-1 (Supplementary Fig. 6f). Together, these results strongly suggest that GLB-12 has antiapoptotic effects by
inhibiting the p38/JNK MAPK pathways. Surprisingly however, in _ced-3_ or p38/JNK MAPK pathways mutants, fecundity was even further reduced following _glb-12_ RNAi compared with WT and
several gonadal defects could still be observed (Fig. 6d; Supplementary Fig. 6g,h). It therefore appears that the p38/JNK MAPK pathways do not mediate all downstream effects of GLB-12 on
reproduction, suggesting that GLB-12 influences additional pathways. Finally, we assessed if GLB-12 influences germline apoptosis by its O2.− redox signal. We indeed observed that one of the
two antioxidants used, ascorbic acid, further augmented apoptosis levels following _glb-12_ RNAi (Fig. 7a; Supplementary Fig. 7a), while the GLB-12 mutants incapable of producing O2.− or
without native GLB-12 localization were unable to rescue the increase in apoptosis following _glb-12_ RNAi (Fig. 7b,c; Supplementary Fig. 7b,c). The antiapoptotic effect of GLB-12 also
appears to be modulated by SOD-1 and SOD-4: loss of SOD-1, leading to a reduced conversion of O2.− to H2O2, enhanced relative germline apoptosis levels following _glb-12_ RNAi (Fig. 7d;
Supplementary Fig. 7d), SOD-1 reporter expression in the _sod-1_ mutant, which restores the conversion of O2.− to H2O2, significantly suppressed the increase in germline apoptosis compared
with _sod-1_ worms following _glb-12_ RNAi (Fig. 7e; Supplementary Fig. 7e) and artificially targeting the H2O2 scavenger PRDX-2 to colocalize with GLB-12, thereby again removing H2O2 and
thus mimicking loss of SOD-1, further augmented apoptosis levels following _glb-12_ RNAi (Fig. 7f; Supplementary Fig. 7f). Following _glb-12_ RNAi, loss of the extracellular SOD-4 did not
significantly affect apoptosis levels compared with WT worms. However, when both SOD-1 and SOD-4 were lost, apoptosis levels were lower compared to when only SOD-1 was lost and comparable to
what is observed in WT animals (Fig. 7d; Supplementary Fig. 7d). This supports that both the intra- and extracellular H2O2 levels are important for GLB-12-based signalling. In line with
this, scavenging H2O2 by targeting PRDX-2 to both the intra- and extracellular side appeared to lead to a lower increase in apoptosis following _glb-12_ RNAi compared to when PRDX-2 was only
present intracellular (Fig. 7f; Supplementary Fig. 7f). SOD-1 and SOD-4 loss also had opposite effects on the relative amount of phosphorylated PMK-1 following GLB-12 depletion, which
further supports their opposite roles in GLB-12-mediated apoptosis (Supplementary Fig. 6f). In general, the role of SOD-1 and especially SOD-4 in GLB-12-mediated apoptosis appeared less
pronounced compared to their role in GLB-12-mediated fecundity. This could indicate that other redundant factors are also involved in GLB-12-mediated apoptosis. Lastly, no added effect
following _glb-12_ RNAi was observed when natural occurring H2O2 scavengers catalase or peroxiredoxin were lost (Supplementary Fig. 7g). Surprisingly again, when each of these scavengers
together with SOD-1 is lost, whereby not only less H2O2 is produced but also less is removed, the apoptosis increase is less severe than when only SOD-1 is lost (Supplementary Fig. 7h).
These results are thus comparable to the effects of these double mutations on the brood size following GLB-12 depletion. As mentioned, the potential role of these H2O2 scavengers in
GLB-12-based signalling is not clear at this moment. DISCUSSION In this study, we identified a novel role within the globin superfamily by showing that GLB-12 of _C. elegans_ functions as a
redox signalling protein in the reproductive system. Based on our findings, we propose a model whereby GLB-12 acts as a superoxide generator in the somatic gonad, after which this O2.−
signal is modulated directly or indirectly by an intracellular and an extracellular SOD, creating a transmembrane H2O2 gradient that acts as a redox signal. This signal then modulates
reproduction, including p38/JNK MAPK-dependent germ cell apoptosis (Fig. 8). The role of GLB-12 in redox chemistry is supported by its biochemical characteristics. We observed that GLB-12
shows a hexacoordinated haem, extremely low ligand affinity, a relatively low redox potential and the ability to transfer electrons to other molecules in a continuous manner. Haem
hexacoordination in globins has previously been associated with increased redox kinetics for the haem iron9,10,11, and, as a result, hexacoordinated globins have been hypothesized to
function in redox biology7. For example, hexacoordinated globins have been proposed to function in electron transfer33,34, to work as redox sensors to control behaviour9, to regulate lipid
oxidation35 or to function as NO scavengers36,37. However, it has thus far been very difficult to link these observations to the biochemical mechanisms used by these globins _in vivo_. We
found that, by using structural, biochemical and mutational approaches, GLB-12 actively converts O2 to O2.−, which in turn is used as a redox signalling molecule. As this hexacoordinated
globin can actively support redox signalling, it provides a reference model for the function of globins and globin-like proteins in other organisms. It is already well appreciated that redox
signals can originate from endogenous sources, such as membrane-bound NADPH oxidases or mitochondria, but the control mechanisms on ROS as endogenous signalling molecules are still poorly
understood. ROS signal specificity can be achieved by strict compartmentalization: by tightly controlled spatio-temporal expression of the ROS generators in proximity to the downstream
target, efficient signal transduction can be realized4. This specificity in spatial expression is indeed observed for GLB-12: (1) at the tissue level, GLB-12 is only present in a specific
part of the somatic gonad, wherefrom it acts to regulate germline reproduction; (2) at the subcellular level, GLB-12 is bound to the plasma membrane by myristoylation and palmitoylation,
whereby this localization increases the effectiveness of the GLB-12 signal. In addition, these types of protein acylation localize proteins to dynamic membrane subdomains that have been
associated with specific redox signalling events26. Given that GLB-12 produces O2.− and that O2.− is diffusible and short lived, we therefore reason that the location of GLB-12 serves as a
spatial determinant for downstream signals, both on a tissue and intracellular level. In this context, our observation that GLB-12 shows a 40-fold lower O2.− production rate compared with
cytochrome P450 might indicate that high ROS levels are not required when the redox signal is strictly localized. On top of localizing the enzymatic source of a redox signal with its
potential downstream target, an additional layer of regulation can be achieved by alterations in the local reduction/oxidation capacity4. We observed that GLB-12 acts together with the main
cytoplasmic SOD-1, whereby the latter enhances the spontaneous oxidation of O2.− into O2 and H2O2, and this interaction indeed increases the effectiveness of the H2O2 redox signal. To our
surprise, also the extracellular SOD-4 appears to influence the GLB-12 signal and this in an opposite manner compared to SOD-1. These results clearly show that SODs have essential roles in
the regulation of redox signalling and are not merely generic antioxidants. We hypothesize that H2O2, produced by GLB-12/SOD-1, influences ligand release from the somatic gonad, while H2O2
produced by SOD-4 could either influence ligand release or affect receptor activity on the germline plasma membrane (Fig. 8). These observations are in line with the increasing amount of
evidence that the intra- and extracellular redox states work in concert to influence cell signalling38,39. Furthermore, the interaction between GLB-12 and these two SODs adds a surprising
third level of regulation in this redox signal, whereby the amount of O2.− and H2O2 on the intra- and extracellular side of the plasma membrane are important determinants of the downstream
signal. It is interesting to note that, while plasma membranes form a physical barrier to ROS, ROS transport across membranes could occur via selective membrane channels. This has been
observed for both O2.− and H2O2 with certain classes of anion channels29 and aquaporins4,40,41,42. This ROS transport across membranes has therefore been hypothesized to participate in the
regulation of redox signalling and could also be involved in GLB-12-mediated signalling. Overall, our results show that redox signalling by GLB-12 is regulated by multiple control layers.
Interesting to note is that NADPH oxidase, a known ROS generator, also displays a very specific subcellular localization that is related to its function, and also appears to interact with
SOD enzymes26,43. These shared characteristics between GLB-12 and NADPH oxidases indicate that redox signalling proteins function according to similar principles. Furthermore, the presence
of a transmembrane redox gradient in GLB-12-mediated signalling presents a fascinating additional principle in how redox signalling can be further modulated. We linked the redox signalling
function of GLB-12 with _C. elegans_ reproduction. Reduction of GLB-12 levels causes decreased fecundity, smaller gonads, increased germline apoptosis levels and several defects during
oocyte development. We further showed that GLB-12 has antiapoptotic effects by inhibiting the p38/JNK MAPK pathways. These highly conserved pathways have been shown to be sensitive to
environmental stress, including oxidative stress, in several organisms44,45. Also in _C. elegans_, their combined role in modulating germ cell apoptosis in response to stressors such as
oxidative and heavy metal toxicity has been demonstrated31. The results presented here provide a link between a redox signalling protein and p38/JNK MAPK-mediated germline apoptosis.
Importantly, GLB-12-based redox regulation of the p38/JNK MAPK pathways, using low local levels of superoxide, is opposite from the effects of excessive oxidative stress on this pathway.
Because GLB-12 acts specifically from the somatic gonad and not from the germline, it likely influences the release of ligands and/or the activation of receptors of the p38/JNK MAPK
pathways. In other organisms, it has indeed been observed that ROS can influence receptor activation upstream of the p38/JNK MAPK pathways44. Our results presented here indicate that, when
p38/JNK MAPK ligands and receptors in the _C. elegans_ reproductive system will be identified, they could form an interesting model to study how GLB-12-mediated redox signalling can
influence the activity of downstream signalling cascades. Finally, in _C. elegans_, about half of all germ cells die by p53-independent ‘physiological’ apoptosis, and it is thought that this
process increases the quality of the surviving germ cells by the relocation of cytoplasmic components46. As fecundity is further reduced by combining _glb-12_ RNAi with p38/JNK pathway
inhibition, GLB-12 may be required to generate or allocate this cytoplasmic material via modulation of germline apoptosis levels. In the absence of GLB-12, apoptosis is then increased as a
compensatory measure to sustain reproduction, albeit at lower levels. This would explain why preventing the increase in cell death during GLB-12 depletion leads to a further decrease in
brood size. In conclusion, we have identified a globin that acts as a source of redox signalling. The specific biochemical characteristics of GLB-12 show how a globin can fulfil this
function, while the precise subcellular localization and the interaction with the SOD antioxidant enzymes increase the effectiveness of the redox signal. The opposite effect of the intra-
and extracellular SOD on GLB-12 signalling is particularly intriguing; it indicates the importance of transmembrane redox gradients and further deepens the concepts of redox signalling
compartmentalization. The notion that highly localized ROS gradients influence important biological processes such as reproduction could open up a new area of redox biology. Finally, given
the widespread occurrence of globins, the majority with unknown function, our results add an important member to the group of proteins that drive redox signalling. METHODS GENERAL METHODS
AND STRAINS Maintenance of _C. elegans_ was carried out according to standard procedures. _C. elegans_ strains were cultured at 20 °C on cholesterol-supplemented nutrient agar (OXOID) plates
containing a lawn of freshly grown _Escherichia coli_ K12 cells. To obtain synchronized cultures, gravid adults were lysed by hypochlorite treatment and eggs were allowed to hatch overnight
in S buffer. Worm strains used in this study were Bristol N2 wild type, NL2099 [_rrf-3(pk1426)_ II], TU3311 [uIs60], NL3511 [_ppw-1(pk1425)_ I], RB798 [_rrf-1(ok589)_ I], OD95
[_unc-119(ed3)_ III; ltIs37 IV; ltIs38], GA187 [_sod-1(tm776)_ II], GA184 [_sod-2(tgk257)_ II], GA186 [_sod-3(tm760)_ X], GA416 [_sod-4(gk101)_ III], GA502 [_sod-5(tm1146)_ II], RB1653
[_ctl-3(ok2042)_ II], VC1151 [_prdx-3(gk529)_ III], VC289 [_prdx-2(gk169)_ II], MT1522 [_ced-3(n717)_ IV], FX536 [_ced-13(tm536)_ X], TJ1 [_cep-1(gk138)_ I], MT8735 [_egl-1(n1084n3082)_ V],
FK171 [_mek-1(ks54)_ X], KU4 [_sek-1(km4)_ X], _mek-1(ks54) sek-1(km4),_ JT366 [_vhp-1(sa366)_ II], KB3 [_kgb-1(um3)_ IV], KU25 [_pmk-1(km25)_ IV], HT1593 [_unc-119(ed3)_ III], EG6699
[ttTi5605 II; _unc-119(ed3)_ III; oxEx1578], which were provided by the _Caenorhabditis_ Genetics Center funded by the National Institutes of Health National Center for Research Resources.
Standard crossing was used to generate double mutants. MOLECULAR BIOLOGY Translational reporters and genetic constructs were made using fusion PCR47, whereby 20 bp overlapping extensions
were used to stitch PCR products together. Individual PCR products were first amplified separately, purified and used as template for the subsequent fusion PCR reaction at ∼0.1–1 ng per 50
μl PCR reaction. Final PCR products were also purified and injected into the gonads of young adult hermaphrodites (HT1593 [_unc-119(ed3)_ III]) to generate extrachromosomal arrays, whereby a
PCR product coding for the _unc-119_ gene was used as a co-injection marker. Primer sequences can be found in Supplementary Table 2. Because we observed that the inherent expression
variation that is associated with extrachromosomal arrays can be seen over different animals within each injected line and that different lines show the same type of variation, 3–5
hermaphrodites were injected for each construct and the resulting transgenic lines were pooled. Expression analysis was performed on at least 10 animals per construct and representative
images are shown. For brood size and germline apoptosis analysis, respectively at least 6 or 10 animals were analysed per transgenic line, per condition and per biological replicate. The
translational reporters contain the target gene and ∼3 kb upstream and 0.5 kb downstream of the target gene, to include endogenous promoter and 3′UTR elements. For the different constructs,
the exact size of these regions can be found in the Supplementary Figures, in the graphics describing these constructs. The _gfp_ or _mCherry_ gene was fused at the 3′ side of the target
gene, and was preceded by the sequence 5′ggagctggtgcaggcgctggagccggtgcc 3′ coding for a GA-linker region. Translational reporter constructs were injected at a concentration of 50 ng μl−1,
together with the _unc-119_ gene at a concentration of 20 ng μl−1. Transformed reporter lines were analysed using a Nikon Eclipse TE2000-5 confocal microscope. An RNAi-resistent _glb-12_
gene (_glb-12__RR_) was generated using the guidelines described by Green _et al._48 In short, the nucleotide sequence was recoded by shuffling alternative codons encoding the same amino
acid, without changing the original amino acid sequence or the overall codon bias, until there were no long (>9 bp) stretches of homology between the original and recoded _glb-12_ gene.
The five _glb-12_ introns were replaced by the three synthetic introns that are also found in eGFP. The _glb-12__RR_ gene was fused at the 3′ side to the coding sequence for the
self-cleaving peptide T2A49, 5′gagggcagaggaagtctgctaacatgcggtgacgtcgaggagaatcctggccca 3′, followed by the _gfp_ gene. When the ribosome encounters the T2A sequence, a ‘ribosomal-skip’ or
‘STOP&GO’ occurs, releasing the GLB-12 polypeptide while translation of the messenger RNA for GFP continues. This results in the simultaneous expression of GLB-12 and GFP, even though
the two proteins are not fused together. The entire construct was fused to the same up- and downstream regions used for the _glb-12_ translational reporter. The exact size of these different
elements for this construct is shown schematically and can be found in the Supplementary Figures. The _glb-12__RR_ genes coding for the mutants K88L, R95L and R129L were generated by
site-directed mutagenesis. The cytosolic _glb-12__RR_ gene was generated by removing the region coding for the first 30 AA of GLB-12, which is the N-terminal membrane-targeting region. All
_glb-12__RR_ genes were validated by sequencing. The constructs coding for the intracellular and extracellular targeted PRDX-2 were generated by combining the _prdx-2_ gene with the
regulatory and coding regions of the _glb-12_ gene and the _sod-4b_ gene, respectively. These construct artificially target PRDX-2 to the intracellular location of GLB-12 or extracellular
location of SOD-4B, respectively. These constructs are schematically described in the Supplementary Figures. These schemes also show the exact size of the different elements. The gene coding
for roGFP2-ORP1 was codon optimized with the web tool, _C. elegans_ codon adapter50, to a codon adaptation index of 0.6 and with the introduction of three artificial introns. The resulting
gene was fused to the upstream and downstream region of the _glb-12_ gene, together with the sequence coding for the first 30 AA of GLB-12. This construct targets the roGFP2-ORP1 probe to
the intracellular location of GLB-12. Also this construct is schematically represented in the Supplementary Figures. We observed that the presence of the four different _glb-12_ introns, in
combination with the _glb-12_ upstream and downstream region, were essential for somatic gonad expression. These introns were separately amplified as short PCR fragments with the following
oligo’s: 5′gtgagttttgagcttgattc 3′ and 5′ccgacttgctggaaaataat 3′ for intron 1, 5′gagttcatggagcaggttag 3′ and 5′tctggttctgattttgttcca 3′ for intron 2 and 3, and 5′tgagattgtgggatcagt 3′ and
5′aaatcatatttgttgggtga 3′ for intron 4. The resulting PCR products were co-injected with all constructs that carry the _glb-12_ upstream and downstream region but lack these intronic
regions: all _glb-12__RR_ constructs, the intracellular _prdx-2_ construct and the _roGFP2-orp1_ construct. The _glb-12_ introns were co-injected at 2–6 ng μl−1. The _glb-12__RR_ and
_prdx-2_ constructs were injected at 25 ng μl−1, together with 20 ng μl−1 for the _unc-119_ gene. A control strain was generated by only injecting 20 ng μl−1 for the _unc-119_ gene. The
_roGFP2-orp1_ construct was injected at 50 ng per μl, together with 20 ng μl−1 for the _unc-119_ gene. To create the transgenic strain overexpressing _glb-12_ together with _roGFP2-orp1_, a
PCR product of the _glb-12_ gene, including the 2,120 bp upstream and 473 bp downstream region of the _glb-12_ gene, was co-injected at 10 ng μl−1. The transgenic strain carrying only the
_roGFP2-orp1_ construct was further crossed in the strains GA187 [_sod-1(tm776)_ II] and GA416 [_sod-4(gk101)_ III]. To create the transgenic strain overexpressing _glb-12_, a PCR product of
the _glb-12_ gene, including the 2,120 bp upstream and 473 bp downstream region of the _glb-12_ gene, was injected at 10 ng μl−1, together with 20 ng μl−1 for the _unc-119_ gene in the
_unc-119_ mutant. For the control strain, we only injected the _unc-119_ rescue gene in the _unc-119_ mutant. To overexpress GLB-12 in a _sod-1_ and _sod-4_ mutant background, we injected
the endogenous _glb-12_ gene (10 ng μl−1) together with a co-injection marker, _Pmyo-2::mCherry::unc-54UTR_ (2.5 ng μl−1), in both these mutants. The control strains were generated by only
injecting the _Pmyo-2::mCherry_ marker. To assess if GLB-12 is expressed in the germline, the GLB-12 translational reporter described above, including the 2,120 bp upstream and 473 bp
downstream region of the _glb-12_ gene was cloned in the pCFJ150 vector by Gibson cloning and injected into strain EG6699[ttTi5605 II; _unc-119(ed3)_ III; oxEx1578] to create integrated
single-copy transgenes using the MosSCI method51. This GLB-12 reporter thus carries the endogenous promoter, intronic and 3′UTR regions, which should mimic endogenous expression as closely
as possible. Furthermore, the MosSCI integration site ttTi5605 in the strain EG6699 is known to allow stable germline expression of integrated constructs carrying regulatory regions that
drive germline expression51. Besides a much weaker expression, the integrated reporter showed a comparable expression pattern compared with the extrachromosomal GLB-12 translational
reporter. No expression was seen within the germline. For localization in human neuroblastoma SH-SY5Y cells, cDNAs of wt _glb-12_, _glb-12_Δmyr, _glb-12_Δpalm, and _glb-12_ΔmyrΔpalm were
cloned into the pEGFP-N1 vector (Clontech) using BglII and HindIII restriction enzymes (Biolabs, Westburg). Ligation of cDNAs in the vectors was performed using T4 DNA ligase (Novagen). The
myristoylation site, glycine at position 2, was modified to alanine and is annotated as GLB-12Δmyr. The palmitoylation site, cysteine at position 6, was mutated to alanine and is annotated
as GLB-12Δpalm. The mutant where both fatty acylation sites are mutated to alanine is annotated as GLB-12ΔmyrΔpalm. These same mutations were also introduced in the GLB-12 translational
reporter for _C. elegans_, which has been described above. All mutations were done with the Quickchange site directed mutagenesis kit (Stratagene) and validated by sequencing. Human
neuroblastoma SH-SY5Y cells (ATCC CRL-2266) were cultured as recommended by the manufacturer’s protocol. Cells were transfected with 3 μl of lipofectamine 2,000 and 0.5 μg of pEGFP-N1
plasmide. After 4 h, transfection medium was replaced by growth medium and SH-SY5Y-cells were allowed to express the GFP-tagged proteins for 24 h. For GLB-12Δpalm, colocalization was
performed with the MitoTracker Deep Red probe (Invitrogen) according to the manufacturer’s protocol. Localization was examined with an UltraVIEW Vox ERS microscope (Perkin-Elmer), and images
were created with the Volocity 6.0.1 software. FEEDING RNAI RNAi was applied by feeding bacteria expressing dsRNA to the worms. The RNAi clone targeting _glb-12_ was derived from the
Ahringer RNAi library13. An _in silico_ analysis of the specificity of _glb-12_ RNAi with the online tool E-RNAi52 presented no aspecific targets. RNAi induction was performed as described
by Timmons _et al._53: an overnight culture of RNAi bacteria was inoculated in LB medium with 100 μg ml−1 ampicilline and 12.5 μg ml−1 tetracycline and incubated at 37 °C with shaking. The
following day, cultures were diluted 1:100 and grown to OD595=0.4 at 37 °C with shaking, after which sterile IPTG was added to a final concentration of 0.4 mM. Cultures were again incubated
at 37 °C with shaking for 4 h, and finally spiked with another 100 μg ml−1 ampicilline and 12.5 μg ml−1 tetracycline. IPTG was added to a final concentration of 0.8 mM. NGM plates with 100
μg ml−1 ampicilline, 12.5 μg ml−1 tetracycline and 0.4 mM IPTG were seeded with the bacterial cultures, air dried and left overnight at room temperature. Finally, synchronized L1 worms were
placed on these NGM plates seeded with the freshly induced RNAi bacteria. FECUNDITY ASSAY Two days after synchronized L1 worms were placed on plates containing RNAi bacteria, individual L4
hermaphrodite were transferred to NGM plates with a 25 μl spot of four times concentrated, induced RNAi bacteria and maintained at 20 °C, except for the temperature-sensitive strain _rrf-3
(pk1426)_, which was kept at 17 °C. Animals were transferred to fresh plates each day until they stopped laying eggs. Twenty-four hours after adults were transferred, hatched larvae and
unhatched eggs on each plate were counted. The fecundity of each worm was calculated as the total number of hatched and unhatched eggs produced. At least 18 worms evenly spread over at least
three independent replicas were analysed for each strain and for each condition. Afterwards, fecundity percentages of _glb-12_ RNAi compared with control RNAi were calculated for each
biological replicate within each genotype, after which statistical analysis was performed on fecundity percentages between genotypes. GERMLINE APOPTOSIS ASSAY For all germline apoptosis
assays, one-day-old adult animals were used. Animals with clearly underdeveloped or abnormal looking gonads were not included in this analysis. Apoptotic cells observed in the late pachytene
region of the germline were scored. For all germline apoptotis assays, at least 10 worms per biological replicate were scored, one gonadal arm per animal was used, and at least three
biological replicates were carried out for each condition. Apoptotic germ cells were visualized by acridin orange (AO) staining, unless otherwise noted. AO (1 mg ml−1) was added to a 9-cm
diameter NGM plate carrying first-day adult animals for overnight incubation. The following day, worms were rinsed from the plate, washed, mounted under coverslips in 20 μl of a 12.5-mM
sodium azide solution, and immediately analysed. The strain MT1522 [_ced-3(n717)_ IV] was used to validate the specific staining of apoptotic cells by AO. Differential interference contrast
microscopy instead of acridin orange staining was used to identify apoptotic germ cells in engulfment-defective mutants, as well as in WT animals that were used to analyse the removal speed
of apoptotic corpses. Finally, for the latter experiment, animals were anaesthetized for 10 min with 10 mM levamisole instead of sodium azide. After this, animals were mounted on agar pads
for analysis and time-lapse contrast microscopy was performed with through-focus z-stack images every 4 min, for a total of 75 min. ASCORBIC ACID AND N-ACETYLCYSTEINE TREATMENT Ascorbic acid
and N-acetylcysteine were added into NGM media from a freshly made 100 mM stock solution the day before the plates were used. Worms were placed on plates containing these antioxidants
starting from the fourth larval stage on. _IN VITRO_ SUPEROXIDE MEASUREMENTS To test the capacity of haem proteins to produce superoxide _in vitro_, we applied the method of Hayashi _et
al._16 to create a reducing environment for haem proteins, and included lucigenin that produces light upon reaction with superoxide, to quantify superoxide production. In brief, the
components of the reduction system are an NADPH-generating system, consisting of glucose-6-phosphate, glucose-6-phosphate dehydrogenase and NADP+ and an electron-mediating system, consisting
of ferredoxin and ferredoxin-NADP reductase. We omitted catalase from the original system, as this might lead to quick removal of the produced superoxide. Concentrations of the different
components, including the different haem proteins tested here, were as described in the original paper. haem proteins tested were myoglobin from horse heart (Sigma M1882), cytochrome P450
2B4 from rabbit liver (Sigma C7552) and cytochrome C from horse heart (Sigma C2506). Lucigenin was added to a final concentration of 100 μM. Measurements were performed in a 200-μl working
volume in a 96-well microtiter plate. Glucose-6-phosphate was added last to start the reduction. Immediately following this, light emission was recorded in a Victor2 1420 Multilabel Counter
(Perkin-Elmer, Wellesley, MA), whereby for each condition the average of 20 consecutive measurements was taken. Runs were repeated three times to allow statistical analysis. _IN VIVO_
HYDROGEN PEROXIDE MEASUREMENTS A GFP-based probe specific for hydrogen peroxide, roGFP2-ORP1 (ref. 28), was used to measure relative levels of _in vivo_ hydrogen peroxide levels at the
subcellular location of GLB-12 and this specifically within the somatic gonad. To this end, a transgenic _C. elegans_ line was generated that expressed roGFP2-ORP1 under the control of the
_glb-12_ regulatory regions, as described above. To increase roGFP2-ORP1 expression levels, animals were cultivated at 22 °C instead of 20 °C. Hydrogen peroxide levels were measured in
first-day adult hermaphrodites. Animals were removed from the plate, anaesthetised in a fresh mixture of 10 mM levamisol dissolved in M9 for 15–30 min before transferring them to an agarose
pad under a coverslip for imaging. To measure _in vivo_ hydrogen peroxide levels following exposure to 20 mM hydrogen peroxide, a freshly made hydrogen peroxide solution was added to the
animals already placed on the agarose pad. Imaging started immediately following this and was completed within 10 min. Animals were imaged using an inverted Zeiss LSM510 confocal microscope
equipped with × 40 magnification objective lens (oil immersion) and Zeiss Zen 2009 software. Laser intensities and microscope settings that were compatible with ratiometric measurements were
determined in an initial run and were kept constant for all subsequent experiments. Excitation of roGFP2-ORP1 was performed sequentially by 405 and 488 nm laser lines, emission was detected
at 500–550 nm. Only those animals that showed clearly visible expression of roGFP2-ORP1 in the somatic gonad were used for image analysis. One or two single focal plane images were taken
for each animal. At least five worms per biological replicate and per condition were scored, one gonadal arm per animal was used, and at least 3 biological replicates were carried out for
each condition. Image processing was based on the method used by Morgan _et al._54 In brief, images were saved as 16-bit tiff files and further processed by ImageJ. Images were first
converted to 32-bit tiff files, the background of both 405 nm images and 488 nm images was subtracted using an upper and lower threshold and background values were set to ‘not a number’
(NaN). Relative H2O2 levels in these processed images were quantified by (1) selecting a polygonal region, which showed the probe within the somatic gonad and which showed no interfering
background fluorescence from the gut, (2) calculating the average pixel intensities within this region for both the 405 nm and the 488 nm image and (3) calculating the ratio of the 405 nm
average pixel intensity over the 488 nm average pixel intensity. RECOMBINANT EXPRESSION OF GLB-12 To express recombinant GLB-12, young adult worms were collected and total RNA was prepared
using the TriZol method (Invitrogen) followed by LiCl precipitation (Ambion). cDNA was prepared using the OneStep RT-PCR kit (Qiagen). Cycling conditions were as followed: 30 min at 50 °C
for the RT reaction, followed by 15 min at 95 °C for the activation of the HotStar Taq DNA polymerase, followed by 35 cycles of 60 sec at 94 °C, 60 s at 54 °C and 90 s at 72 °C. cDNA of
_glb-12_ was cloned into the pET23a-vector (Novagen) using NdeI and XhoI restriction enzymes (Biolabs) and T4 Ligase (Novagen). The expression plasmid was then transformed into _Escherichia
coli_ strain BL21(DE3)pLysS (Invitrogen). Cells were grown at 25 °C in TB medium containing 200 μg ml−1 ampicillin, 30 μg ml−1 chloramphenicol and 1 mM δ-amino-levulinic acid. The culture
was induced at A550=0.8 OD with IPTG (final concentration 0.04 mM). After overnight growth, _E. coli_ cells were collected. Recombinant GLB-12 was spectroscopically localized in the
cytosolic fraction and was purified to homogeneity using (i) ammonium sulfate precipitation, after which the 90% pellet was dissolved and dialyzed against 5 mM Tris-HCl pH 8.5, (ii)
DEAE-Sepharose fast flow chromatography and (iii) Sephacryl S200 gel filtration in 50 mM Tris-HCl pH 8.5, 150 mM NaCl, 0.5 mM EDTA. The fractions containing the pure globin were pooled and
concentrated. UV–VIS UV–vis spectra were measured in a 250–700 nm range on a Cary-5 UV–vis–NIR spectrophotometer (Varian). Ferrous CO-bound and reduced deoxy protein samples were prepared by
flushing 1 ml of 100 mM potassium phosphate buffer pH 7.0 for 15 min with CO- and N2-gas, respectively, in a sealed cuvette. After addition of 10 μl of a satured solution of sodium
dithionite, highly concentrated, recombinant purified protein was added to the flushed sealed cuvette to obtain a final concentration of 50 μM. Ferric CN-ligated GLB-12 was prepared by
adding an excess of 40 mM of KCN. CYCLIC VOLTAMMETRY Cyclic Voltammetry was performed using electrodes modified with a gelatin layer. The presence of this gelatin layer allows the
incorporation of proteins and so the analysis of their electrochemical behaviour. Measurements were performed in a three electrode cell using a SCE containing two compartments (Radiometer
Analytical, France) and a platinum counter electrode. The working electrodes were gold electrodes with a diameter of 1.6 mm (BAS, UK) which were pretreated by mechanical and electrochemical
polishing according to the following procedure. Before its first use the electrode surface was briefly scoured by a silicon carbide emery paper of 1,200 grit to obtain a fresh surface. To
smoothen the resulting relatively rough surface it was further subjected to sequential polishing by polishing cloth covered with alumina powder of 1, 0.3 and 0.05 mm particle size (Buehler,
USA) for, respectively, 5, 10 and 20 min. To remove any adherent Al2O3 particles the electrode surface was rinsed thoroughly with doubly deionised water and cleaned in an ultrasonic bath
containing deionised water (Branson 3210, USA) for 2 min. Before immobilizing gelatin onto the electrode, the gold surface was modified with a self-assembled monolayer (SAM) of
6-mercaptohexanol (unless otherwise indicated). The latter was done by immersing the electrode in a water solution containing 1 mmol L−1 6-mercaptohexanol (MH) for 18 h at room temperature.
The modified gold electrodes were consequently rinsed with water to remove any physically adsorbed mercaptohexanol. To immobilize gelatin onto the electrode, gelatin B (gelB) powder (5 m%)
was dissolved in 10 mM HEPES buffer solution at 40 °C and further mixed with globin (GLB) and/or cytochrome C peroxidase (CCP) solutions (50 μM). An amount of 7 μl of this gelatin solution
was brought onto the surface of the electrode by using a syringe. The electrode was then exposed to air for 2 h at 4 °C (drop drying). The final electrodes are denoted as GLB|CCP|GelB
(1.5:1.5:7 ratio), GLB|GelB (3:7 ratio) or CCP|GelB (3:7 ratio). Additional optimization of this analysis showed that highly comparable results can be obtained when proteins are dissolved in
a small sample volume instead of a gelatin layer. Cyclic voltammetry for dissolved protein samples was conducted in an electrochemical cell designed by Hagen55. A droplet of the protein
solutions (10–50 μM) was placed in the cell equipped with a Pt-counter and a SCE electrodes. Gold disk electrodes (diameter 1.6 mm, BASi, USA) modified with 6-mercaptohexanol (Sigma-Aldrich)
were used as working electrodes. A gelatin layer was used to determine the redox potential of GLB-12, the effect of varying pH on this, and the interaction of GLB-12 with CCP. Except for
the pH depence, these measurements were repeated using dissolved protein samples and showed highly comparable results. All other measurements were performed on dissolved protein samples. All
measurements were carried out under nitrogen atmosphere at room temperature (22±2 °C). REAL-TIME QUANTITATIVE RT-PCR Total RNA was isolated from harvested _C. elegans_ worms using the
RNeasy Midi Kit from Qiagen (Hilden, Germany), according to the manufacturer's instructions. A DNase I (Zymo Research, Orange, California) digestion step was subsequently performed to
remove genomic DNA. First strand cDNA was synthesized using an oligo(dT) primer and a Moloney murine leukemia virus reverse transcriptase (Fermentas, Vilnius, Lithuania). Quantitative RT-PCR
was carried out using a Rotor-Gene 2000 centrifugal real-time cycler (Corbett Research, Mortlake, Australia) using the Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen). Each reaction
contained: 12.5 μl of the Platinum SYBR Green qPCR SuperMix-UDG, 200 nM of forward and reverse primers and 5 μl cDNA (1:40 RNA dilution), to a final volume of 25 μl. Amplification was
performed in 0.1 ml real-time PCR tubes (Corbett Research) placed in the 72-well rotor of the Rotor-Gene instrument. The cycling conditions were as follows: 50 °C for 2 min, initial
denaturation at 95 °C for 2 min, followed by 45 cycles of 15 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C (gain set at 8 for SYBR Green). Following the final cycle, melting curve analysis was
performed to examine the PCR specificity in each reaction tube (absence of primer dimers and other nonspecific products). All PCR reactions were performed in triplicate. _Ct_ values were
transformed to relative quantities and were normalized using the geometric mean of three reference genes56. Four biological replicates were carried out for each experiment. Primer sequences
can be found in Supplementary Table 2. CRYSTALLIZATION AND STRUCTURE DETERMINATION Atomic coordinates and structure factors determined from the GLB-12 crystal have been deposited in the
Protein Data Bank, with entry code 4bja. Crystals of GLB-12 were grown at 4 °C by the hanging-drop vapour diffusion method, by mixing 1 μl protein solution with 1 μl crystallization well
solution containing 2 M (NH4)2SO4, 0.2 M Na-acetate, 0.1 M Na-acetate pH 4.6. Crystals usually grew in about two months, and were cryo-protected with the same crystallization well solution
supplemented with 30% glycerol, prior to cryo-cooling in liquid nitrogen. The crystals belong to the space group _P_6522, with unit cell parameters _a_=50.4 Å, _b_=50.4 Å, _c_=245.3 Å,
γ=120°, and one GLB-12 molecule per asymmetric unit. Diffraction data were collected to 1.65 Å resolution using synchrotron radiation (BM14 beamline, ESRF, Grenoble, France). Raw data were
processed with Mosflm57 and Scala58 (Table 1). The structure was solved by single-wavelength anomalous dispersion (SAD) method, based on the haem-Fe atom anomalous signal. The haem-Fe atom
position and the initial phases were calculated by using the AutoSol pipeline implemented in PHENIX59. The GLB-12 model was built with the program COOT60 and restrained refined to the
maximum resolution using REFMAC61. At the end of the refinement stages (including anisotropic B-factor refinement), 110 water molecules, 1 sulfate ion, and 4 acetate molecules were located
through inspection of difference Fourier maps. The final Rfactor value was 17.8%, and Rfree 23.4%. No electron density was detected for residues 1–18, 167–181 and 216–266. The programs
Procheck62 and Surfnet63 were used to assess the stereochemical quality of the protein structures and to explore the protein matrix cavities. TEM—HIGH-PRESSURE FREEZING AND
FREEZE-SUBSTITUTION To analyse the subcellular localization of GLB-12, the GLB-12 translational reporter in combination with anti-GFP antibodies was used. N2 WT worms were included as
negative control. Copper membrane carriers (1.5 mm × 0.2 mm) (Leica, Microsystems, Vienna, Austria) treated with 1% lecithin were used and filled with 20% (w/v) Bovine Serum Albumin (BSA).
Three to four one-day-old adult nematodes were transferred to the membrane carrier, immersed in the BSA and then immediately frozen in a high-pressure freezer (EM PACT, Leica, Microsystems).
Freeze substitution was carried out using a Leica EM AFS (Leica, Microsystems). Carriers were transferred from EM pact to AFS under liquid nitrogen and placed in an eppendorf filled with
dry acetone. Over a period of 5 days, animals were freeze-substituted as follows: −90 °C for 27 h, 2 °C per hour increase for 15 h, −60 °C for 12 h, 2 °C per hour increase for 15 h, −30 °C
for 32 h, 2 °C per hour increase for 17 h. At 4 °C carriers were rinsed 3 times with dry acetone for 20 min each time. TEM—EMBEDDING AND SECTIONING Samples were infiltrated stepwise in
LR-White, hard grade (London, Resin, Basingstoke, UK) and embedded in closed capsules. Polymerization was performed by UV illumination of the AFS for 24 h at 0 °C, 2 °C per hour increase for
10 h followed by 24 h at 20 °C, 2 °C per hour increase for 8 h followed by 72 h at 37 °C. Ultrathin (70 nm) sections were cut using a Leica Ultracut S ultramicrotome (Leica, Vienna,
Austria) with a diamond knife (Diatome, Ltd., Biel, Switzerland) and collected on formvar-coated copper single slot grids (Agar Scientific, Stansed, UK). TEM—IMMUNOGOLD LABELLING All steps
of the immunolabelling were performed in a humid chamber at room temperature. Grids were floated upside down on 25 μl of aliquots of blocking solution (5% BSA, 1% Fish skin gelatin in
Phosphate buffered saline (PBS)) for 30 min followed by a wash step for five times 5 min in incubation buffer (IB: 1% BSA in PBS). Incubation of primary antibodies for 120 min, Goat
anti-GFP-biotin antibody, 1:300 (Rockland 600–106–215) followed by washing five times 5 min in IB. The grids were then incubated with unconjugated bridging antibodies, Rabbit anti biotin,
1:10,000 (Rockland 100–4,198) for 30 min. After washing five times 5 min in IB, the grids were incubated in Protein A gold (PAG) (10 nm, Cell Biology, Utrecht University) and washed twice, 5
min each, with IB; three times 5 min with PBS; and five times 2 min with double distilled water. Control experiments consisted of treating sections with bridging antibodies and/or PAG 10 nm
alone. After post-staining in a Leica EM AC20 (Leica, Microsystems) for 30 min in uranyl acetate at 20 °C and for 7 min in lead citrate at 20 °C sections were examined with a JEOL JEM 1010
(Jeol, Ltd, Tokyo, Japan) transmission electron microscope operating at 60 kV. Pictures were digitized using a Ditabis system (Pforzheim, Germany). GERM CELL QUANTIFICATION ASSAY Animals for
germ cell quantification assays were cultivated in an identical manner as for the germline apoptosis assay, only the staining with acridin orange was omitted. Briefly, one-day-old adult
animals were used to score number of germ cells, and animals with clearly underdeveloped or abnormal looking gonads were not included in this analysis. Germ cells were scored within a fixed
900 μm2 square region in the late pachytene region of the germline, whereby the entire width of the germline fell within this square. Within this square, the number of germ cells in the
upper half of the germline, starting from the widest point, were determined by differential interference contrast microscopy. For each genotype and condition, at least five worms per
biological replicate were scored, one gonadal arm per animal was used, and at least three biological replicates were carried out. WESTERN BLOTTING Western blotting was performed according to
standard procedures. Synchronized populations were grown on NGM with induced RNAi, collected as one-day-old adults, and flash frozen in 100 μl volumes. After worm homogenization, protein
content was determined using the bicinchoninic acid method. Equal amounts of total protein content were loaded on a 12.5% SDS–PAGE gel. The primary rabbit polyclonal antibodies anti-PMK-1
and anti-phospho-p38 MAPK monoclonal antibody 28B10 (Cell Signalling) were used at a 1:1,000 and 1:500 dilution, respectively, and detected with an HRP-conjugated secondary anti-rabbit
antibody (No. A0545; Sigma) (1:5,000). Incubation with Supersignal West Pico chemiluminescent substrate (Pierce) generated light that was recorded on chemiluminescence film. Densitometry was
performed using ImageJ software. Full scans of Western blots are presented in Supplementary Fig. 8. The specificity of anti-PMK-1 and anti-phospho-p38 MAPK was determined by using the PMK-1
mutant _KU25 [pmk-1(km25) IV]._ Three biological replicates were run. STATISTICAL ANALYSIS Statistical comparisons of fecundity and germline apoptosis levels were performed using a
two-sided _t_-test, assuming unequal variance, on three or more independent biological replicates. Statistical comparisons of _in vitro_ superoxide production was performed using a two-sided
_t_-test, assuming unequal variance, on three or more technical replicates. ADDITIONAL INFORMATION ACCESSION CODES: Crystal structure data have been deposited in the Protein Data Bank under
accession code 4bja. HOW TO CITE THIS ARTICLE: De Henau, S. _et al._ A redox signalling globin is essential for reproduction in _Caenorhabditis elegans_. _Nat. Commun._ 6:8782 doi:
10.1038/ncomms9782 (2015). ACCESSION CODES ACCESSIONS PROTEIN DATA BANK * 4bja REFERENCES * Finkel, T. Signal transduction by reactive oxygen species. _J. Cell. Biol._ 194, 7–15 (2011).
Article CAS Google Scholar * Ray, P. D., Huang, B. W. & Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. _Cell Signal._ 24, 981–990
(2012). Article CAS Google Scholar * D'Autréaux, B. & Toledano, M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. _Nat. Rev. Mol. Cell
Biol._ 8, 813–824 (2007). Article Google Scholar * Dickinson, B. C. & Chang, C. J. Chemistry and biology of reactive oxygen species in signaling or stress responses. _Nat. Chem. Biol._
7, 504–511 (2011). Article CAS Google Scholar * Reeder, B. J. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. _Antioxid. Redox Signal._ 13,
1087–1123 (2010). Article CAS Google Scholar * Duff, S. M., Wittenberg, J. B. & Hill, R. D. Expression, purification, and properties of recombinant barley (Hordeum sp.) hemoglobin.
Optical spectra and reactions with gaseous ligands. _J. Biol. Chem._ 272, 16746–16752 (1997). Article CAS Google Scholar * Kakar, S., Hoffman, F. G., Storz, J. F., Fabian, M. &
Hargrove, M. S. Structure and reactivity of hexacoordinate hemoglobins. _Biophys. Chem._ 152, 1–14 (2010). Article CAS Google Scholar * de Sanctis, D. et al. Structure-function
relationships in the growing hexa-coordinate hemoglobin sub-family. _IUBMB Life_ 56, 643–651 (2004). Article CAS Google Scholar * Yoon, J. et al. Structure and properties of a
bis-histidyl ligated globin from _Caenorhabditis elegans_. _Biochemistry_ 49, 5662–5670 (2010). Article CAS Google Scholar * Dewilde, S. et al. Biochemical characterization and ligand
binding properties of neuroglobin, a novel member of the globin family. _J Biol Chem_ 276, 38949–38955 (2001). Article CAS Google Scholar * Kiger, L. et al. Electron transfer function
versus oxygen delivery: a comparative study for several hexacoordinated globins across the animal kingdom. _PLoS ONE_ 6, e20478 (2011). Article CAS ADS Google Scholar * Hoogewijs, D. et
al. Wide diversity in structure and expression profiles among members of the _Caenorhabditis elegans_ globin protein family. _BMC Genomics_ 8, 356 (2007). Article Google Scholar * Kamath,
R. S. et al. Systematic functional analysis of the _Caenorhabditis elegans_ genome using RNAi. _Nature_ 421, 231–237 (2003). Article CAS ADS Google Scholar * De Wael, K. et al.
Electrochemical study of gelatin as a matrix for the immobilization of horse heart cytochrome c. _Talanta_ 82, 1980–1985 (2010). Article CAS Google Scholar * Petlicki, J. & van de
Ven, T. G. M. The equilibrium between the oxidation of hydrogen peroxide by oxygen and the dismutation of peroxyl or superoxide radicals in aqueous solutions in contact with oxygen. _J.
Chem. Soc. Faraday Trans._ 94, 2763–2767 (1999). Article Google Scholar * Hayashi, A., Suzuki, T. & Shin, M. An enzymic reduction system for metmyoglobin and methemoglobin, and its
application to functional studies of oxygen carriers. _Biochim. Biophys. Acta_ 310, 309–316 (1973). Article CAS Google Scholar * Couture, M., Burmester, T., Hankeln, T. & Rousseau, D.
L. The heme environment of mouse neuroglobin. Evidence for the presence of two conformations of the heme pocket. _J. Biol. Chem._ 276, 36377–36382 (2001). Article CAS Google Scholar *
Pesce, A. et al. Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity. _Structure_ 11, 1087–1095 (2003). Article CAS Google Scholar * Vallone, B.,
Nienhaus, K., Brunori, M. & Nienhaus, G. U. The structure of murine neuroglobin: novel pathways for ligand migration and binding. _Proteins_ 56, 85–92 (2004). Article CAS Google
Scholar * de Sanctis, D. et al. Crystal structure of cytoglobin: the fourth globin type discovered in man displays heme hexa-coordination. _J. Mol. Biol._ 336, 917–927 (2004). Article CAS
Google Scholar * Hargrove, M. S. et al. Crystal structure of a nonsymbiotic plant hemoglobin. _Structure_ 8, 1005–1014 (2000). Article CAS Google Scholar * Pesce, A. et al. HisE11 and
HisF8 provide bis-histidyl heme hexa-coordination in the globin domain of _Geobacter sulfurreducens_ globin-coupled sensor. _J. Mol. Biol._ 386, 246–260 (2009). Article CAS Google Scholar
* Bologna, G., Yvon, C., Duvaud, S. & Veuthey, A. L. N-terminal myristoylation predictions by ensembles of neural networks. _Proteomics_ 4, 1626–1632 (2004). Article CAS Google
Scholar * Ren, J. et al. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. _Protein Eng. Des. Sel._ 21, 639–644 (2008). Article CAS Google Scholar * Shahinian, S.
& Silvius, J. R. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. _Biochemistry_ 34, 3813–3822 (1995). Article CAS Google Scholar
* Ushio-Fukai, M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. _Antioxid. Redox Signal._ 11, 1289–1299 (2009). Article CAS Google Scholar * Veal, E. A.,
Day, A. M. & Morgan, B. A. Hydrogen peroxide sensing and signaling. _Mol. Cell_ 26, 1–14 (2007). Article CAS Google Scholar * Gutscher, M. et al. Proximity-based protein thiol
oxidation by H2O2-scavenging peroxidases. _J. Biol. Chem._ 284, 31532–31540 (2009). Article CAS Google Scholar * Madesh, M. et al. Selective role for superoxide in InsP3 receptor-mediated
mitochondrial dysfunction and endothelial apoptosis. _J. Cell. Biol._ 170, 1079–1090 (2005). Article CAS Google Scholar * Gartner, A., Boag, P. R. & Blackwell, T. K. _Germline
Survival and Apoptosis_ WormBook (2008). * Salinas, L. S., Maldonado, E. & Navarro, R. E. Stress-induced germ cell apoptosis by a p53 independent pathway in _Caenorhabditis elegans_.
_Cell Death Differ._ 13, 2129–2139 (2006). Article CAS Google Scholar * Mizuno, T. et al. The _Caenorhabditis elegans_ MAPK phosphatase VHP-1 mediates a novel JNK-like signaling pathway
in stress response. _EMBO J._ 23, 2226–2234 (2004). Article CAS Google Scholar * Brittain, T., Skommer, J., Raychaudhuri, S. & Birch, N. An antiapoptotic neuroprotective role for
neuroglobin. _Int. J. Mol. Sci._ 11, 2306–2321 (2010). Article CAS Google Scholar * Fago, A., Mathews, A. J., Moens, L., Dewilde, S. & Brittain, T. The reaction of neuroglobin with
potential redox protein partners cytochrome b5 and cytochrome c. _FEBS Lett._ 580, 4884–4888 (2006). Article CAS Google Scholar * Reeder, B. J., Svistunenko, D. A. & Wilson, M. T.
Lipid binding to cytoglobin leads to a change in haem co-ordination: a role for cytoglobin in lipid signalling of oxidative stress. _Biochem. J._ 434, 483–492 (2011). Article CAS Google
Scholar * Dordas, C. et al. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. _Plant J._ 35, 763–770 (2003). Article CAS
Google Scholar * Ohwaki, Y., Kawagishi-Kobayashi, M., Wakasa, K., Fujihara, S. & Yoneyama, T. Induction of class-1 non-symbiotic hemoglobin genes by nitrate, nitrite and nitric oxide in
cultured rice cells. _Plant Cell Physiol._ 46, 324–331 (2005). Article CAS Google Scholar * Chaiswing, L. & Oberley, T. D. Extracellular/microenvironmental redox state. _Antioxid.
Redox Signal._ 13, 449–465 (2010). Article CAS Google Scholar * Fisher, A. B. Redox signaling across cell membranes. _Antioxid. Redox Signal._ 11, 1349–1356 (2009). Article CAS Google
Scholar * Bienert, G. P. et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. _J. Biol. Chem._ 282, 1183–1192 (2007). Article CAS Google Scholar *
Dynowski, M., Schaaf, G., Loque, D., Moran, O. & Ludewig, U. Plant plasma membrane water channels conduct the signalling molecule H2O2 . _Biochem. J._ 414, 53–61 (2008). Article CAS
Google Scholar * Hara-Chikuma, M. et al. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. _Nat. Commun._ 6,
7454 (2015). Article CAS Google Scholar * Fukai, T. & Ushio-Fukai, M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. _Antioxid. Redox Signal._ 15,
1583–1606 (2011). Article CAS Google Scholar * McCubrey, J. A., Lahair, M. M. & Franklin, R. A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways.
_Antioxid. Redox Signal._ 8, 1775–1789 (2006). Article CAS Google Scholar * Wada, T. & Penninger, J. M. Mitogen-activated protein kinases in apoptosis regulation. _Oncogene_ 23,
2838–2849 (2004). Article CAS Google Scholar * Gumienny, T. L., Lambie, E., Hartwieg, E., Horvitz, H. R. & Hengartner, M. O. Genetic control of programmed cell death in the
_Caenorhabditis elegans_ hermaphrodite germline. _Development_ 126, 1011–1022 (1999). CAS PubMed Google Scholar * Hobert, O. PCR fusion-based approach to create reporter gene constructs
for expression analysis in transgenic _C. elegans_. _Biotechniques_ 32, 728–730 (2002). Article CAS Google Scholar * Green, R. A. et al. Expression and imaging of fluorescent proteins in
the _C. elegans_ gonad and early embryo. _Methods Cell Biol._ 85, 179–218 (2008). Article CAS Google Scholar * Ahier, A. & Jarriault, S. Simultaneous expression of multiple proteins
under a single promoter in _Caenorhabditis elegans_ via a versatile 2A-based toolkit. _Genetics_ 196, 605–613 (2014). Article CAS Google Scholar * Redemann, S. et al. Codon
adaptation-based control of protein expression in _C. elegans_. _Nat. Methods_ 8, 250–252 (2011). Article CAS Google Scholar * Frøkjaer-Jensen, C. et al. Single-copy insertion of
transgenes in _Caenorhabditis elegans_. _Nat. Genet._ 40, 1375–1383 (2008). Article Google Scholar * Horn, T. & Boutros, M. E-RNAi: a web application for the multi-species design of
RNAi reagents--2010 update. _Nucleic Acids Res._ 38, W332–W339 (2010). Article CAS Google Scholar * Timmons, L., Court, D. L. & Fire, A. Ingestion of bacterially expressed dsRNAs can
produce specific and potent genetic interference in _Caenorhabditis elegans_. _Gene_ 263, 103–112 (2001). Article CAS Google Scholar * Morgan, B., Sobotta, M. C. & Dick, T. P.
Measuring E(GSH) and H2O2 with roGFP2-based redox probes. _Free Radic. Biol. Med._ 51, 1943–1951 (2011). Article CAS Google Scholar * Hagen, W. R. Direct electron transfer of redox
proteins at the bare glassy carbon electrode. _Eur. J. Biochem._ 182, 523–530 (1989). Article CAS Google Scholar * Vandesompele, J. et al. Accurate normalization of real-time quantitative
RT-PCR data by geometric averaging of multiple internal control genes. _Genome Biol._ 3, RESEARCH0034 (2002). Article Google Scholar * Leslie, A. G.M. _MOSFLM User Guide, Mosflm Version
6.2.3_ MRC Laboratory of Molecular Biology: Cambridge, UK, (2003). * Evans, P. Scaling and assessment of data quality. _Acta Crystallogr. D Biol. Crystallogr._ 62, 72–82 (2006). Article
Google Scholar * Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. _Acta Crystallogr. D Biol. Crystallogr._ 66, 213–221 (2010). Article
CAS Google Scholar * Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. _Acta Crystallogr. D Biol. Crystallogr._ 60, 2126–2132 (2004). Article Google Scholar
* Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. _Acta Crystallogr. D Biol. Crystallogr._ 53, 240–255 (1997).
Article CAS Google Scholar * Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: a program to check the stereochemical quality of protein structure. _J. Appl.
Crystallography_ 26, 283–291 (1993). Article CAS Google Scholar * Laskowski, R. A. SURFNET: A program for visualizing molecular surfaces, cavities and intermolecular interactions. _J.
Mol. Graph._ 13, 323–330 (1995). Article CAS Google Scholar * Battistuzzi, G. et al. Redox reactivity of the heme Fe3+/Fe 2+ couple in native myoglobins and mutants with peroxidase-like
activity. _J. Biol. Inorg. Chem._ 12, 951–958 (2007). Article CAS Google Scholar * Raphael, A. L. & Gray, H. B. Axial ligand replacement in horse heart cytochrome c by semisynthesis.
_Proteins_ 6, 338–340 (1989). Article CAS Google Scholar * Shumyantseva, V. V. et al. A new format of electrodes for the electrochemical reduction of cytochromes P450. _J. Inorg.
Biochem._ 100, 1353–1357 (2006). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr K. Matsumoto and Dr T. Mizuno for kindly providing the _mek-1(ks54)
sek-1(km4)_ double mutant, the antibody anti-PMK-1 and technical advice on antibody use; Dr D. Kim for kindly providing the pDK177 RNAi strain; Dr M. Ubbink and Dr Q. Bashir for providing
CCP; Dr K. Oegema and the OD lab for sharing technical expertise; M. Couvreur for assistance in generating transgenic lines; and Dr T. Dansen for the final support. Some strains were
provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). S.D.H. and F.G. are PhD fellows of the Fund for Scientific Research (FWO).
Financial support to S.D. and L.M. was provided by the University of Antwerp (BOF UA TOP 2006), to K.D.W., S.D. and S.T. by the University of Antwerp (BOF-GOA) and to S.D., L.M., B.P.B., by
FWO project G.0247.09. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biology, Ghent University, Ghent, B-9000, Belgium Sasha De Henau, Matthew Vangheel, Caroline Vlaeminck,
Jacques R. Vanfleteren, Wim Bert & Bart P. Braeckman * Department of Biomedical Sciences, University of Antwerp, Antwerp, B-2000, Belgium Lesley Tilleman, Evi Luyckx, Francesca Germani,
Luc Moens & Sylvia Dewilde * Department of Chemistry, University of Antwerp, Antwerp, B-2000, Belgium Stanislav Trashin, Martje Pauwels & Karolien De Wael * Department of Physics,
University of Genova, Genova, I-16146, Italy Alessandra Pesce * Department of Biosciences, University of Milano, Milano, I-20133, Italy Marco Nardini & Martino Bolognesi * CNR-IBF and
CIMAINA, University of Milano, Milano, I-20133, Italy Martino Bolognesi Authors * Sasha De Henau View author publications You can also search for this author inPubMed Google Scholar * Lesley
Tilleman View author publications You can also search for this author inPubMed Google Scholar * Matthew Vangheel View author publications You can also search for this author inPubMed Google
Scholar * Evi Luyckx View author publications You can also search for this author inPubMed Google Scholar * Stanislav Trashin View author publications You can also search for this author
inPubMed Google Scholar * Martje Pauwels View author publications You can also search for this author inPubMed Google Scholar * Francesca Germani View author publications You can also search
for this author inPubMed Google Scholar * Caroline Vlaeminck View author publications You can also search for this author inPubMed Google Scholar * Jacques R. Vanfleteren View author
publications You can also search for this author inPubMed Google Scholar * Wim Bert View author publications You can also search for this author inPubMed Google Scholar * Alessandra Pesce
View author publications You can also search for this author inPubMed Google Scholar * Marco Nardini View author publications You can also search for this author inPubMed Google Scholar *
Martino Bolognesi View author publications You can also search for this author inPubMed Google Scholar * Karolien De Wael View author publications You can also search for this author
inPubMed Google Scholar * Luc Moens View author publications You can also search for this author inPubMed Google Scholar * Sylvia Dewilde View author publications You can also search for
this author inPubMed Google Scholar * Bart P. Braeckman View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors contributed extensively
to the work presented in this paper. S.D.H., J.R.V. and B.P.B. designed the phenotypical, expression, genetic, molecular and part of the biochemical analysis. S.D.H., M.V. and C.V. carried
out the phenotypical, expression, molecular and genetic analysis. W.B. gave technical support for TEM. L.T., E.L., S.D. and L.M. gathered biochemical and expression data. S.T., M.P. and K.D.
carried out cyclic voltametry. A.P., F.G., M.N. and M.B. gathered the crystallization data. The manuscript was prepared by S.D.H. and all authors discussed the results and implications and
commented on the manuscript at all stages. CORRESPONDING AUTHORS Correspondence to Sasha De Henau or Bart P. Braeckman. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figures 1-8 and Supplementary Tables 1-2 (PDF 1929 kb) RIGHTS AND PERMISSIONS This work is
licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license,
unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce
the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE De Henau, S., Tilleman, L.,
Vangheel, M. _et al._ A redox signalling globin is essential for reproduction in _Caenorhabditis elegans_. _Nat Commun_ 6, 8782 (2015). https://doi.org/10.1038/ncomms9782 Download citation *
Received: 09 February 2015 * Accepted: 02 October 2015 * Published: 01 December 2015 * DOI: https://doi.org/10.1038/ncomms9782 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative