Play all audios:
Extracellular matrix is a key regulator of normal homeostasis and tissue phenotype1. Important signals are lost when cells are cultured ex vivo on two-dimensional plastic substrata. Many of
these crucial microenvironmental cues may be restored using three-dimensional (3D) cultures of laminin-rich extracellular matrix (lrECM)2. These 3D culture assays allow phenotypic
discrimination between nonmalignant and malignant mammary cells, as the former grown in a 3D context form polarized, growth-arrested acinus-like colonies whereas the latter form
disorganized, proliferative and nonpolar colonies3. Signaling pathways that function in parallel in cells cultured on plastic become reciprocally integrated when the cells are exposed to
basement membrane–like gels4,5,6,7. Appropriate 3D culture thus provides a more physiologically relevant approach to the analysis of gene function and cell phenotype ex vivo. We describe
here a robust and generalized method for the culturing of various human breast cell lines in three dimensions and describe the preparation of cellular extracts from these cultures for
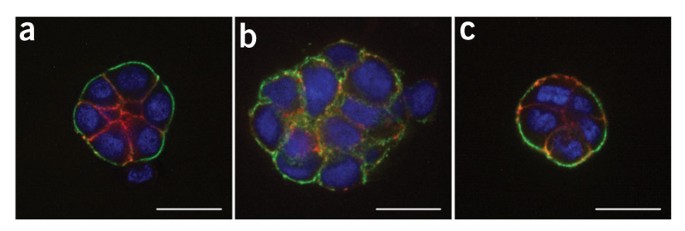
molecular analyses. The procedure below describes the 3D 'embedded' assay, in which cells are cultured embedded in an lrECM gel8 (Fig. 1). By lrECM, we refer to the solubilized extract
derived from the Engelbreth-Holm-Swarm mouse sarcoma cells9. For a discussion of user options regarding 3D matrices, see Box 1. Alternatively, the 3D 'on-top' assay, in which cells are
cultured on top of a thin lrECM gel overlaid with a dilute solution of lrECM, may be used as described in Box 2 (Fig. 1 and Fig. 2).
The protocol described here has been the work of many members of the Bissell laboratory over many years. We apologize to those whose work could not be cited owing to space limitations and
have cited reviews where possible. This work was supported by grants from the Office of Biological and Environmental Research of the US Department of Energy (DE-AC03-76SF00098 and a
Distinguished Fellow Award to M.J.B.), the US National Cancer Institute (CA64786 to M.J.B.; CA57621 to Zena Werb and M.J.B.) and the Breast Cancer Research Program of the US Department of
Defense (Innovator Award DAMD17-02-1-438 to M.J.B).
Life Sciences Division, Lawrence Berkeley National Laboratory, MS 977R225A, Berkeley, 94720, California, USA
Time-lapse movies of HMT-3522 T4-2 cells treated with vehicle and cultured in the 3D on-top assay for 5 d. Phase contrast images were taken at 1-h intervals. (MOV 1420 kb)
Time-lapse movies of HMT-3522 T4-2 cells treated with an EGFR inhibitor, AG1478, and cultured in the 3D on-top assay for 5 d. Phase contrast images were taken at 1-h intervals. (MOV 1193 kb)
Anyone you share the following link with will be able to read this content: