Play all audios:
ABSTRACT Afferent lymph–borne dendritic cells essentially rely on the chemokine receptor CCR7 for their transition from the subcapsular lymph node sinus into the parenchyma, a migratory step
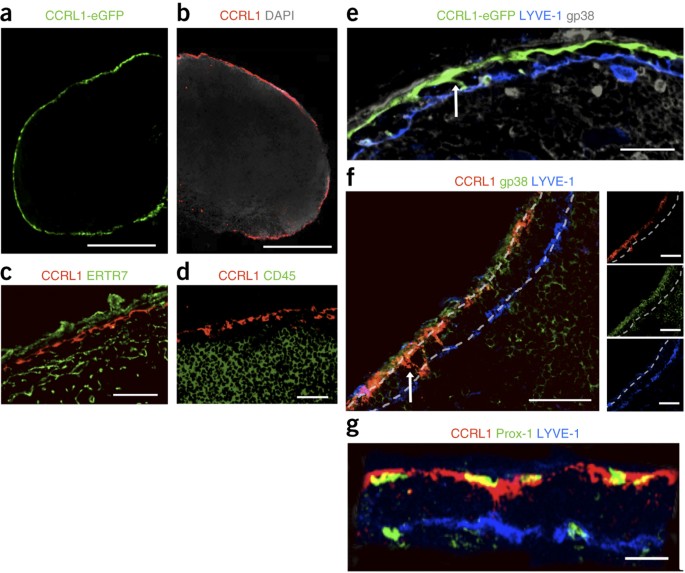
driven by putative gradients of CCR7 ligands. We found that lymph node fringes indeed contained physiological gradients of the chemokine CCL21, which depended on the expression of CCRL1,
the atypical receptor for the CCR7 ligands CCL19 and CCL21. Lymphatic endothelial cells lining the ceiling of the subcapsular sinus, but not those lining the floor, expressed CCRL1, which
scavenged chemokines from the sinus lumen. This created chemokine gradients across the sinus floor and enabled the emigration of dendritic cells. _In vitro_ live imaging revealed that
spatially confined expression of CCRL1 was necessary and sufficient for the creation of functional chemokine gradients. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only
$17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS INFLUENZA INDUCES LUNG
LYMPHANGIOGENESIS INDEPENDENT OF YAP/TAZ ACTIVITY IN LYMPHATIC ENDOTHELIAL CELLS Article Open access 12 September 2024 ATYPICAL CHEMOKINE RECEPTORS IN THE IMMUNE SYSTEM Article 07 May 2024 B
CELL ZONE RETICULAR CELL MICROENVIRONMENTS SHAPE CXCL13 GRADIENT FORMATION Article Open access 22 July 2020 REFERENCES * von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in
lymph nodes. _Nat. Rev. Immunol._ 3, 867–878 (2003). Article CAS PubMed Google Scholar * Shakhar, G. et al. Stable T cell–dendritic cell interactions precede the development of both
tolerance and immunity _in vivo_. _Nat. Immunol._ 6, 707–714 (2005). Article CAS PubMed PubMed Central Google Scholar * Lindquist, R.L. et al. Visualizing dendritic cell networks _in
vivo_. _Nat. Immunol._ 5, 1243–1250 (2004). Article CAS PubMed Google Scholar * Förster, R. et al. CCR7 coordinates the primary immune response by establishing functional
microenvironments in secondary lymphoid organs. _Cell_ 99, 23–33 (1999). Article PubMed Google Scholar * Schneider, M.A., Meingassner, J.G., Lipp, M., Moore, H.D. & Rot, A. CCR7 is
required for the _in vivo_ function of CD4+CD25+ regulatory T cells. _J. Exp. Med._ 204, 735–745 (2007). Article CAS PubMed PubMed Central Google Scholar * Ohl, L. et al. CCR7 governs
skin dendritic cell migration under inflammatory and steady-state conditions. _Immunity_ 21, 279–288 (2004). Article CAS PubMed Google Scholar * Vander Lugt, B. et al. CCR7 plays no
appreciable role in trafficking of central memory CD4 T cells to lymph nodes. _J. Immunol._ 191, 3119–3127 (2013). Article CAS PubMed Google Scholar * Förster, R., Davalos-Misslitz, A.C.
& Rot, A. CCR7 and its ligands: balancing immunity and tolerance. _Nat. Rev. Immunol._ 8, 362–371 (2008). Article CAS PubMed Google Scholar * Weber, M. et al. Interstitial dendritic
cell guidance by haptotactic chemokine gradients. _Science_ 339, 328–332 (2013). Article CAS PubMed Google Scholar * Braun, A. et al. Afferent lymph-derived T cells and DCs use
different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. _Nat. Immunol._ 12, 879–887 (2011). Article CAS PubMed Google Scholar *
Gosling, J. et al. Cutting edge: Identification of a novel chemokine receptor that binds dendritic cell- and T cell-active chemokines including ELC, SLC, and TECK. _J. Immunol._ 164,
2851–2856 (2000). Article CAS PubMed Google Scholar * Townson, J.R. & Nibbs, R.J. Characterization of mouse CCX–CKR, a receptor for the lymphocyte-attracting chemokines TECK/mCCL25,
SLC/mCCL21 and MIP-3β/mCCL19: comparison to human CCX–CKR. _Eur. J. Immunol._ 32, 1230–1241 (2002). Article CAS PubMed Google Scholar * Bachelerie, F. et al. International Union of
Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. _Pharmacol. Rev._ 66, 1–79 (2014). Article
CAS PubMed PubMed Central Google Scholar * Bachelerie, F. et al. New nomenclature for atypical chemokine receptors. _Nat. Immunol._ 15, 207–208 (2014). Article CAS PubMed Google
Scholar * Heinzel, K., Benz, C. & Bleul, C.C. A silent chemokine receptor regulates steady-state leukocyte homing _in vivo_. _Proc. Natl. Acad. Sci. USA_ 104, 8421–8426 (2007). Article
CAS PubMed PubMed Central Google Scholar * Mäkinen, T. et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. _Genes Dev._ 19, 397–410 (2005).
Article CAS PubMed PubMed Central Google Scholar * Comerford, I., Milasta, S., Morrow, V., Milligan, G. & Nibbs, R. The chemokine receptor CCX-CKR mediates effective scavenging of
CCL19 in vitro. _Eur. J. Immunol._ 36, 1904–1916 (2006). Article CAS PubMed Google Scholar * Comerford, I. et al. The atypical chemokine receptor CCX-CKR scavenges homeostatic chemokines
in circulation and tissues and suppresses Th17 responses. _Blood_ 116, 4130–4140 (2010). Article CAS PubMed Google Scholar * Wendland, M. et al. Lymph node T cell homeostasis relies on
steady state homing of dendritic cells. _Immunity_ 35, 945–957 (2011). Article CAS PubMed Google Scholar * Moussion, C. & Girard, J.P. Dendritic cells control lymphocyte entry to
lymph nodes through high endothelial venules. _Nature_ 479, 542–546 (2011). Article CAS PubMed Google Scholar * Stein, J.V. et al. The CC chemokine thymus-derived chemotactic agent 4
(TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high
endothelial venules. _J. Exp. Med._ 191, 61–76 (2000). Article CAS PubMed PubMed Central Google Scholar * Gunn, M.D. et al. Mice lacking expression of secondary lymphoid organ chemokine
have defects in lymphocyte homing and dendritic cell localization. _J. Exp. Med._ 189, 451–460 (1999). Article CAS PubMed PubMed Central Google Scholar * Vassileva, G. et al. The
reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. _J. Exp. Med._ 190, 1183–1188 (1999). Article CAS PubMed PubMed Central Google Scholar
* Rot, A. & von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. _Annu. Rev. Immunol._ 22, 891–928 (2004). Article CAS PubMed
Google Scholar * Sánchez-Madrid, F. & del Pozo, M.A. Leukocyte polarization in cell migration and immune interactions. _EMBO J._ 18, 501–511 (1999). Article PubMed PubMed Central
Google Scholar * Okada, T. et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. _PLoS Biol._ 10.1371/journal.pbio.0030150 (3
May 2005). * Castellino, F. et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4 T cell-dendritic cell interaction. _Nature_ 440, 890–895 (2006). Article CAS
PubMed Google Scholar * Middleton, J. et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. _Cell_ 91, 385–395 (1997). Article CAS PubMed Google Scholar *
Pruenster, M. et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. _Nat. Immunol._ 10, 101–108 (2009). Article CAS PubMed
Google Scholar * Haessler, U., Pisano, M., Wu, M.M. & Swartz, M.A. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and
CCL19. _Proc. Natl. Acad. Sci. USA_ 108, 5614–5619 (2011). Article PubMed PubMed Central Google Scholar * Schumann, K. et al. Immobilized chemokine fields and soluble chemokine gradients
cooperatively shape migration patterns of dendritic cells. _Immunity_ 32, 703–713 (2010). Article CAS PubMed Google Scholar * Chai, Q. et al. Maturation of lymph node fibroblastic
reticular cells from myofibroblastic precursors is critical for antiviral immunity. _Immunity_ 38, 1013–1024 (2013). Article CAS PubMed PubMed Central Google Scholar * Crick, F.
Diffusion in embryogenesis. _Nature_ 225, 420–422 (1970). Article CAS PubMed Google Scholar * Naumann, U. et al. CXCR7 functions as a scavenger for CXCL12 and CXCL11. _PLoS ONE_ 5, e9175
(2010). Article CAS PubMed PubMed Central Google Scholar * Boldajipour, B. et al. Control of chemokine-guided cell migration by ligand sequestration. _Cell_ 132, 463–473 (2008).
Article CAS PubMed Google Scholar * Venkiteswaran, G. et al. Generation and dynamics of an endogenous, self-generated signaling gradient across a migrating tissue. _Cell_ 155, 674–687
(2013). Article CAS PubMed Google Scholar * Donà, E. et al. Directional tissue migration through a self-generated chemokine gradient. _Nature_ 503, 285–289 (2013). Article CAS PubMed
Google Scholar * Ulvmar, M.H., Hub, E. & Rot, A. Atypical chemokine receptors. _Exp. Cell Res._ 317, 556–568 (2011). Article CAS PubMed PubMed Central Google Scholar * Nibbs,
R.J.B. & Graham, G.J. Immune regulation by atypical chemokine receptors. _Nat. Rev. Immunol._ 13, 815–829 (2013). Article CAS PubMed Google Scholar * Graham, G.J., Locati, M.,
Mantovani, A., Rot, A. & Thelen, M. The biochemistry and biology of the atypical chemokine receptors. _Immunol. Lett._ 145, 30–38 (2012). Article CAS PubMed Google Scholar * Colditz,
I.G., Schneider, M.A., Pruenster, M. & Rot, A. Chemokines at large: _In-vivo_ mechanisms of their transport, presentation and clearance. _Thromb. Haemost._ 97, 688–693 (2007). Article
CAS PubMed Google Scholar * Lee, K.M. et al. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. _Blood_ 118, 6220–6229 (2011). Article
CAS PubMed PubMed Central Google Scholar * Fra, A.M. et al. Cutting edge: scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. _J.
Immunol._ 170, 2279–2282 (2003). Article CAS PubMed Google Scholar * Nakano, H. et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune
responses. _Nat. Immunol._ 10, 394–402 (2009). Article CAS PubMed PubMed Central Google Scholar * Tanaka, Y., Mamalaki, C., Stockinger, B. & Kioussis, D. In-vitro negative selection
of αβ-T-cell receptor transgenic thymocytes by conditionally immortalized thymic cortical epithelial-cell lines and dendritic cells. _Eur. J. Immunol._ 23, 2614–2621 (1993). Article CAS
PubMed Google Scholar * Friedl, P. & Brocker, E.B. Reconstructing leukocyte migration in 3D extracellular matrix by time-lapse videomicroscopy and computer-assisted tracking. _Methods
Mol. Biol._ 239, 77–90 (2004). PubMed Google Scholar * Overwijk, W.W. et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T
cells. _J. Exp. Med._ 198, 569–580 (2003). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank J. Caamano for reading the manuscript and for
suggestions; C. Bleul (Novartis) for CCRL1-eGFP mice; M. Gunn (Duke University) for plt mice; and D. Kioussis (Medical Research Council, National Institute for Medical Research) for the TEP
cell line. Supported by the Medical Research Council (G0802838 to A.R. and G9818340 to the Medical Research Council Centre), The European Union Marie Curie Actions (CRITICS to M.H.U. and
A.R.), the Wellcome Trust (WT090962MA to I.N.-B. and A.R.), the European Research Council (322645 to R.F.), Deutsche Forschungsgemeinschaft (SFB738-B5 and EXC62, 'Rebirth', to
R.F.) and Boehringer Ingelheim Fonds (A.B.). AUTHOR INFORMATION Author notes * Maria H Ulvmar & Asolina Braun Present address: Present addresses: Department of Immunology, Genetics and
Pathology, Rudbeck Laboratory, Uppsala University, Uppsala, Sweden (M.H.U.), and Institute of Microbiology and Immunology, University of Melbourne, Australia (A.B.)., * Maria H Ulvmar and
Kathrin Werth: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * MRC Centre for Immune Regulation, School of Immunity and Infection, University of Birmingham,
Birmingham, UK Maria H Ulvmar, Poonam Kelay, Elin Hub, Kathrin Eller, Li Chan, Beth Lucas, Igor Novitzky-Basso, Kyoko Nakamura & Antal Rot * Institute of Immunology, Hannover Medical
School, Hannover, Germany Kathrin Werth, Asolina Braun, Tim Worbs & Reinhold Förster * Division of Nephrology, Medical University of Graz, Graz, Austria Kathrin Eller * Institute of
Laboratory Animal Science, University of Veterinary Medicine, Vienna, Austria Thomas Rülicke * Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow, UK Robert J
B Nibbs Authors * Maria H Ulvmar View author publications You can also search for this author inPubMed Google Scholar * Kathrin Werth View author publications You can also search for this
author inPubMed Google Scholar * Asolina Braun View author publications You can also search for this author inPubMed Google Scholar * Poonam Kelay View author publications You can also
search for this author inPubMed Google Scholar * Elin Hub View author publications You can also search for this author inPubMed Google Scholar * Kathrin Eller View author publications You
can also search for this author inPubMed Google Scholar * Li Chan View author publications You can also search for this author inPubMed Google Scholar * Beth Lucas View author publications
You can also search for this author inPubMed Google Scholar * Igor Novitzky-Basso View author publications You can also search for this author inPubMed Google Scholar * Kyoko Nakamura View
author publications You can also search for this author inPubMed Google Scholar * Thomas Rülicke View author publications You can also search for this author inPubMed Google Scholar * Robert
J B Nibbs View author publications You can also search for this author inPubMed Google Scholar * Tim Worbs View author publications You can also search for this author inPubMed Google
Scholar * Reinhold Förster View author publications You can also search for this author inPubMed Google Scholar * Antal Rot View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS M.H.U., K.W., A.B., E.H., K.E., T.W., R.F. and A.R. designed the experiments and evaluated the data; M.H.U., K.W., A.B., P.K., E.H., L.C. and I.N.-B.
did the experiments; T.W. wrote evaluation software; M.H.U., K.W., A.B., P.K., E.H., R.F. and A.R. prepared the figures; M.H.U., K.W., A.B. and E.H. contributed to the preparation of the
manuscript; B.L. and K.N. produced cell lines; R.J.B.N. provided a mouse strain; T.R. rederived mouse strains; and R.F. and A.R. conceived of the project, directed the research and wrote the
manuscript. CORRESPONDING AUTHORS Correspondence to Reinhold Förster or Antal Rot. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. INTEGRATED
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 SPECIFICITY CONTROLS FOR STAINING WITH ANTIBODY TO CCRL1. Acetone-fixed frozen sections of WT LN stained (A) with anti-CCRL1 antibody (red)
and (B) with control isotype IgG. Acetone-fixed cryo sections of WT LN (C) and CCRL1 ko LN (D) stained with anti-CCRL1 (red), anti-LYVE-1 (blue) and anti-gp38 (green) antibodies. Scale bars
50 μm. Representative of more than 3 studies. SUPPLEMENTARY FIGURE 2 CCRL1 EXPRESSION IN THE LNS IS CONFINED TO THE GP38HILYVE-1LO LEC SUBSET. Flow cytometry analysis of LECs in lymph nodes
from CCRL1-eGFP mice. (A-B) Stromal cells in the live cell gate (A), gated from the CD45neg, Ter119neg cells (B). (C) LECs are defined as gp38high CD31high cells, BECs as gp38low CD31high,
FRC as gp38high CD31neg, and DN stromal cells are defined as gp38neg CD31neg. (D) gp38high CD31high LECs analysed for expression of LYVE-1 and eGFP. (E-F) Analysis of the eGFPneg and eGFPpos
gated LEC subsets for expression of gp38 (E) and Lyve-1 (F). Geometric mean (MFI) and LYVE-1 positive cells in gate L1 are shown. eGFP positive cells in the gp38high CD31low, FRCs (G), the
gp38low CD31high, BECs, (H) the gp38low CD31low DN stromal cells (I) and CD45pos gated cells (J). Representative graphs from inguinal LN from 3 CCRL1-eGFP mice. SUPPLEMENTARY FIGURE 3
TEP-CCRL1 CELLS BIND AND SCAVENGE CCR7 LIGANDS. (A) CCRL1 expressed in TEP cells scavenges CCL19. TEP-CCRL1 or TEP-mock cells were incubated at 37°C with 10nM CCL19-Alexa647 for the time
indicated. Cells were washed with cold PBS 3% FBS and analyzed by flow cytometry revealing accumulation of CCL19 over time in TEP-CCRL1 cells but not TEP-mock cells (B,C). The expression of
CCRL1 in monolayers inhibits CCL19- (B) and CCL21-induced (C) _in vitro_ transmigration of BM-DCs. Monolayers of TEP-CCRL1 and empty vector-transfected control TEP-mock cells were grown to
confluence on the lower side of Transwell insert filters with 5μm pores. BM-DCs were added to triplicate inserts and allowed to migrate in response to 0.8nM murine recombinant CCL19 or CCL21
or RPMI in the lower well. After incubation at 37o C for 3 h, BM-DCs were collected from the bottom well, stained by an anti-CD11c Ab and analyzed by FACS using counting beads. Data shown
are mean and STDEV for triplicate wells from one representative experiment each from three and four performed using CCL19 and CCL21, respectively. Significance indicated: *: P < 0.05. In
all experiments migration indices (ratios of BM-DCs migrated to chemokine and to RPMI) were 1.6±0.2 and 3.5±0.3 for CCL19 and 1.7±0.9 and 6.6±4.2 for CCL21, across TEP-CCRL1 and TEP-mock
monolayers, respectively (median±STDEV; for both chemokines significant difference between TEP-CCRL1 and TEP-mock, p<0.05, Mann-Whitney, U-test). SUPPLEMENTARY FIGURE 4 LOWER PROPORTIONS
OF CCR7+CD86+ DCS IN SKIN-DRAINING LNS OF CCRL1-DEFICIENT MICE. (A) Gating strategy to identify “migratory” CD86pos CCR7pos DC in inguinal LNs of wild type (WT)and CCRL1 deficient (KO) mice.
After single cell gate and exclusion of dead cells, total CD45+ cells were analysed for CD11c and CD11b expression as displayed for representative wild type and CCRL1 deficient LNs.
Populations of CD11bhigh (blue gate) and CD11blow (green gate) DCs were further analysed for CCR7 and CD86 expression to identify CCR7+CD86+ “migratory” DCs. To identify LDCs total DCs (red
gate) were analysed for langerin against CCR7. This gate encompasses both epidermal-derived Langerhans cells and langerinpos dermal DCs. (B) Proportions of both CD11bhighCD11c+CD86highCCR7+
and CD11blowCD11c+CD86highCCR7+ DCs are significantly lower in CCRL1 deficient mice (KO, white box) compared to wild-type (WT grey box), Significance indicated: *, _P_ < 0.00. Data are
from four litters of 8 week old F2 female mice. n=9 per group. (C) Representative histogram of CCR7 expression in WT (blue) versus CCRL1 ko (red) gated on all DCs, representative of 3
independent experiments. (E) Geometric mean fluorescent intensity of CCR7 in gated CD86high cells from WT (grey box) and KO (white box). F2 littermates, n=4 and 5 for CCRL1 ko and WT mice,
respectively. Box-plot graphs show median values with quartiles and minimum/maximum. SUPPLEMENTARY FIGURE 5 CELL POPULATIONS IN LNS OF WILD-TYPE AND CCRL1-DEFICIENT MICE. (A) LN cellularity
in WT (grey box) and CCRL1 ko mice (white box) determined by FACS using counting beads after LN digestion. (B-E) Absolute cell numbers (left) and relative proportions (right) of main immune
cell populations. (B) T cells (CD3+), (C) B cells (B220+CD19+) (D) Myeloid cells (CD11c-CD11b+) and (E) DC (CD11c+). Data are from F2 generation 8 week old females; n=6-7 in A-C and n=5 in
D-E across three and two litters, respectively. Significance indicated: *, _P_ < 0.05, **, _P_ < 0.01. Box-plot graphs show median values with quartiles and minimum/maximum.
SUPPLEMENTARY FIGURE 6 DC-BORNE ANTIGEN–INDUCED T CELL PROLIFERATION IN WILD-TYPE AND CCRL1-DEFICIENT LNS (A-D) Representative proliferation curves of CellTrace violet labelled pmel (Thy1.1+
Vbeta13 TCR+) T-cells after transfer of 5x104 cognate antigen loaded, LPS matured WT BM-DCs into the footpad of WT or CCRL1 deficient mice. Proliferating T cells in draining LNs of WT (A)
and CCRL1 deficient (B), and non-draining LNs of WT (C) and CCRL1 deficient (D) mice. Data expressed as % of pmel cells showing dilution of the CellTrace dye. (E-F) Cumulative data from
draining (E) and non-draining (F) LNs of WT and CCRL1 deficient mice; n=18 and 19 (E) and 9 and 8 (F), respectively. (G) Homing of pmel T-cells into resting WT and CCRL1 ko LNs after i.v.
transfer. Cumulative data from 10 WT and 8 CCRL1 deficient mice. Significance indicated,*, _P_ < 0.001. Box-plot graphs show median values with quartiles and minimum/maximum.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–6 (PDF 2021 kb) CCRL1-EXPRESSING TEP CELLS CREATE GRADIENTS OF CCR7 LIGANDS. (MOV 4545 KB) CCRL1
'PATTERNS' FUNCTIONAL _IN VITRO_ GRADIENTS OF CCL19. (MOV 8923 KB) SOURCE DATA SOURCE DATA TO FIG. 1 SOURCE DATA TO FIG. 2 SOURCE DATA TO FIG. 3 SOURCE DATA TO FIG. 4 RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ulvmar, M., Werth, K., Braun, A. _et al._ The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in
lymph nodes. _Nat Immunol_ 15, 623–630 (2014). https://doi.org/10.1038/ni.2889 Download citation * Received: 03 December 2013 * Accepted: 04 April 2014 * Published: 11 May 2014 * Issue
Date: July 2014 * DOI: https://doi.org/10.1038/ni.2889 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative