Play all audios:
ABSTRACT Aberrant activity in brain regions underlying various aspects of executive cognition has been reported in patients with schizophrenia and in their healthy relatives, suggesting an
association with genetic liability. The aim of this study was to investigate brain responses to selective aspects of cognitive control in unaffected siblings who are at increased genetic
risk of schizophrenia. Altogether, 65 non-affected siblings, 70 patients with schizophrenia spectrum disorders, and 235 normal controls participated in this study.
Blood-oxygen-Ievel-dependent functional magnetic resonance imaging was conducted while participants performed a cognitive control task (‘flanker task’) to identify brain activity and
connectivity associated with response inhibition and conflict monitoring, and suppression. Behaviorally, similar to patients with schizophrenia, siblings were less accurate when inhibiting
prepotent responses relative to normal controls. During response inhibition, again similar to patients with schizophrenia, siblings showed decreased activity in the anterior cingulate (ACC),
along with increased functional coupling with the dorsolateral prefrontal cortex (PFC) when compared to normal controls. Our findings show altered ACC activity and PFC connectivity in
unaffected siblings and patients with schizophrenia during response inhibition. These results suggest that such changes in the neural activity underlying aspects of cognitive control may
represent a potential intermediate phenotype for the investigation of the genetic basis of schizophrenia. SIMILAR CONTENT BEING VIEWED BY OTHERS CONTROL OF RESPONSE INTERFERENCE: CAUDATE
NUCLEUS CONTRIBUTES TO SELECTIVE INHIBITION Article Open access 01 December 2020 A COGNITIVE NEUROGENETIC APPROACH TO UNCOVERING THE STRUCTURE OF EXECUTIVE FUNCTIONS Article Open access 06
August 2022 WHITE MATTER DISCONNECTION OF LEFT MULTIPLE DEMAND NETWORK IS ASSOCIATED WITH POST-LESION DEFICITS IN COGNITIVE CONTROL Article Open access 29 March 2023 INTRODUCTION Genetic
studies using complex phenotypes such as clinical diagnosis or symptom dimensions have provided few and inconsistent results on the genetic variants associated with schizophrenia. An
alternative approach is the use of intermediate phenotypes (Gottesman and Gould, 2003). They are narrower biological constructs that are closer to the effects of the risk genes than the
diagnosis itself. They include biological markers that are associated with the disorder and are expressed more frequently in non-affected family members than in the general population
(Gottesman et al, 2003; Tan et al, 2008). The idea that the effects of genes on neuroimaging-based susceptibility-related phenotypes have greater penetrance for the identification of genetic
effects led to the development of the ‘imaging genetics’ approach (Weinberger et al, 2001). Cognitive function has emerged as an attractive intermediate phenotype for schizophrenia for
multiple reasons, including its objective measurement, relative clinical stability during the course of illness, impact on disability, heritability, and link to genetic risk (Toulopoulou et
al, 2010). Although cognitive symptoms are core features of schizophrenia (Goldberg and Weinberger, 1988), they represent a complex construct _per se._ Altered cognitive control has been
frequently reported in this disorder (Carter et al, 2001; Goldberg et al, 1988). Cognitive control allows adaptive variation of thoughts and behavior to current goals based on contextual
information, and includes multiple cognitive processes such as response inhibition, interference control, attention, and working memory (WM). Twin and family studies have suggested a genetic
substrate for cognitive control (Swan and Carmelli, 2002). Interestingly, heritability of cognitive control capacity has also been associated with liability for schizophrenia. Unaffected
siblings (SIBs) of patients with schizophrenia show impaired performance on those tasks that tap cognitive control functions such as WM, set-shifting, and response inhibition (Goldberg et
al, 1995). Recent results from a large study that included first-degree relatives including co-twins by Toulopoulou et al, (2010) suggest that a significant portion of the phenotypic
correlation between schizophrenia and cognitive measures can be explained by shared genetic effects. Neuroimaging studies show that a network of brain regions including lateral-PFC, dorsal
anterior cingulate (ACC), and parietal cortex mediates cognitive control (Badre and Wagner, 2004; Blasi et al, 2006; Kerns et al, 2004). PFC and parietal cortex are implicated in the dynamic
tuning of cognitive control via top-down modulation of attentional processes and via response suppression, whereas ACC is responsible for detection and direct or PFC-mediated suppression of
cognitive conflict (see Mansouri et al, 2009 for a review), and response inhibition (Swick and Turken, 2002). Furthermore, lateral-PFC and ACC are both anatomically (Koski and Paus, 2000)
and functionally connected (Badre et al, 2004), and interact in regulating cognitive control during higher cognitive demands (Medalla and Barbas, 2009), particularly during response
inhibition (Stevens et al, 2009). Studies in patients with schizophrenia (SCZs) have shown altered function of these regions during cognitive tasks (Callicott et al, 2003). In particular,
SCZs show reduced activity in ACC during commission errors for stimuli that invoked strong conflict (Carter et al, 2001), conflict resolution (Carter et al, 2001), and inhibition of
prepotent responses (Fallgatter et al, 2003). This functional alteration in cognitive control regions in the context of altered performance may be due to the subjects not engaging or not
being able to engage in the task at hand (Ford et al, 2004). Similarly, previous studies have shown that SCZs show prefrontal hyperactivity when compared to healthy subjects (NCs) with
similar behavioral performance, but SCZs who fail to sustain the prefrontal network, reflected as prefrontal hypoactivity, manifest lower accuracy (Callicott et al, 2003; Manoach et al,
2001). There are also reports of the lack of a relationship between behavioral performance and prefrontal responses associated with cognitive control (Minzenberg et al, 2009). Thus, the role
of performance differences on brain correlates underlying cognitive control needs to be clarified. Importantly, the extent to which altered activity in ACC during cognitive control and
response inhibition, in particular, can indicate that genetic liability for schizophrenia has yet to be determined. In the current study, we used functional resonance imaging (fMRI) to
elucidate the role of cortical responses associated with cognitive control on genetic risk for schizophrenia. We focused on brain activity related to response inhibition and interference
suppression, two crucial processes that underlie cognitive control. The former is the ability to inhibit prepotent behavioral responses that are premature, inappropriate or incorrect; the
latter requires the capability to detect and filter out irrelevant or conflicting information. We tested the hypothesis that individuals at risk for schizophrenia when challenged with
demands on cognitive control, similar to SCZs, would also be impaired at the behavioral level as well as at the neural level, which will be reflected as decreased recruitment of ACC and
altered functional coupling of ACC with lateral-PFC. MATERIALS AND METHODS SUBJECTS The sample consisted of 370 subjects: 65 SIBs, 70 SCZs, and 235 NCs (Table 1). Subjects were recruited
nationwide as part of an ongoing family study of schizophrenia at the Clinical Brain Disorders Branch Sibling Study (Protocol 95-M-0150) at the NIH. All of the patients had a diagnosis of
schizophrenia-spectrum disorder, and 77.1% of them met the DSM-IV-TR diagnostic criteria for schizophrenia. Exclusion criteria are detailed in Supplementary Materials. A minority of SIBs had
a past lifetime history of a non-psychotic mental illness and/or substance abuse and/or dependence (see Supplementary Materials), but none met the DSM-IV-TR criteria at the time of
evaluation and only five of the SIBs were receiving psychotropic medicines. Although the prevalence of smoking was expectedly frequent in SCZs, SIBs and NCs included a similar minority of
smokers (<10% in each group). All participants gave written informed consent, approved by the Institutional Review Board of the National Institute of Mental Health, to take part in the
experiment. TASK All subjects performed a modified version of the flanker task (Blasi et al, 2006; see Supplementary Figure S1). Briefly, subjects saw a set of five symbols that included a
central arrow pointing left or right, flanked by two pairs of symbols (arrows, boxes or X’s), one on each side. This task included four experimental conditions: ‘congruent’, ‘incongruent’,
‘neutral’, and ‘No-Go’. In all the conditions except ‘No-Go’, subjects were asked to indicate the direction of the central arrow by a button press as quickly and accurately as possible. In
the ‘congruent’ condition, the central arrow was flanked by pairs of arrows orientated in the same direction. In the ‘incongruent’ condition, the flanking arrows were orientated in a
direction opposite to the central arrow to evaluate interference monitoring and suppression. In the ‘neutral’ condition pairs of boxes flanked the central arrow. In the ‘No-Go’ condition two
pairs of X’s flanking the central arrow required subjects to withhold their motor response and served to evaluate response inhibition. Each trial was presented for 800 ms and a fixation
crosshair (inter-trial-interval=2200–5200 ms) was shown in between. A total of 145 pseudorandomized trials (No-Go/neutral/incongruent/congruent=33/31/40/41) were presented. Performance was
recorded through a fiber-optic response box, which allowed the measurement of correct responses and their reaction time (RT). IMAGE ACQUISITION Blood-oxygen-level-dependent (BOLD)-fMRI was
performed on a GE Signa3.0 Tesla magnet. A gradient echo BOLD-echo-planar imaging sequence was used to acquire 300 images. Each image consisted of 26 4-mm-thick axial slices, covering the
entire cerebrum and most of the cerebellum (TR/TE=2000/28 ms; FOV=24 cm; matrix=64 × 64; gap=1 mm; flip-angle=90). DATA ANALYSIS DEMOGRAPHICS, BEHAVIORAL DATA One-way ANOVAs and _χ_2
analyses were used to compare demographic data across diagnostic groups. General linear models (GLM) with repeated measures for task conditions and with age, gender, and premorbid-IQ as
indexed by wide range achievement test (WRAT) served as covariates of no interest were used to evaluate performance differences across diagnostic groups. To test planned comparisons, linear
t-contrasts were also computed. To exclude potential effects of current psychotropic treatment in SIBs, we re-run the behavioral analyses excluding the five SIBs on psychotropic drugs at the
time of the data acquisition. IMAGING Data were pre-processed and analyzed using Statistical Parametrical Mapping (SPM5; http://www.fil.ion.ucl.ac.uk, see Supplementary Materials). For each
stimulus type, a stick function was convolved with a canonical hemodynamic response function at each voxel. Six subject-specific movement parameters obtained from the realignment procedure
were included in the model as covariates of no interest, taking into account the effects of subject motion. All data sets underwent rigorous quality control check to exclude motion artifacts
(>2 mm translation, <1.5 degrees rotation). We also included a regressor of no interest for incorrect and missed responses. For correct trials only, linear contrasts were computed
producing voxel-wise t-statistical maps for interference monitoring and suppression (incongruent>congruent), and response inhibition (No-Go). The whole sample group maps of these
contrasts that are orthogonal to the diagnosis were eventually used to mask random effects second-level analyses (mask size was 340 092 and 370 737 mm3 for incongruent>congruent and
No-Go, respectively). ANCOVAs with age, gender, WRAT, and reaction times (RTs, only for conditions requiring button press) as nuisance variables were used to identify significant differences
in brain activation across the diagnostic groups. Pairwise diagnostic differences were tested with linear t-contrasts. In those brain regions where both SCZs and their SIBs had abnormal
activity relative to NCs, the average cluster brain activations was extracted and pairwise compared using planned comparisons. To examine the cognitive control-dependent modulation of
functional coupling of ACC with the rest of the brain, a psychophysiological interaction (PPI) analysis was performed. This analysis allows the evaluation of regional specific responses in
terms of the interaction between the neural activity of different brain regions and an experimental condition. Based on our strong _a priori_ hypothesis on the role of ACC in cognitive
control we chose this region, as identified through WFU-pickatlas toolbox (http://www.rad.wfubmc.1edu/fmri), as seed for the PPI. The first eigenvariate of individual time-courses was
extracted from the seed, mean-centered, high-pass filtered, and deconvolved. A new GLM was then computed at each individual subject level using three regressors: a physiological regressor
(the time course response from the seed), a psychological regressor (No-Go _vs_ congruent for response inhibition, and incongruent _vs_ congruent during interference monitoring and
suppression, respectively), and a psychophysiological interaction term, calculated as the de-meaned scalar product of the physiological and psychological regressors. To identify differences
in brain connectivity across diagnostic groups, individual PPI contrasts were entered into random effects ANCOVAs as for the activation analyses. To exclude potential effects of current
psychotropic treatment in SIBs, we re-run the imaging analyses excluding the SIBs on psychotropic drugs at the time of the data acquisition. Furthermore, to exclude that group differences in
neural activity and connectivity were unduly driven by differences in demographics and behavioral performance, we selected a subsample of 228 subjects matched also for age, WRAT, accuracy,
and RT (Supplementary Table S1). Given the small number of female SCZs and male SIBs, we could not match for this variable; nevertheless, we added this variable in ANCOVAs as a nuisance
variable to account for this difference and also ran further confirmatory analyses in a gender-matched subgroup (see Supplementary Materials). For both the task activation and PPI ANCOVA
analyses, a statistical threshold of _P_<0.05 corrected for multiple comparisons with family-wise error small volume-correction (FWE-SVC) was used to identify significant differences
within anatomical regions of interest (ROI) associated with task effects (Blasi et al, 2006). ROls were created using WFU-pickatlas and comprised the following _a priori_ regions: ACC
(BA24/32), and lateral-PFC (BA9/10/44/45/46/47). A single mask including ACC and bilateral PFC was used to perform FWE-SVC for activation responses, whereas a bilateral PFC ROI was used for
the ACC-PPI connectivity analyses. All coordinates are reported in MNI system. Linear correlations between the first eigenvariate of the signal of the clusters showing the effect of
diagnosis on brain responses (activity and connectivity) associated with response inhibition and accuracy were performed in each diagnosis group separately. In SCZs, correlations between
brain responses and treatment variables (chlorpromazine equivalents) were analyzed. To correct for potential dependence of behavioral (accuracy and RT) and neural responses (ACC activation
and PPI in lateral-PFC) to the flanker task within members of the same family, we also performed confirmatory one-way ANOVAs on behavioral and neural measures across all diagnostic groups,
and between SIBs and SCZs with the robust variance correction as applied in Stata10.0 (see Supplementary Materials) as in Rasetti et al, 2011. This type of correction estimate uses a robust
covariance matrix to estimate the standard error by taking into account within-cluster (family) correlation (data not independent within-groups but dependent across group clusters, ie
families). This estimate is then used for adjusting appropriately neural and behavioral responses results for within-family correlations. RESULTS BEHAVIORAL RESULTS ACCURACY There was a main
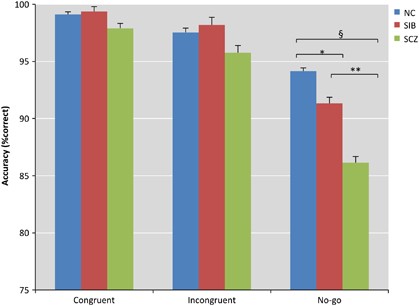
effect of diagnosis (Figure 1): SCZs had significantly lower accuracy compared to SIBs and NCs (_P_<0.0001). There was a trend towards significance for task condition [F(3,1 092)=2.1988;
_P_=0.087)], with the lowest performance on the No-Go condition (_P_<0.0001). Additionally, accuracy during the incongruent condition was significantly lower relative to the congruent
(_P_<0.0001). There was also an interaction of diagnosis-by-task condition with SIBs (_P_<0.02) and SCZs (_P_<0.00001) having worse accuracy during response inhibition but not
during interference monitoring (_t_-contrast incongruent>congruent by groups, _P_=0.8) compared to NCs. Robust-variance corrected analyses confirmed the effect of diagnosis on accuracy
during No-Go (_P_<0.001), but not in the other task conditions (_P_>0.1). REACTION TIME There was a main effect of diagnosis [F(2,364)=4.8058, _P_=0.00871]: SIBs (_P_=0.04) as well as
SCZs (_P_<0.0001) were slower relative to NCs. There was also a main effect of task condition [F(2,728)=6.8929, _P_=0.001] with incongruent being the slowest condition (_P_<0.05).
Interestingly, there was no significant diagnosis-by-task condition interaction (Supplementary Figure S2). Robust-variance corrected analyses confirmed the effect of diagnosis on RT for
congruent and incongruent (_P_<0.005). All the behavioral results were similar and statistically significant after excluding SIBs on psychotropic drugs (data not shown). IMAGING RESULTS
EFFECT OF TASK CONDITIONS During response inhibition, participants showed predominantly right-Iateralized activation in the following brain regions: dorsolateral-PFC (DLPFC, BA9/46),
ventrolateral-PFC (VLPFC, BA44/47), supplementary motor area (BA6), ACC (BA32), insula, caudate, thalamus, precuneus (BA7/40), and occipital regions. Interference monitoring and suppression
was associated with greater activity in a network of brain regions including parietal cortex (BA7/40), VLPFC (BA44/45/47), insula (BA13), DLPFC (BA9/46), ACC (BA24/32), caudate, putamen, and
thalamus. EFFECT OF DIAGNOSIS Response inhibition: SCZs and SIBs showed decreased activation in ACC during ‘No-Go’ trials (BA24/32; _xyz_=0,15,36; _k_=70; _Z_=4.17, _P_=0.00002,
FWE-SVC=0.02; Figure 2a). Planned comparison analyses confirmed decreased activation in the ACC of SIBs (Figure 2b; _P_=0.007; FWE-SVC=0.001) when compared only with NCs, but showed no
significant difference between SIBs and SCZs (_P_>0.2). This decreased engagement is not an effect of more frequent errors in SCZs and SIBs across all the trials, as these analyses were
based only on correct trials. Interference monitoring and suppression: SCZs showed decreased activation in ACC (BA24), bilateral DLPFC (BA9), right supplementary motor area (BA6), bilateral
caudate, and thalamus (Supplementary Figure S4). None of these clusters, however, showed a significant difference between NCs and SIBs. The effects of diagnosis on task-related response
associate with response inhibition, and interference monitoring and suppression were confirmed in the matched subsample (see Supplementary Figure S6). PPI: During response inhibition, there
was increased PPI between ACC and a network of brain regions that included bilateral VLPFC, right DLPFC, posterior parietal (BA7), thalamus, and putamen (see Supplementary Figure S5)
relative to the rest of the brain. Both SCZs and SIBs showed marginally significant greater PPI during response inhibition in the left lateral-PFC (BA10/46; _xyz_=−39,48,24, _k_=6, _Z_=3.46,
_P_=0.00027, FWE-SVC=0.09; Figure 3) when compared to NCs. Planned comparison analyses indicated increased PPI of ACC with this region in SIBs (_P_=0.02) when compared with NCs. A further
analysis in the matched subsample confirmed the significance of this comparison (_P_=0.003, FWE-SVC=0.03; see Supplementary Figure S7). Robust-variance corrected analyses confirmed the
effect of diagnosis on No-Go activation and PPI during response inhibition (_P_=0.001). All the imaging results were similar and statistically significant after excluding SIBs on
psychotropic drugs (data not shown). We did not find any effect of diagnosis on the context-dependent coupling of ACC during interference monitoring and suppression. PPI of ACC was weakly
but positively correlated (_r_=0.12, _P_=0.05) with No-Go accuracy in NCs as well as in SIBs (_r_=0.25, _P_=0.04 after removing one outlier). We did not find other brain response–behavior
correlations in any diagnosis group. Treatment variables were not significantly associated with brain responses in SCZs. DISCUSSION The present study investigated whether brain responses
underlying cognitive control, particularly those germane to ACC, are associated with genetic liability for schizophrenia in a cohort of SIBs. Behaviorally, SIBs showed decreased accuracy
relative to NCs during response inhibition. Similar to SCZs, SIBs also showed decreased dorsal ACC activation during this task in comparison to NCs, and this physiological difference
occurred when they were not making errors. Furthermore, both SIBs and SCZs showed altered context-related modulation of functional connectivity, as measured by the PPI, between ACC and
lateral-PFC during response inhibition relative to NCs. In our study, SIBs showed overall decreased accuracy during response inhibition relative to NCs. Most studies have identified impaired
response inhibition in SCZs during a Stop-task (Badcock et al, 2002; Enticott et al, 2006), although negative findings have also been reported (Rubia et al, 2001). A recent study using
masked negative priming has reported that voluntary but not unconscious response inhibition is impaired in schizophrenia (Huddy et al, 2009) suggesting, together with another study based on
a Stop-task (Badcock et al, 2002), that impairments in response inhibition are not due to slower processing speed _per se_. Decreased performance on neuropsychological tests that tap into
attentional control functions has been reported in unaffected relatives of patients with schizophrenia (Cannon et al, 1994; Goldberg et al, 1995). More specifically, Groom et al, (2008)
found a longer latency of response inhibition on the Hayling Sentence Completion Task in both siblings and patients. Notably, we observed similar behavioral impairments during response
inhibition in unaffected siblings also in the context of similar performance on the other task conditions, thus suggesting a possible role of this measure in genetic liability to
schizophrenia (see below). Most importantly, our study identified altered neural correlates of response inhibition processing in unaffected siblings of patients with schizophrenia.
Converging evidence from multi-modal neuroimaging studies indicates altered ACC function in siblings of patients with schizophrenia. Most studies show decreased ACC activation in siblings on
a variety of executive cognition tasks during fMRI (Callicott et al, 2003; Filbey et al, 2008; Sepede et al, 2010; Whalley et al, 2004), although some studies have reported increased ACC
activity (Thermenos et al, 2004) or no difference (Becker et al, 2008; MacDonald et al, 2006; Vink et al, 2006; Zandbelt et al, 2011). We found decreased activation in ACC specifically
during response inhibition in unaffected siblings relative to normal controls. These findings are consistent with previous studies in patients with schizophrenia (Arce et al, 2006; Ford et
al, 2004; Kaladjian et al, 2007; Rubia et al, 2001) and in their siblings (Blackwood et al, 1999). Notably, to exclude that the present physiologic results were affected by behavioral
differences, we analyzed only correct trials and confirmed these results in a sample that was matched for task performances. Thus, our data suggest that the abnormal ACC engagement most
likely relates to the neural strategy for performing response inhibition, and not to its success or failure. Moreover, whereas our sample of SIBs included a minority of individuals with a
past history of psychiatric treatment for nonpsychotic disorders, none were in continuing treatment and the groups also did not differ in smoking frequency, suggesting that obvious secondary
confounders are not likely explanations for the results. Interestingly, siblings of patients also have functional alterations in PFC (Callicott et al, 2003; Thermenos et al, 2004) and in
PFC connectivity (Rasetti et al, 2011), and we demonstrated alterations in ACC-PFC coupling during more demanding conditions of cognitive control. ACC is functionally connected with DLPFC
within a fronto-cingulate-parietal network (Wang et al, 2010) that supports cognitive control (Stevens et al, 2009). Recently, Brazdil reported hierarchically organized intrinsic effective
connectivity within an ACC-PFC circuit, so that ACC modulates DLPFC during attentional tasks (Brazdil et al, 2007). Other reports have suggested a bidirectional connectivity between these
two regions as being critical for optimal attentional control (Wang et al, 2010). Increased connectivity between PFC and ACC found in our patient and sibling samples could reflect a neural
processing strategy to compensate for suboptimal function of these two regions as suggested by other studies in patients with schizophrenia and aging (Sambataro et al, 2009, 2012). This may
account for the correct performance observed during the analyzed trials. Indeed, we did find a weak positive correlation between ACC-PFC connectivity and better performance during response
inhibition in normal controls as well as in the siblings. The lack of a significant PFC-ACC connectivity–performance correlation in patients may be suggestive of failed compensation due to
reduced PFC efficiency (Callicott et al, 2003). ACC-PFC coupling is crucial to enhance signal relative to noise and filter out irrelevant information. Primate studies have shown that the ACC
projection neurons innervate excitatory pyramidal neurons in PFC responsible for response selection via large boutons with inhibitory neurons that come into play at high cognitive task
demands (Medalla et al, 2009). At low cognitive loads, pyramidal neurons in BA9 are modulated only via PFC pathways. When cognitive demands become higher, ACC neurons enhance cognitive
control both by, (1) suppressing excessive PFC noise through large boutons projecting on calbindin inhibitory neurons, and (2) reversing decisions via greater PFC activity of previous
signals as well as enhancing new signals through activity of large boutons (Medalla et al, 2009). Therefore, decreased ACC function might result in suppressed activity of PFC inhibitory
interneurons, and consequently decreased cognitive control during challenging cognitive tasks (Medalla et al, 2009). Alternatively, decreased ACC activation and increased coupling within the
PFC-ACC network may suggest that siblings, similarly to patients with schizophrenia, are not optimally engaged in the task and examine each trial on a trial-by-trial basis, and possibly
have diminished prepotent response prior to the No-Go trials when compared to normal controls (Ford et al, 2004; Kaladjian et al, 2007). Unfortunately, there was no way we could assess the
prepotent response behaviorally with the task paradigm we used. In our study, altered ACC activity and connectivity were not unduly driven by group differences in task performance, as we
only included the brain responses related to correct trials in the imaging analyses. Furthermore, we were able to replicate these results in a subsample matched for performance and
demographic variables across diagnostic groups, thus excluding any biasing effects. Of note, we did not find altered interference processing in unaffected siblings. Previous studies did not
identify altered ACC response in healthy first-degree relatives, but decreased lateral-PFC response together with poor behavioral responses during conflict tasks including the Stroop-task
(Becker et al, 2008; MacDonald et al, 2006). Performance differences across diagnostic groups, the presence of psychiatric disorders in relatives, and age heterogeneity due to the inclusion
of first-degree relatives including offspring, parents, and siblings may explain differences between our findings and those of these earlier reports. Furthermore, the Stroop paradigm elicits
a stronger conflict relative to Eriksen's flanker congruency task (see above). These tasks differ critically in the underlying processing demands and sensitivity to conflict. In the
Stroop-task, interference consists of color-naming mismatch (Stroop effect) that is different from the flanker task where conflict is based on location (flanker incongruency effect). In the
Stroop-task, conflict between different categories translates into engagement of additional neural processes which require greater effort to adjust responses to contextual information (Lehle
and Hubner, 2008). The candidate intermediate phenotypes reported would be expected to be present in high-risk individuals. We could not test this possibility as the average age of the
unaffected siblings in our sample was beyond the typical age of risk for developing schizophrenia. Future studies with siblings of a younger age could help inform this issue. In conclusion,
the present study shows that unaffected siblings of patients with schizophrenia evince altered brain function during response inhibition. Our results suggest that impaired response
inhibition, which is associated with altered function of an ACC-PFC network, is at the least familiar and may reflect genetic risk for schizophrenia and as such may be an intermediate
phenotype for genetic studies of schizophrenia. Further work addressing the heritability of this phenotype is warranted. REFERENCES * Arce E, Leland DS, Miller DA, Simmons AN, Winternheimer
KC, Paulus MP (2006). Individuals with schizophrenia present hypo- and hyperactivation during implicit cueing in an inhibitory task. _Neuroimage_ 32: 704–713. Article PubMed Google Scholar
* Badcock JC, Michie PT, Johnson L, Combrinck J (2002). Acts of control in schizophrenia: dissociating the components of inhibition. _Psychol Med_ 32: 287–297. Article CAS PubMed Google
Scholar * Badre D, Wagner AD (2004). Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. _Neuron_ 41: 473–487.
Article CAS PubMed Google Scholar * Becker TM, Kerns JG, Macdonald AW, Carter CS (2008). Prefrontal dysfunction in first-degree relatives of schizophrenia patients during a stroop task.
_Neuropsychopharmacology_ 33: 2619–2625. Article PubMed Google Scholar * Blackwood DH, Glabus MF, Dunan J, O’Carroll RE, Muir WJ, Ebmeier KP (1999). Altered cerebral perfusion measured by
SPECT in relatives of patients with schizophrenia. Correlations with memory and P300. _Br J Psychiatry_ 175: 357–366. Article CAS PubMed Google Scholar * Blasi G, Goldberg TE, Weickert
T, Das S, Kohn P, Zoltick B _et al_ (2006). Brain regions underlying response inhibition and interference monitoring and suppression. _Eur J Neurosci_ 23: 1658–1664. Article PubMed Google
Scholar * Brazdil M, Mikl M, Marecek R, Krupa P, Rektor I (2007). Effective connectivity in target stimulus processing: a dynamic causal modeling study of visual oddball task. _Neuroimage_
35: 827–835. Article PubMed Google Scholar * Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR (2003). Complexity of prefrontal cortical dysfunction in
schizophrenia: more than up or down. _Am J Psychiatry_ 160: 2209–2215. Article PubMed Google Scholar * Cannon TD, Zorrilla LE, Shtasel D, Gur RE, Gur RC, Marco EJ _et al_ (1994).
Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. _Arch Gen Psychiatry_ 51: 651–661. Article CAS PubMed Google Scholar * Carter CS,
MacDonald AW, Ross LL, Stenger VA (2001). Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. _Am J
Psychiatry_ 158: 1423–1428. Article CAS PubMed Google Scholar * Enticott PG, Ogloff JRP, Bradshaw JL (2006). Associations between laboratory measures of executive inhibitory control and
self-reported impulsivity. _Pers Indiv Differ_ 41: 285–294. Article Google Scholar * Fallgatter AJ, Bartsch AJ, Zielasek J, Herrmann MJ (2003). Brain electrical dysfunction of the anterior
cingulate in schizophrenic patients. _Psychiatry Res_ 124: 37–48. Article PubMed Google Scholar * Filbey FM, Russell T, Morris RG, Murray RM, McDonald C (2008). Functional magnetic
resonance imaging (fMRI) of attention processes in presumed obligate carriers of schizophrenia: preliminary findings. _Ann Gen Psychiatry_ 7: 18. Article PubMed PubMed Central Google
Scholar * Ford JM, Gray M, Whitfield SL, Turken U, Glover G, Faustman WO _et al_ (2004). Acquiring and inhibiting prepotent responses in schizophrenia: event-related brain potentials and
functional magnetic resonance imaging. _Arch Gen Psychiatry_ 61: 119–129. Article PubMed Google Scholar * Goldberg TE, Torrey EF, Gold JM, Bigelow LB, Ragland RD, Taylor E _et al_ (1995).
Genetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder. _Schizophr Res_ 17: 77–84. Article CAS PubMed
Google Scholar * Goldberg TE, Weinberger DR (1988). Probing prefrontal function in schizophrenia with neuropsychological paradigms. _Schizophr Bull_ 14: 179–183. Article CAS PubMed
Google Scholar * Gottesman II, Gould TD (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. _Am J Psychiatry_ 160: 636–645. Article PubMed Google Scholar
* Groom MJ, Jackson GM, Calton TG, Andrews HK, Bates AT, Liddle PF _et al_ (2008). Cognitive deficits in early-onset schizophrenia spectrum patients and their non-psychotic siblings: a
comparison with ADHD. _Schizophr Res_ 99: 85–95. Article CAS PubMed Google Scholar * Huddy VC, Aron AR, Harrison M, Barnes TR, Robbins TW, Joyce EM (2009). Impaired conscious and
preserved unconscious inhibitory processing in recent onset schizophrenia. _Psychol Med_ 39: 907–916. Article CAS PubMed Google Scholar * Kaladjian A, Jeanningros R, Azorin JM, Grimault
S, Anton JL, Mazzola-Pomietto P (2007). Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. _Schizophr Res_ 97: 184–193. Article
PubMed Google Scholar * Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS (2004). Anterior cingulate conflict monitoring and adjustments in control. _Science_ 303: 1023–1026.
Article CAS PubMed Google Scholar * Koski L, Paus T (2000). Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. _Exp
Brain Res_ 133: 55–65. Article CAS PubMed Google Scholar * Lehle C, Hubner R (2008). On-the-fly adaptation of selectivity in the flanker task. _Psychon Bull Rev_ 15: 814–818. Article
PubMed Google Scholar * MacDonald AW, Becker TM, Carter CS (2006). Functional magnetic resonance imaging study of cognitive control in the healthy relatives of schizophrenia patients.
_Biol Psychiatry_ 60: 1241–1249. Article PubMed Google Scholar * Manoach DS, Halpern EF, Kramer TS, Chang Y, Goff DC, Rauch SL _et al_ (2001). Test-retest reliability of a functional MRI
working memory paradigm in normal and schizophrenic subjects. _Am J Psychiatry_ 158: 955–958. Article CAS PubMed Google Scholar * Mansouri FA, Tanaka K, Buckley MJ (2009).
Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. _Nat Rev Neurosci_ 10: 141–152. Article CAS PubMed Google Scholar * Medalla M, Barbas
H (2009). Synapses with inhibitory neurons differentiate anterior cingulate from dorsolateral prefrontal pathways associated with cognitive control. _Neuron_ 61: 609–620. Article CAS
PubMed PubMed Central Google Scholar * Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009). Meta-analysis of 41 functional neuroimaging studies of executive function in
schizophrenia. _Arch Gen Psychiatry_ 66: 811–822. Article PubMed PubMed Central Google Scholar * Rasetti R, Sambataro F, Qiang C, Callicott JH, Mattay VS, Weinberger DR (2011). Altered
cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804. _Arch Gen Psychiatry_ 68: 1207–1217. Article PubMed Google Scholar * Rubia K,
Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A _et al_ (2001). An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. _Schizophr Res_
52: 47–55. Article CAS PubMed Google Scholar * Sambataro F, Reed JD, Murty VP, Das S, Tan HY, Callicott JH _et al_ (2009). Catechol-O-methyltransferase valine(158)methionine polymorphism
modulates brain networks underlying working memory across adulthood. _Biol Psychiatry_ 66: 540–548. Article CAS PubMed PubMed Central Google Scholar * Sambataro F, Safrin M, Lemaitre
HS, Steele SU, Das SB, Callicott JH _et al_ (2012). Normal aging modulates prefrontoparietal networks underlying multiple memory processes. _Eur J Neurosci_ 36: 3559–3567. Article PubMed
PubMed Central Google Scholar * Sepede G, Ferretti A, Perrucci MG, Gambi F, Di Donato F, Nuccetelli F _et al_ (2010). Altered brain response without behavioral attention deficits in
healthy siblings of schizophrenic patients: an event-related fMRI study. _Neuroimage_ 49: 1080–1090. Article PubMed Google Scholar * Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD (2009).
Brain network dynamics during error commission. _Hum Brain Mapp_ 30: 24–37. Article PubMed PubMed Central Google Scholar * Swan GE, Carmelli D (2002). Evidence for genetic mediation of
executive control: a study of aging male twins. _J Gerontol B Psychol Sci Soc Sci_ 57: P133–P143. Article PubMed Google Scholar * Swick D, Turken AU (2002). Dissociation between conflict
detection and error monitoring in the human anterior cingulate cortex. _Proc Natl Acad Sci USA_ 99: 16354–16359. Article CAS PubMed Google Scholar * Tan HY, Callicott JH, Weinberger DR
(2008). Intermediate phenotypes in schizophrenia genetics redux: is it a no brainer? _Mol Psychiatry_ 13: 233–238. Article CAS PubMed Google Scholar * Thermenos HW, Seidman LJ, Breiter
H, Goldstein JM, Goodman JM, Poldrack R _et al_ (2004). Functional magnetic resonance imaging during auditory verbal working memory in nonpsychotic relatives of persons with schizophrenia: a
pilot study. _Biol Psychiatry_ 55: 490–500. Article PubMed Google Scholar * Toulopoulou T, Goldberg TE, Mesa IR, Picchioni M, Rijsdijk F, Stahl D _et al_ (2010). Impaired intellect and
memory: a missing link between genetic risk and schizophrenia? _Arch Gen Psychiatry_ 67: 905–913. Article PubMed Google Scholar * Vink M, Ramsey NF, Raemaekers M, Kahn RS (2006). Striatal
dysfunction in schizophrenia and unaffected relatives. _Biol Psychiatry_ 60: 32–39. Article PubMed Google Scholar * Wang L, Liu X, Guise KG, Knight RT, Ghajar J, Fan J (2010). Effective
connectivity of the fronto-parietal network during attentional control. _J Cogn Neurosci_ 22: 543–553. Article PubMed Google Scholar * Weinberger DR, Egan MF, Bertolino A, Callicott JH,
Mattay VS, Lipska BK _et al_ (2001). Prefrontal neurons and the genetics of schizophrenia. _Biol Psychiatry_ 50: 825–844. Article CAS PubMed Google Scholar * Whalley HC, Simonotto E,
Flett S, Marshall I, Ebmeier KP, Owens DG _et al_ (2004). fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. _Brain_ 127: 478–490. Article
CAS PubMed Google Scholar * Zandbelt BB, van Buuren M, Kahn RS, Vink M (2011). Reduced proactive inhibition in schizophrenia is related to corticostriatal dysfunction and poor working
memory. _Biol Psychiatry_ 70: 1151–1158. Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS This research was supported by the Intramural Research Program of the National
Institute of Mental Health, NIH, Bethesda, MD 20892, USA. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Brain Center for Motor and Social Cognition, Istituto Italiano di Tecnologia@UNIPr,
Parma, Italy Fabio Sambataro * Genes, Cognition, and Psychosis Program, Clinical Brain Disorders Branch, National Institute of Mental Health Intramural Research Program, National Institutes
of Health, Bethesda, MD, USA Fabio Sambataro, Venkata S Mattay, Kristina Thurin, Martin Safrin, Roberta Rasetti, Giuseppe Blasi, Joseph H Callicott & Daniel R Weinberger * Lieber
Institute for Brain Development, Johns Hopkins Medical Campus, Baltimore, MD, USA Venkata S Mattay & Daniel R Weinberger Authors * Fabio Sambataro View author publications You can also
search for this author inPubMed Google Scholar * Venkata S Mattay View author publications You can also search for this author inPubMed Google Scholar * Kristina Thurin View author
publications You can also search for this author inPubMed Google Scholar * Martin Safrin View author publications You can also search for this author inPubMed Google Scholar * Roberta
Rasetti View author publications You can also search for this author inPubMed Google Scholar * Giuseppe Blasi View author publications You can also search for this author inPubMed Google
Scholar * Joseph H Callicott View author publications You can also search for this author inPubMed Google Scholar * Daniel R Weinberger View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Daniel R Weinberger. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL
INFORMATION Supplementary Information accompanies the paper on the Neuropsychopharmacology website SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIALS (DOC 564 KB) POWERPOINT SLIDES POWERPOINT
SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sambataro, F., Mattay, V.,
Thurin, K. _et al._ Altered Cerebral Response During Cognitive Control: A Potential Indicator of Genetic Liability for Schizophrenia. _Neuropsychopharmacol_ 38, 846–853 (2013).
https://doi.org/10.1038/npp.2012.250 Download citation * Received: 09 August 2012 * Revised: 18 October 2012 * Accepted: 08 November 2012 * Published: 05 December 2012 * Issue Date: April
2013 * DOI: https://doi.org/10.1038/npp.2012.250 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * response inhibition * functional magnetic resonance
imaging * anterior cingulate * heritability * intermediate phenotype