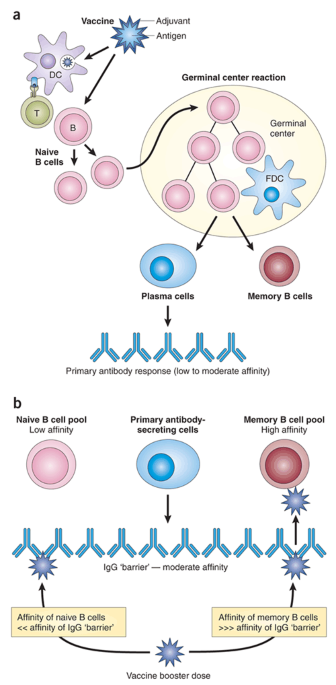
Play all audios:
ABSTRACT Some recently introduced vaccines that have excellent efficacy records have been developed without a clear understanding of their mechanism of protection. In fact, successful
vaccines have often emerged out of empirical observations and have only rarely been the result of a rational use of the continuously increasing immunological knowledge available to
scientists. However, _a posteriori_ deciphering of the biological bases for the efficacy of successful vaccines should be an essential component of research efforts directed at the
development of new vaccines for the most challenging infectious diseases. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS A GUIDE TO VACCINOLOGY: FROM BASIC PRINCIPLES TO NEW DEVELOPMENTS
Article 22 December 2020 EMERGING CONCEPTS IN THE SCIENCE OF VACCINE ADJUVANTS Article 06 April 2021 VACCINATION AGAINST RAPIDLY EVOLVING PATHOGENS AND THE ENTANGLEMENTS OF MEMORY Article 09
October 2024 REFERENCES * Janeway, C.A. Use of concentrated human serum γ-globulin in the prevention and attenuation of measles. _Bull. N. Y. Acad. Med._ 21, 202–222 (1945). CAS PubMed
PubMed Central Google Scholar * Keller, M.A. & Stiehm, E.R. Passive immunity in prevention and treatment of infectious diseases. _Clin. Microbiol. Rev._ 13, 602–614 (2000). Article
CAS PubMed PubMed Central Google Scholar * Fox, J.P. Modes of action of poliovirus vaccines and relation to resulting immunity. _Rev. Infect. Dis._ 6 (Suppl. 2), S352–S355 (1984).
Article PubMed Google Scholar * De Quadros, C.A. Polio. in _The Vaccine Book_ (eds. Bloom, B.R. & Lambert, P.H.) 189–196 (Academic Press, Amsterdam, 2003). Chapter Google Scholar *
Strebel, P.M., Papania, M.J. & Halsey, N.A. Measles vaccine. in _Vaccines_ (eds. Plotkin, S.A. & Orenstein, W.A.) 389–440 (Elsevier, Philadelphia, 2004). Google Scholar * Lampe,
R.M., Weir, M.R., Scott, R.M. & Weeks, J.L. Measles reimmunization in children immunized before 1 year of age. _Am. J. Dis. Child._ 139, 33–35 (1985). CAS PubMed Google Scholar *
Fukuda, K., Levandowski, R.A., Bridges, C.B. & Cox, N.J. Inactivated influenza vaccines. in _Vaccines_ (eds. Plotkin, S.A. & Orenstein, W.A.) 339–370 (Elsevier, Philadelphia, 2004).
Google Scholar * Belshe, R., Maassab, H.G. & Mendelman, P.M. Influenza vaccine—live. in _Vaccines_ (eds. Plotkin, S.A. & Orenstein, W.A.) 371–388 (Elsevier, Philadelphia, 2004).
Google Scholar * Davies, J.R. & Grilli, E.A. Natural or vaccine-induced antibody as a predictor of immunity in the face of natural challenge with influenza viruses. _Epidemiol. Infect._
102, 325–333 (1989). Article CAS PubMed PubMed Central Google Scholar * Treanor, J.J. et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza
vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. _Vaccine_ 18, 899–906 (1999). Article CAS
PubMed Google Scholar * Belshe, R.B. et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. _J. Infect. Dis._ 181,
1133–1137 (2000). Article CAS PubMed Google Scholar * Manz, R.A., Hauser, A.E., Hiepe, F. & Radbruch, A. Maintenance of serum antibody levels. _Annu. Rev. Immunol._ (in the press).
* McHeyzer-Williams, L.J. & McHeyzer-Williams, M. Antigen-specific memory B cell development. _Annu. Rev. Immunol._ (in the press). * Gourley, T.S., Wherry, E.J., Masopust, D. &
Ahmed, R. Generation and maintenance of immunological memory. _Semin. Immunol._ 16, 323–333 (2004). Article CAS PubMed Google Scholar * Tokoyoda, K., Egawa, T., Sugiyama, T., Choi, B.I.
& Nagasawa, T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. _Immunity_ 20, 707–718 (2004). Article CAS PubMed Google Scholar * Ochsenbein,
A.F. et al. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary
lymphoid organs. _Proc. Natl. Acad. Sci. USA_ 97, 13263–13268 (2000). Article CAS PubMed PubMed Central Google Scholar * Zinkernagel, R.M. On differences between immunity and
immunological memory. _Curr. Opin. Immunol._ 14, 523–536 (2002). Article CAS PubMed Google Scholar * Bernasconi, N.L., Traggiai, E. & Lanzavecchia, A. Maintenance of serological
memory by polyclonal activation of human memory B cells. _Science_ 298, 2199–2202 (2002). Article CAS PubMed Google Scholar * Pinschewer, D.D. et al. Kinetics of protective antibodies
are determined by the viral surface antigen. _J. Clin. Invest._ 114, 988–993 (2004). Article CAS PubMed PubMed Central Google Scholar * Nicol, M. et al. _Haemophilus influenzae_ type b
conjugate vaccine diluted tenfold in diphtheria-tetanus-whole cell pertussis vaccine: a randomized trial. _Pediatr. Infect. Dis. J._ 21, 138–141 (2002). Article PubMed Google Scholar *
Cassidy, W.M. et al. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. _Pediatrics_
107, 626–631 (2001). Article CAS PubMed Google Scholar * Ahman, H., Kayhty, H., Vuorela, A., Leroy, O. & Eskola, J. Dose dependency of antibody response in infants and children to
pneumococcal polysaccharides conjugated to tetanus toxoid. _Vaccine_ 17, 2726–2732 (1999). Article CAS PubMed Google Scholar * Bosnak, M., Dikici, B., Bosnak, V. & Haspolat, K.
Accelerated hepatitis B vaccination schedule in childhood. _Pediatr. Int._ 44, 663–665 (2002). Article PubMed Google Scholar * Zinkernagel, R.M. et al. Antigen localisation regulates
immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. _Immunol. Rev._ 156, 199–209 (1997). Article CAS PubMed Google Scholar * Deloye, F.,
Doussau, F. & Poulain, B. Action mechanisms of botulinum neurotoxins and tetanus neurotoxins. _C.R. Seances Soc. Biol. Fil._ 191, 433–450 (1997). CAS PubMed Google Scholar * Turton,
K., Chaddock, J.A. & Acharya, K.R. Botulinum and tetanus neurotoxins: structure, function and therapeutic utility. _Trends Biochem. Sci._ 27, 552–558 (2002). Article CAS PubMed Google
Scholar * Matsuda, M., Kamei, M., Sugimoto, N., Ma, Y. & Hashizume, S. Characteristics of toxin-neutralization by anti-tetanus human monoclonal antibodies directed against the three
functional domains [A], [B] and [C] of the tetanus toxin molecule and a reliable method for evaluating the protective effects of monoclonal antibodies. _Eur. J. Epidemiol._ 8, 1–8 (1992).
Article CAS PubMed Google Scholar * Gupta, R.K. & Siber, G.R. Comparative analysis of tetanus antitoxin titers of sera from immunized mice and guinea pigs determined by toxin
neutralization test and enzyme-linked immunosorbent assay. _Biologicals_ 22, 215–219 (1994). Article CAS PubMed Google Scholar * Siegrist, C.A. et al. Co-administration of CpG
oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. _Vaccine_ 23, 615–622 (2004). Article CAS PubMed Google Scholar * Pihlgren, M.
et al. CpG-motifs enhance initial and sustained primary tetanus-specific antibody secreting cell responses in spleen and bone marrow, but are more effective in adult than in neonatal mice.
_Vaccine_ 21, 2492–2499 (2003). Article CAS PubMed Google Scholar * Usinger, W.R. & Lucas, A.H. Avidity as a determinant of the protective efficacy of human antibodies to
pneumococcal capsular polysaccharides. _Infect. Immun._ 67, 2366–2370 (1999). CAS PubMed PubMed Central Google Scholar * Golaz, A. et al. Evaluation of a single dose of
diphtheria-tetanus toxoids among adults in Odessa, Ukraine, 1995: immunogenicity and adverse reactions. _J. Infect. Dis._ 181 Suppl 1, S203–S207 (2000). Article PubMed Google Scholar *
Banatvala, J., Van Damme, P. & Van Hattum, J. Boosters for hepatitis B. European Consensus Group on Hepatitis B Immunity. _Lancet_ 356, 337–338 (2000). Article CAS PubMed Google
Scholar * Goldblatt, D., Vaz, A.R. & Miller, E. Antibody avidity as a surrogate marker of successful priming by _Haemophilus influenzae_ type b conjugate vaccines following infant
immunization. _J. Infect. Dis._ 177, 1112–1115 (1998). Article CAS PubMed Google Scholar * Goldblatt, D., Miller, E., McCloskey, N. & Cartwright, K. Immunological response to
conjugate vaccines in infants: follow up study. _Br. Med. J._ 316, 1570–1571 (1998). Article CAS Google Scholar * McVernon, J., Johnson, P.D., Pollard, A.J., Slack, M.P. & Moxon, E.R.
Immunologic memory in _Haemophilus influenzae_ type b conjugate vaccine failure. _Arch. Dis. Child._ 88, 379–383 (2003). Article CAS PubMed PubMed Central Google Scholar * Kelly, D.F.,
Moxon, E.R. & Pollard, A.J. _Haemophilus influenzae_ type b conjugate vaccines. _Immunology_ 113, 163–174 (2004). Article CAS PubMed PubMed Central Google Scholar * Trotter, C.L.,
Andrews, N.J., Kaczmarski, E.B., Miller, E. & Ramsay, M.E. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. _Lancet_ 364, 365–367 (2004). Article
CAS PubMed Google Scholar * Davidkin, I., Peltola, H., Leinikki, P. & Valle, M. Duration of rubella immunity induced by two-dose measles, mumps and rubella (MMR) vaccination. A
15-year follow-up in Finland. _Vaccine_ 18, 3106–3112 (2000). Article CAS PubMed Google Scholar * Thomas, S.L. & Hall, A.J. What does epidemiology tell us about risk factors for
herpes zoster? _Lancet Infect. Dis._ 4, 26–33 (2004). Article PubMed Google Scholar * Kaufmann, S.H.E. & McMichael, A. Annulling a dangerous liaison: vaccination strategies against
AIDS and tuberculosis. _Nat Med._ 11, Suppl 1, S33–S44 (2005). Article CAS PubMed PubMed Central Google Scholar * Salmaso, S. et al. Sustained efficacy during the first 6 years of life
of 3-component acellular pertussis vaccines administered in infancy: the Italian experience. _Pediatrics_ 108, E81 (2001). Article CAS PubMed Google Scholar * Giuliano, M. et al.
Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. _J. Pediatr._ 132, 983–988 (1998). Article
CAS PubMed Google Scholar * Gans, H.A. et al. IL-12, IFN-γ, and T cell proliferation to measles in immunized infants. _J. Immunol._ 162, 5569–5575 (1999). CAS PubMed Google Scholar *
Alonso, P.L. et al. Efficacy of the RTS,S/AS02A vaccine against _Plasmodium falciparum_ infection and disease in young African children: randomised controlled trial. _Lancet_ 364, 1411–1420
(2004). Article CAS PubMed Google Scholar * Ballou, W.R. et al. Update on the clinical development of candidate malaria vaccines. _Am. J. Trop. Med. Hyg._ 71, 239–247 (2004). Article
PubMed Google Scholar * Hoskins, T.W., Davies, J.R., Smith, A.J., Miller, C.L. & Allchin, A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at
Christ's Hospital. _Lancet_ 1, 33–35 (1979). Article CAS PubMed Google Scholar * Powers, D.C. & Belshe, R.B. Vaccine-induced antibodies to heterologous influenza A H1N1 viruses:
effects of aging and “original antigenic sin”. _J. Infect. Dis._ 169, 1125–1129 (1994). Article CAS PubMed Google Scholar * Smith, D.J., Forrest, S., Ackley, D.H. & Perelson, A.S.
Variable efficacy of repeated annual influenza vaccination. _Proc. Natl. Acad. Sci. USA_ 96, 14001–14006 (1999). Article CAS PubMed PubMed Central Google Scholar * Voordouw, A.C. et al.
Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. _J. Am. Med. Assoc._ 292, 2089–2095 (2004). Article CAS Google Scholar * Robbins, F.C.
The history of polio vaccine development. in _Vaccines_ (eds. A., P.S. & Orenstein, W.A.) 17–30 (Elsevier, Philadelphia, 2004). Google Scholar * Bresee, J.S., Glass, R.I., Parashar, U.
& Gentsch, J.G. Rotavirus. in _The Vaccine Book_ (eds. Bloom, B.R. & Lambert, P.H.) 225–243 (Academic Press, Amsterdam, 2003). Chapter Google Scholar * Hackell, J.G. Pneumococcus,
pneumococcal disease and prevention. in _The Vaccine Book_ (eds. Bloom, B.R. & Lambert, P.H.) 257–277 (Academic Press, Amsterdam, 2003). Chapter Google Scholar * Hausdorff, W.P.,
Bryant, J., Paradiso, P.R. & Siber, G.R. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. _Clin. Infect.
Dis._ 30, 100–121 (2000). Article CAS PubMed Google Scholar * Bosch, F.X. et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological
study on cervical cancer (IBSCC) Study Group. _J. Natl. Cancer Inst._ 87, 796–802 (1995). Article CAS PubMed Google Scholar * Kaplan, S.L. et al. Decrease of invasive pneumococcal
infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. _Pediatrics_ 113, 443–449 (2004). Article
PubMed Google Scholar * Siegrist, C.A. Neonatal and early life vaccinology. _Vaccine_ 19, 3331–3346 (2001). Article CAS PubMed Google Scholar * Gans, H.A. et al. Deficiency of the
humoral immune response to measles vaccine in infants immunized at age 6 months. _J. Am. Med. Assoc._ 280, 527–532 (1998). Article CAS Google Scholar * Vazquez, M. et al. Effectiveness
over time of varicella vaccine. _J. Am. Med. Assoc._ 291, 851–855 (2004). Article CAS Google Scholar * Pihlgren, M. et al. Unresponsiveness to lymphoid-mediated signals at the neonatal
follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. _J. Immunol._ 170,
2824–2832 (2003). Article CAS PubMed Google Scholar * Pihlgren, M. et al. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. _Eur. J.
Immunol._ 31, 939–946 (2001). Article CAS PubMed Google Scholar * Goriely, S. et al. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. _J.
Immunol._ 166, 2141–2146 (2001). Article CAS PubMed Google Scholar * De Wit, D. et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human
newborns. _Blood_ 103, 1030–1032 (2004). Article CAS PubMed Google Scholar * Goriely, S. et al. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal
dendritic cells. _J. Exp. Med._ 199, 1011–1016 (2004). Article CAS PubMed PubMed Central Google Scholar * Ota, M.O. et al. Hepatitis B immunisation induces higher antibody and memory
Th2 responses in new-borns than in adults. _Vaccine_ 22, 511–519 (2004). Article CAS PubMed Google Scholar * Marchant, A. et al. Newborns develop a Th1-type immune response to
_Mycobacterium bovis_ bacillus Calmette-Guerin vaccination. _J. Immunol._ 163, 2249–2255 (1999). CAS PubMed Google Scholar * Ota, M.O. et al. Influence of Mycobacterium bovis bacillus
Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. _J. Immunol._ 168, 919–925 (2002). Article CAS PubMed Google Scholar * Dagan, R. et al. Immunization
against hepatitis A in the first year of life: priming despite the presence of maternal antibody. _Pediatr. Infect. Dis. J._ 19, 1045–1052 (2000). Article CAS PubMed Google Scholar *
Shapiro, E.D. et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. _N. Engl. J. Med._ 325, 1453–1460 (1991). Article CAS PubMed Google Scholar * Bjorkholm,
B., Hagberg, L., Sundbeck, G. & Granstrom, M. Booster effect of low doses of tetanus toxoid in elderly vaccinees. _Eur. J. Clin. Microbiol. Infect. Dis._ 19, 195–199 (2000). Article CAS
PubMed Google Scholar * Pawelec, G. et al. Is immunosenescence infectious? _Trends Immunol._ 25, 406–410 (2004). Article CAS PubMed Google Scholar * Gomez, I., Marx, F., Gould, E.A.
& Grubeck-Loebenstein, B. T cells from elderly persons respond to neoantigenic stimulation with an unimpaired IL-2 production and an enhanced differentiation into effector cells. _Exp.
Gerontol._ 39, 597–605 (2004). Article CAS PubMed Google Scholar * Powers, D.C. & Belshe, R.B. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody
responses elicited by inactivated influenza virus vaccine. _J. Infect. Dis._ 167, 584–592 (1993). Article CAS PubMed Google Scholar * American Academy of Pediatrics. Tetanus. in _Red
Book 2003. Report of the Committee on Infectious Diseases_ 26th edn. (ed. Pickering, L.K.) 611–616 (American Academy of Pediatrics, Elk Grove Village, Illinois, USA, 2003). * Gupta, P.S.,
Kapoor, R., Goyal, S., Batra, V.K. & Jain, B.K. Intrathecal human tetanus immunoglobulin in early tetanus. _Lancet_ 2, 439–440 (1980). Article CAS PubMed Google Scholar * American
Academy of Pediatrics. Diphtheria. in _Red Book 2003. Report of the Committee on Infectious Diseases_ 26th edn. (ed. Pickering, L.K.) 263–266 (American Academy of Pediatrics, Elk Grove
Village, Illinois, USA, 2003). * Morris, D. & McDonald, J.C. Failure of hyper-immune globulin to prevent whooping cough. _Arch. Dis. Child._ 32, 236–239 (1957). Article CAS PubMed
PubMed Central Google Scholar * Edwards, K.M. Pertussis: an important target for maternal immunization. _Vaccine_ 21, 3483–3486 (2003). Article CAS PubMed Google Scholar * Siber, G.R.
et al. Evaluation of bacterial polysaccharide immune globulin for the treatment or prevention of _Haemophilus influenzae_ type b and pneumococcal disease. _J. Infect. Dis._ 165 Suppl 1,
S129–S133 (1992). Article PubMed Google Scholar * Gershon, A.A. Measles virus (rubeola). in _Principles and Practice of Infectious Diseases_ 4th edn. (eds. Mandel, G.L., Bennett, J.E.
& Dolin, R.) 1519–1528 (Churchill Livingstone, New York, 1995). Google Scholar * American Academy of Pediatrics. Measles. in _Red Book 2003. Report of the Committee on Infectious
Diseases_ 26th edn. (ed. Pickering, L.K.) 419–429 (American Academy of Pediatrics, Elk Grove Village, Illinois, USA, 2003). * Centers for Disease Control and Prevention. Human rabies
prevention—United States, 1999. Recommendations of Advisory Committee (ACIP). _MMWR Morbid. Mortal. Wkly. Rep._ 48, 1–21 (1999). * Hankins, D.G. & Rosekrans, J.A. Overview, prevention,
and treatment of rabies. _Mayo Clin. Proc._ 79, 671–676 (2004). Article PubMed Google Scholar * Kempe, C.H. et al. The use of vaccinia hyperimmune gamma-globulin in the prophylaxis of
smallpox. _Bull. World Health Org._ 25, 41–48 (1961). CAS PubMed PubMed Central Google Scholar * Galmiche, M.C., Goenaga, J., Wittek, R. & Rindisbacher, L. Neutralizing and
protective antibodies directed against vaccinia virus envelope antigens. _Virology_ 254, 71–80 (1999). Article CAS PubMed Google Scholar * American Academy of Pediatrics. Hepatitis A. in
_Red Book 2003. Report of the Committee on Infectious Diseases_ 26th edn. (ed. Pickering, L.K.) 309–318 (American Academy of Pediatrics, Elk Grove Village, Illinois, USA, 2003). * Centers
for Disease Control and Prevention. Postexposure prophylaxis of hepatitis B. _MMWR Morbid. Mortal. Wkly. Rep._ 33, 398–402 (1984). * Terrault, N.A. et al. Prophylaxis in liver transplant
recipients using a fixed dosing schedule of hepatitis B immunoglobulin. _Hepatology_ 24, 1327–1333 (1996). Article CAS PubMed Google Scholar * Storch, G.A. Humanized monoclonal antibody
for prevention of respiratory syncytial virus infection. _Pediatrics_ 102, 648–651 (1998). Article CAS PubMed Google Scholar * Givner, L.B. Monoclonal antibodies against respiratory
syncytial virus. _Pediatr. Infect. Dis. J._ 18, 541–542 (1999). Article CAS PubMed Google Scholar * Fisher, R.G. & Edwards, K.M. Varicella-zoster. _Pediatr. Rev._ 19, 62–67 (1998).
Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors wish to thank C. Brighouse for her editorial assistance. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Departments of Immunology-Pathology and Pediatrics, Centre of Vaccinology, University of Geneva–CMU, 1 rue Michel-Servet, Geneva, 1211, Switzerland Paul-Henri Lambert & Claire-Anne
Siegrist * Transgene SA, 11 rue de Molshelm, Strasbourg, 67082, France Margaret Liu Authors * Paul-Henri Lambert View author publications You can also search for this author inPubMed Google
Scholar * Margaret Liu View author publications You can also search for this author inPubMed Google Scholar * Claire-Anne Siegrist View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Paul-Henri Lambert. ETHICS DECLARATIONS COMPETING INTERESTS Margaret A. Liu is Vice Chairman, Transgene, Strasbourg
(France). This company is involved in immunotherapy. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lambert, PH., Liu, M. & Siegrist, CA. Can
successful vaccines teach us how to induce efficient protective immune responses?. _Nat Med_ 11 (Suppl 4), S54–S62 (2005). https://doi.org/10.1038/nm1216 Download citation * Published: 05
April 2005 * Issue Date: April 2005 * DOI: https://doi.org/10.1038/nm1216 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative