Play all audios:
ABSTRACT Invasive meningococcal disease (IMD) is a global health problem and vaccination has proven the most effective way of disease control. _Neisseria meningitidis_ serogroup X (_Nm_X) is
an emerging threat in the African sub-Saharan meningitis belt, but no vaccine is available today. Leading vaccines against _Nm_ are glycoconjugates, in which capsular polysaccharides
isolated from large-scale pathogen cultures are conjugated to adjuvant proteins. Though safe and efficacious even in infants, high costs and biohazard associated with the production limit
abundant application of glycoconjugate vaccines particularly in the most afflicted nations. An existing _Nm_X vaccine candidate (CPSXn-CRM197) produced by established protocols from _Nm_X
capsule polysaccharide (CPSX) has been shown to elicit high bactericidal immunoglobulin G titres in mice. Here we describe the scalable _in vitro_ synthesis of CPSXiv from chemically pure
precursors by the use of recombinant _Nm_X capsule polymerase. Application of the described coupling chemistry gives CPSXiv-CRM197, which in mouse vaccination experiments behaves identical
to the benchmark CPSXn-CRM197. Excluding any biohazards, this novel process represents a paradigm shift in vaccine production and a premise towards vaccine manufacturing in emerging
economies. SIMILAR CONTENT BEING VIEWED BY OTHERS A STABILIZED GLYCOMIMETIC CONJUGATE VACCINE INDUCING PROTECTIVE ANTIBODIES AGAINST _NEISSERIA MENINGITIDIS_ SEROGROUP A Article Open access
07 September 2020 PROGRESS TOWARDS A GLYCOCONJUGATE VACCINE AGAINST GROUP A STREPTOCOCCUS Article Open access 28 March 2023 PROOF OF CONCEPT FOR A SINGLE-DOSE GROUP B _STREPTOCOCCUS_ VACCINE
BASED ON CAPSULAR POLYSACCHARIDE CONJUGATED TO QΒ VIRUS-LIKE PARTICLES Article Open access 06 October 2023 INTRODUCTION _Neisseria meningitidis (Nm)_ is a leading cause of bacterial
meningitis and sepsis worldwide. The strictly human pathogen causes recurrent devastating epidemics in developing countries, in particular the African sub-Saharan meningitis belt.1 In
Western World countries, _Nm_ infections occur sporadically, but outbreaks have been reported caused by hyper-invasive strains in situations of crowding2,3 and in communities with increased
risk.4,5 As _Nm_ is the only bacterial pathogen that can spread in epidemic waves, outbreaks present a significant danger for individuals with high susceptibility, that is, small children,
adolescents and elderly people.6 A chief problem with invasive meningococcal disease (IMD), often characterised by meningitis and sepsis, is rapid progression. IMD can lead to death within
hours,7 leaving open an extremely short window for medical intervention. Accordingly, case-fatality rates are high, exceeding 20% even in developed countries.8 Moreover, survivors often
suffer from fatal sequelae like limb loss, deafness and neurologic disabilities.9 The benefit of vaccination is obvious on this background and motivated industrial companies (GSK Vaccines,
Pfizer, Baxter, Sanofi-Pasteur, Serum Institute of India10) and international nonprofit organisations like PATH and WHO11,12 to invest into the development of vaccines to combat IMD and thus
improve health and life conditions in afflicted regions. Major virulence factors of _Nm_ are the capsular polysaccharides (CPS).13 Twelve chemically different CPSs have been identified and
determine the twelve _Nm_ serogroups, of which six (_Nm_A, -B, -C, -W, -Y and -X) account for virtually all cases of IMD.13 With the exception of _Nm_B, where the CPS (CPSB) consists of
α2,8-linked polysialic acid identical to host-expressed polysialic acid,14,15 the CPSs coupled to adjuvant proteins provide the basis for modern vaccine formulations, the so called
glycoconjugate vaccines.16 Currently, mono- and multivalent glycoconjugate vaccines are available for _Nm_A, -C, -W and -Y and their application has provided most promising results.16 Though
safe and effective, glycoconjugate vaccines are under debate because of high production costs that may limit broad application in afflicted regions.17 Up until today, the production starts
with mass fermentation of pathogens, a biohazardous step that requests the high-tech infrastructure of modern industrial plants10 and limits the build-up of the production infrastructure in
indigent regions like the countries of the African sub-Saharan meningitis belt. However, the exigence of decentralised vaccine production has gained conspicuous actuality in the 2015
meningitis season, when an _Nm_C outbreak in Niger could not be stopped because of a shortage in vaccine provision.18 Considering that no licenced _Nm_X vaccine exists today and capitalising
on our recent success with exploiting recombinant capsule polymerases (CPs) for _in vitro_ production of bioidentical CPSs,19,20 we focused this study on the production of a synthetic _Nm_X
vaccine. With recombinant CsxA, the CP of _Nm_X, we established reaction conditions that yielded the nature identical _in vitro_ produced CPSX (henceforth referred to as CPSXiv to
differentiate it from CPSXn, the polymer isolated from natural source) in homogenous quality. In a mouse vaccination experiment, a glycoconjugate vaccine prepared from CPSXiv was found to
induce bactericidal antibodies at titres not different form the benchmark.21 The process line exemplified for _Nm_X can be easily adjusted for the production of vaccines against other _Nm_
serogroups and thus should have model character for all glycoconjugate vaccines that start with the biohazardous step of pathogen fermentation. RESULTS _IN VITRO_ SYNTHESIS OF
OLIGOSACCHARIDE PRIMERS Recently we described the small scale production of CPSXiv by use of recombinant CsxA.19 Besides the chemically pure donor sugar UDP-_N_-acetylglucosamine
(UDP-GlcNAc), short priming oligosaccharides (primCPSX) were needed to maximise the reaction efficacy and were obtained through hydrolysis of CPSXn.19 As it was our aim to avoid the use of
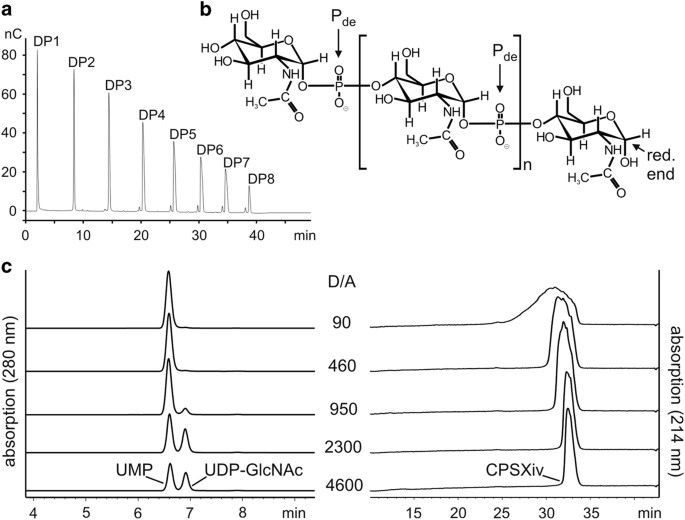
_Neisseria_-derived materials to the greatest possible extent in the current upscaled reactions, we first produced primCPSXiv (Figure 1a). Still starting with primCPSXn, three consecutive
CsxA catalysed reactions (reactions 1–3) were carried out. Priming oligosaccharides in reactions 2 and 3 were derived from purified and hydrolysed CPSXiv produced in reactions 1 and 2,
respectively. In reactions 1–3, priming oligosaccharides were mixed with a 1,000-fold molar excess of UDP-GlcNAc, resulting in a >109 fold dilution of the initially used primCPSXn.
Because acidic hydrolysis cleaves the phosphodiester bond in CPSX proximal to the anomeric C-atom (Figure 1b), oligosaccharides obtained from reaction 3 were enzymatically treated to release
the phosphate group from the non-reducing end and thus maximise the concentration of functional primers. Anion-exchange chromatography (AEC) used to purify this final primCPSXiv pool as
well as high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD; Figure 1a) identified fragments ranging in size between DP1–DP8 (DP, degree of
polymerisation), for which an average DP (avDP) of 4.5 was calculated based on 1H NMR analysis.21 DETERMINATION OF EFFICIENT REACTION CONDITIONS Because the donor-substrate UDP-GlcNAc
(henceforth referred to as donor, D) is the single reaction component of appreciable economic value, completeness of conversion into CPSXiv is a critical aspect. With the primCPSXiv fraction
of avDP4.5 (henceforth referred to as acceptor, A) at hand, a titration experiment was carried out to determine the D/A ratio that allowed complete consumption of UDP-GlcNAc. The donor
concentration was kept at 10 mM and the acceptor increased to give D/A ratios between 4,600 and 90 (Figure 1c). The reactions were run overnight and products separated and visualised using
our recently developed high-performance liquid chromatography based anion exchange chromatography (HPLC-AEC) assay.20 Complete conversion of UDP-GlcNAc to UMP was seen at a D/A ratio of 460.
However, because the basis of the product peak, an indicator of product dispersity, had considerably broadened from D/A ratio of 950 to D/A ratio of 460, we decided for the D/A ratio of 800
to set-up medium-scale CPSXiv productions. SCALING UP CPSXIV PRODUCTION AND PRODUCT CHARACTERISATION The first upscaled reaction was calculated to give 35 mg CPSXiv. As before, UDP-GlcNAc
consumption and product synthesis were visualised by HPLC-AEC (shown for start and end point in Figure 2a). After overnight incubation all UDP-GlcNAc was converted to UMP with the
corresponding CPSXiv peak detected at 214 nm. A close to 100% reaction yield was confirmed when the mixture was analysed by 31P NMR (Figure 2b). Two main peaks representing UMP and Pde
(phosphodiester bonds) appeared and integration of peak areas resulted in a 1:1 ratio. With only trace amounts of GlcNAc-1P, a product of a hydrolytic side activity of CsxA,19 the highly
sensitive 31P NMR analysis corroborated the chosen reaction conditions. To control reproducibility, a second reaction was calculated to give 112 mg and was carried out in triplicate. The
analytical HPLC-AEC profiles obtained for these reactions clearly confirmed the reliability of the CPSXiv production procedure (Supplementary Figure 1). Because the CPSXiv production was
started with ultrapure reagents and yielded close to 100% donor consumption, the product purification steps were limited to salt removal by dialysis and AEC to separate CPSXiv from UMP and
GlcNAc-1P traces (data not shown). Product purity and identity were confirmed by 1H NMR (Figure 2c). If compared with the crude reaction mixture (bottom panel), it is immediately obvious
that the two-step purification procedure delivered homogenously pure CPSXiv (middle panel). Moreover, co-analysis of CPSXn (top panel) demonstrated chemical identity of the two polymers. As
a final step, we compared the size of CPSXiv and CPSXn chains using HPLC size exclusion chromatography. With a relative molecular mass of 308 kDa, CPSXiv perfectly mimicked the natural
product CPSXn with a relative molecular mass of 388 kDa (Supplementary Figure 2a). PREPARATION OF A CPSXIV-BASED GLYCOCONJUGATE VACCINE For the preparation of a CPSXiv-based vaccine we
precisely followed the protocol previously established for CPSXn.21 First, 120 mg of the synthetic polymer were hydrolysed to shorten the CPS chains and the reaction was monitored by 31P
NMR.22 Hydrolysis was stopped after 270 min when 31P NMR analysis roughly indicated an avDP12 (Supplementary Figure 2b). The mixture was loaded onto an AEC column to remove oligosaccharides
shorter than five to six repeating units. Two pools containing different oligosaccharide polydispersions were collected (Supplementary Figure 2c). Pool 2 contained 84.7 mg of
oligosaccharides (70% recovery), which, as identified in an analytical HPAEC-PAD run, exhibited an overlapping dispersity with the benchmark CPSXn of avDP15 (Supplementary Figure 2d).
However, with avDP10 (calculated based on 31P NMR analysis; see Supplementary Figure 1d), pool 2 slightly deviated from the benchmark (avDP15). Finally, the perfect congruence of the 1H NMR
spectra obtained for the oligosaccharide fractions prepared from CPSXiv and CPSXn confirmed their chemical identity (Figure 3a). Conjugation of CPSXiv fragments to the protein carrier
CRM197, a non-toxic variant of the diphtheria toxin,23 followed the coupling technology successfully used by Micoli _et al._21 to produce the CPSXn-based vaccine MenX-ADH-SIDEA-CRM197
(referred to as CPSXn-CRM197).21 As schematically illustrated in (Figure 3b), coupling to CRM197 proceeds via the reducing end of the oligosaccharides (marked by red squares). Successful
coupling was confirmed by HPLC size exclusion chromatography. Similar to our benchmark, CPSXn-CRM197, the new glycoconjugate CPSXiv-CRM197 showed a bipartite elution profile in the absence
of detectable amounts of free CRM197 (Figure 3c). However, in line with the smaller size of the oligomer fraction, the second peak was shifted right. CPSXiv-CRM197 was then purified by
hydrophobic interaction chromatography and, as a final step in the quality control process, established protocols21,24 were used to determine oligosaccharide loading of CRM197 and control
for the presence of free saccharide. These control experiments were done in parallel with CPSXn-CRM197 (Table 1). IMMUNOGENICITY TESTING IN MICE The ability of CPSXiv-CRM197 to elicit
protective antibodies was tested in a mouse model in comparison with CPSXn-CRM197.21 Figure 4a illustrates the immunisation scheme. After the collection of pre-immune sera at day 0, female
BALB/c mice (eight animals per group) were subcutaneously injected with the first dose (day 1; 1 μg saccharide content per dose, formulated in phosphate-buffered saline buffer, pH 7.2, with
aluminum phosphate as adjuvant). The injections were repeated twice at day 14 and day 28. Post immunisation, sera (post) were taken at day 27 (Post 2) and day 42 (Post 3). Control animals
were treated in the same scheme with phosphate-buffered saline plus adjuvant. Immunoglobulin G (IgG) titres induced were detected using our recently developed ELISA (enzyme-linked
immunosorbent assay).21 Comparable IgG titres obtained with the two glycoconjugate vaccines indicate identical immunological properties for CPSXiv-CRM197 and CPSXn-CRM197 (Figure 4b).
Moreover, it is of note that considerable IgG titres had established already after two injections (Post 2 sera) and only increased by factor 2–3 after the third vaccine application
(CPSXn-CRM197: _P_=0.028, CPSXiv-CRM197: _P_=0.0284; Mann–Whitney test). Eventually we measured bactericidal activity against _Nm_X strain Z9615. Here, sera isolated from each group and time
point were pooled and rabbit serum was used as complement source.21 Comparable bactericidal activity (one dilution step is within the variation of the assay) measured in sera collected from
CPSXiv-CRM197 and CPSXn-CRM197 immunised mice convincingly evidenced the value of the described alternative and biohazard-free protocol for the production of an _Nm_X vaccine (Table 2).
DISCUSSION For the first time, we describe in this study the use of _in vitro_ synthesised nature identical CPSXiv for the production of a synthetic _Nm_X glycoconjugate vaccine candidate
(CPSXiv-CRM197). In a mouse immunisation study, CPSXiv-CRM197 competed with the benchmark vaccine CPSXn-CRM197 (ref. 21) in terms of immunogenicity and elicitation of bactericidal
antibodies. We chose _Nm_X to pioneer the enzyme-catalysed vaccine production process, because this serogroup has gained prevalence worldwide17,25–31 and outbreaks in Niger,25 Uganda and
Kenya,31 and in Togo and Burkina Faso17,30 have forcefully evidenced the need for a specific vaccine. Moreover, in the absence of a licensed _Nm_X vaccine, the implementation of an
alternative production scheme should be expedited. As highlighted by the Global Alliance for Vaccines and Immunisation, a key determinant towards improved health and economic conditions in
low-income countries is their access to vaccines (vaccines against poverty).32–34 The production process described in this study should be a quantum leap in this regard. Starting from
well-defined and pyrogen-free chemicals (UDP-GlcNAc and primCPSXiv) and completely excluding biohazards, the process provides a vantage ground for the build-up of the production
infrastructure in indigent regions and thus increase their flexibility to reply to unforeseen needs.18 In addition, stockpiling of vaccines and of vaccine components (for example,
oligosaccharide fractions ready for coupling) as requested by the International Coordinating Group on Vaccine Provision for Epidemic Meningitis would be facilitated with the new technology.
Of utmost importance in this context is that analytical steps to guarantee safety, homogeneity and lot consistency are facilitated in the fermentation-free procedure.35 The described
conditions allowed close to 100% conversion of the donor sugar into polymer. Small oligosaccharides that accrue in hydrolysis and sizing steps provide the optimal starters for new production
rounds and a simple HPLC-based analysis enabled on-line process control. Last, but certainly not the least, the recombinant CsxA can be produced in high quality. Without any further
optimisation, the enzyme purified from only 1 litre of non-pathogenic _E. coli_ M15(pREP4) culture (OD600=3.0; ref. 19) would have sufficed to generate 8 g CPSXiv. This value is remarkable,
given the fact that yields of homogenously pure CPSXn (isolated from fermented bacteria) range between 10 and 600 mg/l.21,36–39 Nevertheless, significant room remains for further process
optimisation, in particular at the level of the recombinant enzyme. First, solid phase coupling and reuse of the enzyme in repeated production rounds would simplify and enhance the process.
Second, the capsule polymerase _per se_ can be engineered towards enhanced yield, solubility and/or stability,20,40 to acquire process-adjusted elongation modes,41 and, eventually, increased
flexibility towards chemically functionalised acceptors. The latter could help reducing the coupling chemistry.20 A difference detected between the tested vaccines was a variation in the
oligosaccharide dispersion (avDP10 in CPSXiv-CRM197 versus avDP15 in CPSXn-CRM197), a feature most likely attributable to the smaller scale of the CPSXiv hydrolysis reaction. Although more
studies are needed to finally decide if this difference is of relevance, the fact that immunisation results did not reflect any associated variance suggests that it is negligible. In
contrast to CPSX, which represents a simple homopolymer of GlcNAc-1P repeating units, CPSs from other _Nm_ serogroups are more complex and _in vitro_ production would need more than one
enzymatic activity. We could recently demonstrate on an analytical scale that the herein-presented technology can potentially be expanded to more complex biosynthesis systems. The acetylated
CPS of _Nm_A could be produced in a one-pot reaction using three different recombinant enzymes,20 we have done pioneering steps towards the fermentation-free production of the dimeric CPSs
of _Nm_W and Y42,43 and, as an example for the most complex CPS expressed by _Nm_, we also described the synthesis of the trimeric CPS of _Nm_L.44 Taken together, this paradigmatic
evaluation of a biohazard-free protocol for the production of an efficient _Nm_X vaccine should pave the way for future decentralised glycoconjugate vaccine production and thus back up the
mission for vaccines against poverty.32 MATERIALS AND METHODS SYNTHESIS AND PURIFICATION OF PRIMCPSXIV CPSXiv was synthesised _in vitro_ using the recombinant capsule polymerase CsxA
expressed as MBP-CsxA-His6 fusion construct.19 Hydrolysis of 0.5 mg CPSXiv (2.5 mg/ml) was performed in 50 mM sodium acetate buffer at pH 4.0 and 80 °C for 6 h. NaOH was added to readjust pH
7 before the mixture was dialysed against water (ZelluTrans, Roth, 1 kDa MWCO) and freeze-dried. Alternatively, primCPSXiv were recycled from pool 1 containing oligosaccharides <DP10
(see Supplementary Figure 1c). For the dephosphorylation of oligosaccharides, we used either acid phosphatase (Worthington Biochemical Corporation, Lakewood, NJ, USA) or calf intestinal
alkaline phosphatase (CIP, NEB) and followed the manufacturer’s guidelines. Although removal of acid phosphatase from primCPSXiv was achieved with Amicon centrifugal devices (10 MWCO), CIP
was removed by AEC. Individual oligosaccharides were separated on an ÄKTA-FPLC (GE Healthcare, Little Chalfont, Buckinghamshire, UK) equipped with a MonoQ HR 5/5 column (GE Healthcare) at a
flow-rate of 1 ml/min. H2O and 1 M NaCl were used as mobile phases M1 and M2, respectively. The samples were separated using a combination of linear gradients (0 to 5% M2 over 1 ml, 5 to 20%
M2 over 10 ml, 20 to 30% M2 over 20 ml). Saccharide-containing fractions were pooled and desalted by dialysis (ZelluTrans, Roth, 1 kDa MWCO) against a 1,000-fold excess of water. Fractions
containing DP3, DP4 and DP5 were isolated and the DP was confirmed by 1H NMR and HPAEC-PAD as described in refs 21,22. UPSCALING OF THE _IN VITRO_ CPSX SYNTHESIS Test reactions in 25 μl of
total reaction volume were performed as described before19 in the presence of varying amounts of primCPSXiv to determine conditions suitable for the production of long CPSX chains and
complete consumption of UDP-GlcNAc. The analysis of the reaction products by HPLC-AEC was performed following established protocols.19,20,44 For upscaling, 80 mg of UDP-GlcNAc were incubated
with 0.6 or 2.5 nmol (62.5 or 250 μg, for overnight or 4 h reactions, respectively) MBP-CsxA-His6 at 37 °C in the presence of primCPSXiv in a total volume of 12.4 ml allowing the synthesis
of a maximum of 35 mg CPSXiv. The synthesis of 112 mg CPSX was upscaled accordingly. UMP and other reaction constituents were removed by Tangential Flow Filtration using Vivaflow 50
membranes with 30 kDa MWCO. Alternatively, the reaction volume was reduced to 5 ml by freeze-drying and applied to an ÄKTA-FPLC (GE Healthcare) equipped with a MonoQ 10/100 GL column (GE
Healthcare) at a flow-rate of 5 ml/min. H2O and 1 M NaCl were used as mobile phases M1 and M2, respectively. The separation was performed using a combination of linear gradients from 0 to
15% M2 over 24 ml and from 15 to 100% M2 over 224 ml. CPSX-containing fractions were pooled, dialysed against H2O and freeze-dried. Acidic hydrolysis was performed for 4.5 h as described
above and the avDP was estimated by 31P NMR.22 The hydrolysate was loaded onto a Q Sepharose column (GE Healthcare) equilibrated with binding buffer (5 mM sodium acetate, pH 7.0). Small
oligosaccharides (<DP5-6) were removed with binding buffer containing 200 mM NaCl (pool I; Supplementary Figure 1c) and longer oligosaccharides were eluted with binding buffer containing
1 M NaCl. GENERATION AND IMMUNOGENICITY OF CPSXN-CRM197 IMMUNISATION PROTOCOL, SERUM ANALYSIS, ELISA AND RSBA All methods concerning the generation and characterisation of CPSXiv-CRM197 as
well as the immunisation protocol and serum analysis were performed as described before.21 _Nm_X strain Z9615 was kindly provided by Gerd Pluschke, Swiss Tropical and Public Health
Institute, Molecular Immunology, Basel, Switzerland. The animal studies were performed in accordance with the Italian law, approved by the local Animal Ethics Committee, and authorised by
the Italian Ministry of Health. REFERENCES * Stephens, D. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium _Neisseria meningitidis_. _Vaccine_ 27 (Suppl
2): B71–B77 (2009). Article Google Scholar * Broderick, M. P., Phillips, C. & Faix, D. Meningococcal disease in US military personnel before and after adoption of conjugate vaccine.
_Emerg. Infect. Dis._ 21, 377–379 (2015). Article CAS Google Scholar * Hill, D. J., Griffiths, N. J., Borodina, E. & Virji, M. Cellular and molecular biology of _Neisseria
meningitidis_ colonization and invasive disease. _Clin. Sci. (Lond)_ 118, 547–564 (2010). Article CAS Google Scholar * Kratz, M. M. et al. Community-Based Outbreak of _Neisseria
meningitidis_ Serogroup C Infection in Men who Have Sex with Men, New York City, New York, USA, 2010-2013. _Emerg. Infect. Dis._ 21, 1379–1386 (2015). Article CAS Google Scholar *
Kupferschmidt, K. Infectious diseases. Bacterial meningitis finds new niche in gay communities. _Science_ 341, 328 (2013). Article CAS Google Scholar * Caesar, N. M., Myers, K. A. &
Fan, X. _Neisseria meningitidis_ serogroup B vaccine development. _Microb. Pathog._ 57, 33–40 (2013). Article CAS Google Scholar * Cartwright, K. A. & Ala’Aldeen, D. A. _Neisseria
meningitidis_: clinical aspects. _J. Infect._ 34, 15–19 (1997). Article CAS Google Scholar * Tan, L. K., Carlone, G. M. & Borrow, R. Advances in the development of vaccines against
_Neisseria meningitidis_. _N. Engl. J. Med._ 362, 1511–1520 (2010). Article CAS Google Scholar * Nadel, S. Prospects for eradication of meningococcal disease. _Arch. Dis. Child_ 97,
993–998 (2012). Article Google Scholar * Costantino, P., Rappuoli, R. & Berti, F. The design of semi-synthetic and synthetic glycoconjugate vaccines. _Expert Opin. Drug Discov_ 6,
1045–1066 (2011). Article CAS Google Scholar * Tiffay, K., Jodar, L., Kieny, M. P., Socquet, M. & Laforce, F. M. The Evolution of the Meningitis Vaccine Project. _Clin. Infect. Dis._
61 (Suppl 5): S396–S403 (2015). Article Google Scholar * Laforce, F. M., Konde, K., Viviani, S. & Preziosi, M. P. The Meningitis Vaccine Project. _Vaccine_ 25 (Suppl 1): A97–A100
(2007). Article Google Scholar * Tzeng, Y. L., Thomas, J. & Stephens, D. S. Regulation of capsule in _Neisseria meningitidis_. _Crit Rev. Microbiol._ 42, 759–772 (2015). PubMed PubMed
Central Google Scholar * Lo, H., Tang, C. M. & Exley, R. M. Mechanisms of avoidance of host immunity by _Neisseria meningitidis_ and its effect on vaccine development. _Lancet Infect.
Dis._ 9, 418–427 (2009). Article CAS Google Scholar * Eckhardt, M. et al. Molecular characterization of eukaryotic polysialyltransferase-1. _Nature_ 373, 715–718 (1995). Article CAS
Google Scholar * Pace, D. Glycoconjugate vaccines. _Expert Opin. Biol. Ther._ 13, 11–33 (2013). Article CAS Google Scholar * Xie, O., Pollard, A. J., Mueller, J. E. & Norheim, G.
Emergence of serogroup X meningococcal disease in Africa: need for a vaccine. _Vaccine_ 31, 2852–2861 (2013). Article Google Scholar * Maurice, J. Vaccine shortage threatens spread of
meningitis in Niger. _Lancet_ 385, 2241 (2015). Article Google Scholar * Fiebig, T. et al. Functional expression of the capsule polymerase of _Neisseria meningitidis_ serogroup X: a new
perspective for vaccine development. _Glycobiology_ 24, 150–158 (2014). Article CAS Google Scholar * Fiebig, T. et al. Molecular cloning and functional characterization of components of
the capsule biosynthesis complex of _Neisseria meningitidis_ serogroup A: toward _in vitro_ vaccine production. _J. Biol. Chem._ 289, 19395–19407 (2014). Article CAS Google Scholar *
Micoli, F. et al. Development of a glycoconjugate vaccine to prevent meningitis in Africa caused by meningococcal serogroup X. _Proc. Natl Acad. Sci. USA_ 110, 19077–19082 (2013). Article
CAS Google Scholar * Berti, F. et al. Relative stability of meningococcal serogroup A and X polysaccharides. _Vaccine_ 30, 6409–6415 (2012). Article CAS Google Scholar * Bröker, M.,
Costantino, P., DeTora, L., McIntosh, E. D. & Rappuoli, R. Biochemical and biological characteristics of cross-reacting material 197 CRM197, a non-toxic mutant of diphtheria toxin: use
as a conjugation protein in vaccines and other potential clinical applications. _Biologicals_ 39, 195–204 (2011). Article Google Scholar * Micoli, F. et al. Meningococcal X polysaccharide
quantification by high-performance anion-exchange chromatography using synthetic N-acetylglucosamine-4-phosphate as standard. _Anal. Biochem._ 442, 259–261 (2013). Article CAS Google
Scholar * Boisier, P. et al. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. _Clin. Infect. Dis._ 44, 657–663 (2007). Article Google
Scholar * Chen, C. et al. A first meningococcal meningitis case caused by serogroup X _Neisseria meningitidis_ strains in China. _Chin. Med. J. (Engl. )_ 121, 664–666 (2008). Article CAS
Google Scholar * Fazio, C. et al. _Neisseria meningitidis_ serogroup X sequence type 2888, Italy. _Emerg. Infect. Dis._ 16, 359–360 (2010). Article Google Scholar * Vicente, D., Esnal, O.
& Perez-Trallero, E. Fatal _Neisseria meningitidis_ serogroup X sepsis in immunocompromised patients in Spain. Virulence of clinical isolates. _J. Infect._ 64, 184–187 (2012). Article
Google Scholar * Kilic, A. et al. _Neisseria meningitidis_ serogroup X sequence type 767 in Turkey. _J. Clin. Microbiol._ 48, 4340–4341 (2010). Article Google Scholar * Delrieu, I. et al.
Emergence of epidemic _Neisseria meningitidis_ serogroup X meningitis in Togo and Burkina Faso. _PLoS One_ 6, e19513 (2011). Article CAS Google Scholar * Mutonga, D. M. et al.
Epidemiology and risk factors for serogroup X meningococcal meningitis during an outbreak in western Kenya, 2005-2006. _Am. J. Trop. Med. Hyg._ 80, 619–624 (2009). Article Google Scholar *
MacLennan, C. A. & Saul, A. Vaccines against poverty. _Proc. Natl Acad. Sci. USA_ 111, 12307–12312 (2014). Article CAS Google Scholar * Shen, A. K. et al. Country ownership and Gavi
transition: comprehensive approaches to supporting new vaccine introduction. _Health Aff. (Millwood)_ 35, 272–276 (2016). Article Google Scholar * Berkley, S. Make vaccine coverage a key
UN health indicator. _Nature_ 526, 165 (2015). Article CAS Google Scholar * Jones, C. Glycoconjugate vaccines: the regulatory framework. _Methods Mol. Biol._ 1331, 229–251 (2015). Article
Google Scholar * Chilukuri, S. R. et al. Process development and immunogenicity studies on a serogroup ‘X’ Meningococcal polysaccharide conjugate vaccine. _Biologicals_ 42, 160–168
(2014). Article CAS Google Scholar * Robinson, J. A. & Apicella, M. A. Isolation and characterization of _Neisseria meningitidis_ Groups A, C, X and Y polysaccharide antigens.
_Infect. Immun._ 1, 8–14 (1970). CAS PubMed PubMed Central Google Scholar * Bundle, D. R., Jennings, H. J. & Kenny, C. P. Studies on the group-specific polysaccharide of _Neisseria
meningitidis_ serogroup X and an improved procedure for its isolation. _J. Biol. Chem._ 249, 4797–4801 (1974). CAS PubMed Google Scholar * Xie, O. et al. Characterization of size,
structure and purity of serogroup X _Neisseria meningitidis_ polysaccharide, and development of an assay for quantification of human antibodies. _Vaccine_ 30, 5812–5823 (2012). Article CAS
Google Scholar * Keys, T. G., Berger, M. & Gerardy-Schahn, R. A high-throughput screen for polysialyltransferase activity. _Anal. Biochem._ 427, 60–68 (2012). Article CAS Google
Scholar * Keys, T. G. et al. Engineering the product profile of a polysialyltransferase. _Nat. Chem. Biol._ 10, 437–442 (2014). Article CAS Google Scholar * Romanow, A. et al.
Biochemical and biophysical characterization of the sialyl-/hexosyltransferase synthesizing the meningococcal serogroup W135 heteropolysaccharide capsule. _J. Biol. Chem._ 288, 11718–11730
(2013). Article CAS Google Scholar * Romanow, A. et al. Dissection of hexosyl- and sialyltransferase domains in the bifunctional capsule polymerases from _Neisseria meningitidis_ W and Y
defines a new sialyltransferase family. _J. Biol. Chem._ 289, 33945–33957 (2014). Article CAS Google Scholar * Litschko, C. et al. The capsule polymerase CslB of _Neisseria meningitidis_
serogroup L catalyzes the synthesis of a complex trimeric repeating unit comprising glycosidic and phosphodiester linkages. _J. Biol. Chem._ 290, 24355–24366 (2015). Article CAS Google
Scholar Download references ACKNOWLEDGEMENTS We thank Daniela Proietti, Evita Balducci and Elena Mori of GSK Vaccines (Siena, Italy) for useful discussions and Gerd Pluschke of Swiss
Tropical and Public Health Institute, Molecular Immunology (Basel, Switzerland), for kindly providing _Nm_X strain Z9615. The study was financed by LOM (impact oriented funding) funds to the
Institute for Cellular Chemistry. AUTHOR INFORMATION Author notes * Timm Fiebig and Maria Rosaria Romano: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS *
Institute for Cellular Chemistry, Hannover Medical School, Hannover, Germany Timm Fiebig, Monika Berger & Rita Gerardy-Schahn * GSK Vaccines, Research, Siena, Italy Maria Rosaria Romano,
Davide Oldrini, Roberto Adamo, Marta Tontini, Barbara Brogioni, Laura Santini, Paolo Costantino & Francesco Berti Authors * Timm Fiebig View author publications You can also search for
this author inPubMed Google Scholar * Maria Rosaria Romano View author publications You can also search for this author inPubMed Google Scholar * Davide Oldrini View author publications You
can also search for this author inPubMed Google Scholar * Roberto Adamo View author publications You can also search for this author inPubMed Google Scholar * Marta Tontini View author
publications You can also search for this author inPubMed Google Scholar * Barbara Brogioni View author publications You can also search for this author inPubMed Google Scholar * Laura
Santini View author publications You can also search for this author inPubMed Google Scholar * Monika Berger View author publications You can also search for this author inPubMed Google
Scholar * Paolo Costantino View author publications You can also search for this author inPubMed Google Scholar * Francesco Berti View author publications You can also search for this author
inPubMed Google Scholar * Rita Gerardy-Schahn View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS RG-S and PC initiated the research; TF,
RG-S, FB and MRR designed the research; TF, MRR, DO, MT, BB, LS and MB performed the research; RG-S, FB, TF, RA and MRR interpreted the results; TF and RG-S supported by FB, MRR, RA and PC
wrote the manuscript. CORRESPONDING AUTHORS Correspondence to Francesco Berti or Rita Gerardy-Schahn. ETHICS DECLARATIONS COMPETING INTERESTS The authors MRR, DO, RA, MT, BB, LS, PC and FB
are full-time employees of GSK Vaccines. The authors TF, MB and RG-S have submitted patent applications for glycoconjugate vaccines against _Nm_X. ADDITIONAL INFORMATION Supplementary
Information accompanies the paper on the _npj Vaccines_ website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (DOC 393 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative
Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in
the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy
of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Fiebig, T., Romano, M., Oldrini, D. _et al._ An efficient
cell free enzyme-based total synthesis of a meningococcal vaccine candidate. _npj Vaccines_ 1, 16017 (2016). https://doi.org/10.1038/npjvaccines.2016.17 Download citation * Received: 10 May
2016 * Revised: 14 July 2016 * Accepted: 03 August 2016 * Published: 15 November 2016 * DOI: https://doi.org/10.1038/npjvaccines.2016.17 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative