Play all audios:
ABSTRACT Genetic deletion of the neurokinin 1 receptor (NK1R) has been shown to decrease the reinforcing properties of opioids, but it is unknown whether pharmacological NK1R blockade has
the same effect. Here, we examined the effect of L822429, a rat-specific NK1R antagonist, on the reinforcing properties of heroin in rats on short (1 h: ShA) or long (12 h: LgA) access to
intravenous heroin self-administration. ShA produces heroin self-administration rates that are stable over time, whereas LgA leads to an escalation of heroin intake thought to model
important dependence-related aspects of addiction. L822429 reduced heroin self-administration and the motivation to consume heroin, measured using a progressive-ratio schedule, in both ShA
and LgA rats. L822429 also decreased anxiety-like behavior in both groups, measured on the elevated plus maze, but did not affect mechanical hypersensitivity observed in LgA rats. Expression
of _TacR1_ (the gene encoding NK1R) was decreased in reward- and stress-related brain areas both in ShA and LgA rats compared with heroin-naïve rats, but did not differ between the two
heroin-experienced groups. In contrast, passive exposure to heroin produced increases in _TacR1_ expression in the prefrontal cortex and nucleus accumbens. Taken together, these results show
that pharmacological NK1R blockade attenuates heroin reinforcement. The observation that animals with ShA and LgA to heroin were similarly affected by L822429 indicates that the SP/NK1R
system is not specifically involved in neuroadaptations that underlie escalation resulting from LgA self-administration. Instead, the NK1R antagonist appears to attenuate acute, positively
reinforcing properties of heroin and may be useful as an adjunct to relapse prevention in detoxified opioid-dependent subjects. SIMILAR CONTENT BEING VIEWED BY OTHERS FACILITATING MGLUR4
ACTIVITY REVERSES THE LONG-TERM DELETERIOUS CONSEQUENCES OF CHRONIC MORPHINE EXPOSURE IN MALE MICE Article 21 December 2020 AM6527, A NEUTRAL CB1 RECEPTOR ANTAGONIST, SUPPRESSES OPIOID
TAKING AND SEEKING, AS WELL AS COCAINE SEEKING IN RODENTS WITHOUT AVERSIVE EFFECTS Article Open access 10 April 2024 NOP RECEPTOR ANTAGONISM ATTENUATES REINSTATEMENT OF ALCOHOL-SEEKING
THROUGH MODULATION OF THE MESOLIMBIC CIRCUITRY IN MALE AND FEMALE ALCOHOL-PREFERRING RATS Article 20 July 2021 INTRODUCTION Studies with genetically modified mice have suggested that
substance P (SP) and its preferred neurokinin 1 receptor (NK1R) have a significant role in opioid reinforcement. Morphine reward, as measured by conditioned place preference (CPP), is absent
in NK1R−/− mice (Murtra et al, 2000), and this is associated with decreased rates of morphine self-administration compared with wild-type mice (Ripley et al, 2002). Neurotoxin lesions
targeting NK1 receptor-containing neurons in the amygdala, but not the nucleus accumbens (NAc), prevented the development of morphine CPP (Gadd et al, 2003), suggesting a role of NK1Rs in
the amygdala in mediating opioid reward. A recent study using intracranial self-stimulation (ICSS) has provided further support for a role of NK1Rs in the rewarding effects of opioids, by
demonstrating that NK1R blockade attenuated the ability of morphine to lower ICSS thresholds (Robinson et al, 2012). Although these data collectively support the possibility that NK1R
antagonists may be useful for the treatment of opioid addiction, demonstration that pharmacological NK1R blockade suppresses self-administration of opioids is lacking. Initial findings
indicated a selective role of NK1Rs in opioid reward and self-administration, in that neither CPP for cocaine (Murtra et al, 2000) nor self-administration of this drug (Ripley et al, 2002)
were affected by a genetic deletion of the NK1R. More recently, however, data have also emerged in support of a role for NK1Rs in the rewarding properties of alcohol. For example, NK1R−/−
mice consume less alcohol in a two-bottle free-choice paradigm, and do not exhibit alcohol CPP (George et al, 2008; Thorsell et al, 2010). The decreased alcohol consumption and reward found
in NK1R-null mutants is unlikely to be the result of compensatory developmental processes caused by constitutive gene deletion because these behavioral effects were replicated by NK1R
antagonist administration in wild-type animals (Thorsell et al, 2010). Taken together, these observations have provided support for the hypothesis that NK1R antagonism may have utility as a
pharmacotherapy for alcoholism. Initial human translation of these findings was obtained through the demonstration that an NK1R antagonist blocked alcohol craving in recently detoxified
alcohol-dependent patients (George et al, 2008). Over time, the motivation for drug seeking and taking is hypothesized to undergo an allostatic shift. Positive reinforcement predominates
during the early stages of the addictive process, but the ability of drug intake to alleviate negative emotional states that emerge during abstinence becomes increasingly important with a
prolonged history of drug abuse (Edwards and Koob, 2010). NK1R antagonists display antistress and antianxiety effects in a wide range of animal models (Ebner and Singewald, 2006), and
thereby have the potential to obviate the stress-reducing, negatively reinforcing effects of addictive drugs. Accordingly, systemic administration of an NK1R antagonist blocked
stress-induced reinstatement of alcohol seeking in rats (Schank et al, 2011). Thus, NK1R antagonist treatment may have a potential for therapeutic utility in addictive disorders through dual
mechanisms, by reducing both negative and positive reinforcing properties. Limited access (1 h per day) to heroin produces stable levels of drug intake. However, extended access (6–23 h per
day) to heroin self-administration in rats can lead to escalated self-administration (Ahmed et al, 2000; Vendruscolo et al, 2011). It has been hypothesized that during limited access
conditions, drug intake is mainly driven by the positive reinforcing properties of heroin, whereas during extended access, brain stress systems are recruited and negative reinforcement
mechanisms predominate (Edwards and Koob, 2010). Here, we asked whether pharmacological blockade of NK1Rs would be able to reduce heroin reinforcement. Because NK1 receptors display
considerable divergence between species, NK1R antagonists developed for activity at the human NK1R may possess limited efficacy in rat studies (Leffler et al, 2009). We therefore used
L822429, a brain-penetrant NK1R antagonist with high affinity for the rat NK1R that produces anxiolytic-like effects after systemic administration (Ebner et al, 2004; Ebner et al, 2008;
Singewald et al, 2008). We examined the effects of L822429 on heroin self-administration in short (ShA; 1 h per day) and long access (LgA; 12 h per day) rats. Furthermore, we tested the
effect of NK1R antagonism on measures of negative emotional-like behavior associated with heroin withdrawal: increased anxiety-like behavior and mechanical sensitivity during withdrawal from
heroin self-administration in ShA and LgA rats. Finally, to determine neurokinin system-related neuroadaptations associated with limited _vs_ excessive heroin intake, _TacR1_ transcript
levels were also assessed in several stress/reward-related brain areas in ShA and LgA rats, and in rats passively receiving repeated moderate doses of heroin or escalating doses of heroin.
MATERIALS AND METHODS SUBJECTS Adult male Wistar rats (Charles River, Raleigh, NC), weighing between 225 and 275 g at the beginning of the experiments, were housed in groups of 2–3 per cage
in a temperature-controlled (22 °C) vivarium on a 12 h/12 h light/dark cycle (lights on at 1800 hours) with _ad libitum_ access to food and water. The animals were allowed to acclimate to
the animal facility for at least 7 days before surgery. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the
Institutional Animal Care and Use Committee of The Scripps Research Institute. ELEVATED PLUS MAZE The elevated plus maze testing was carried out as described previously (Vendruscolo et al,
2003) during withdrawal, just before subsequent self-administration sessions. The apparatus had four elevated arms (52 cm above the floor), 50 cm long and 10 cm wide, set in a cross-like
arrangement, with two opposite arms enclosed by 40 cm high opaque walls and two open arms with a lip (1 mm thick and 5 mm high). A central platform at their intersection (10 × 13.5 cm2)
permitted access to any of the four arms. The central platform was under <10 lx illumination. Each rat was placed on the central platform facing an open arm. The behavior of each animal
was recorded for 5 min. The percentage of time spent in the open arms relative to the combined time spent in both the open and closed arms, and the percentage of entries in the open arms
were used as indices of anxiety-like behavior. Closed-arm entries were used as an index of general locomotion. MEASUREMENT OF MECHANICAL SENSITIVITY: PAW WITHDRAWAL THRESHOLD Mechanical
nociceptive testing was conducted during withdrawal, just before subsequent self-administration sessions according to methods by Chaplan et al (1994) as described previously (Edwards et al,
2012). Briefly, rats were acclimated for 15 min in elevated cages with a wire mesh floor. A series of von Frey filaments were applied perpendicularly to the plantar surface of the hind paw
for 3 s. A sharp withdrawal of the hind paw indicated a positive response. The stimulus was incrementally increased until a positive response was obtained, and then decreased until a
negative result was observed to determine a pattern of responses to apply to the statistical method of Dixon (1980). Baseline mechanical nociceptive thresholds were similar to those reported
for the ages of rats employed in this study (Ririe and Eisenach, 2006). SURGERY Rats were anesthetized with isoflurane (1.5–2.5%) and prepared with chronic intravenous silastic catheters
(Dow Corning, Midland, MI) into the right jugular vein (Vendruscolo et al, 2011). The catheter was secured to the vein with suture thread and passed subcutaneously to exit dorsally on the
animal’s back. After surgery, the catheters were flushed daily with 0.2 ml of a sterile solution containing heparinized (30 USP units/ml) saline and the antibiotic Cefazolin. Rats were
allowed to recover for 7 days before behavioral testing. SELF-ADMINISTRATION PROCEDURE Self-administration sessions were conducted in standard operant conditioning chambers (Med Associates).
Self-administration sessions were carried out as described previously (Vendruscolo et al, 2012). Briefly, rats were trained to press one of the two levers (the active lever) on a
fixed-ratio 1 (FR1) schedule of reinforcement (each response resulted in fluid delivery) to obtain 0.1 ml of heroin (60 μg/kg per infusion) in 1-h sessions. Reinforced responses were
followed by a 20-s timeout period, in which a cue-light (above the active lever) was turned on and lever presses did not result in additional injections. During the acquisition of heroin
self-administration, food and water were not available to the rats while in the test chambers. After the acquisition of heroin self-administration, rats were split into two groups matched
for lever press in the last three sessions of the acquisition phase and were given 1 h (ShA) or 12 h (LgA) of access to heroin self-administration. In this phase, all groups were allowed to
nosepoke for food on an FR3 schedule and water on an FR1 schedule while in the test chambers. (Vendruscolo et al, 2011). PHARMACOLOGICAL TESTING The NK1 receptor antagonist L822429 was
prepared as a suspension for systemic administration (intraperitoneally) in 45% (w/v) 2-hydroxypropyl _β_-cyclodextrin. Rats were administered vehicle or L822429 (15 and 30 mg/kg in 45%
(w/v) 2-hydroxypropyl _β_-cyclodextrin at 2 ml/kg). Doses and pretreatment times were based on previous studies (Schank et al, 2011). For the self-administration studies, rats were given
L822429 1 h before session. ShA and LgA rats received all three doses (0, 15, and 30 mg/kg) in a Latin Square design and tested for heroin self-administration on an FR1 schedule. A regular
FR1 heroin self-administration session without drug treatment was performed between testing days. Operant self-administration on FR1 requires minimal effort from the animal to obtain heroin
and was considered herein a measure of intake. After the FR1 treatment phase was completed, the same ShA and LgA rats were then tested on a progressive-ratio (PR) schedule, under which the
number of lever presses necessary to obtain the next reinforcement increased progressively according to the following progression: 1, 1, 2, 2, 2, 3, 3, 4, 4, 5, 5, 6, 6, 7, 7, 8, 9, 9, 10,
11, 11, 12, 13, 14, 14, etc. (Vendruscolo et al, 2012). The PR session lasted 6 h. Rats received 0 and 30 mg/kg in a Latin Square design (with a regular FR1 session between the PR sessions).
In this test, the workload (‘price’) for the next reinforcement increases progressively and is considered herein a measure of motivation. Rats from a separate cohort were trained to
self-administer heroin and split in ShA and LgA groups as described above. When escalated levels of heroin intake were established for the LgA group (ie, significantly escalated intake over
1 week), ShA and LgA rats were given L822429 (0 or 30 mg/kg, intraperitoneally, 1 h before testing) and tested in the elevated plus maze. Rats were tested only once in this test with a
single dose (ie, either 0 or 30 mg/kg). The same animals were tested for PWT afterward (ie, 1.5 h post injection). After 2 days, rats received the other dose (different from the first day)
and tested for PWT only. Testing was performed during acute withdrawal (12–23 h post heroin self-administration, corresponding to the time of day when ShA and LgA animals would typically be
self-administering heroin). PASSIVE HEROIN ADMINISTRATION To determine the effects of passively administered heroin on gene expression, we chronically treated rats with heroin for 18 days,
either at a constant level (repeated heroin group: 1.25 mg/kg subcutaneously, days 1–18) or via an escalating dose regimen (escalated heroin group: 1.25 mg/kg subcutaneously, days 1–3; 2.5
mg/kg, days 4–6; 5 mg/kg, days 7–9; 10 mg/kg, days 10–12; 20 mg/kg, days 13–18). Animals receiving chronic saline served as a control group (repeated saline group: 1 ml/kg subcutaneously,
days 1–18). All treatments were administered once per day. Paw withdrawal thresholds (PWTs) were measured on day 18 immediately before the final injection, and animals were killed 24 h
following this last injection. REVERSE TRANSCRIPTION AND QUANTITATIVE PCR Prefrontal cortex (PFC), NAc, BNST, amygdala, and hippocampus (Hipp) were collected 20, 10, and 24 h after ShA
self-administration, LgA self-administration, and passive heroin administration, respectively. For self-administration groups, this time point roughly corresponded to the typical withdrawal
time between sessions. RNA was extracted and purified from brain tissue using the PureLink RNA Mini Kit (Ambion, Austin, TX) following the manufacturer’s instructions. cDNA was reverse
transcribed from total RNA using the Superscript III First Strand Synthesis System (Invitrogen, Carlsbad, CA). Gene expression levels were determined by quantitative polymerase chain
reaction (qPCR) using a TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA). Reactions were carried out as described previously (Vendruscolo et al, 2012) and cDNA
concentrations of _TacR1_ were calculated according to the relative quantification (ddCt) method, corrected for differences in PCR efficiency, normalized to glyceraldehyde-3-phosphate
dehydrogenase (Gapdh). Primers used were as follows: TaqMan qPCR utilized commercially available _TacR1_ (Rn00562004_ml) and _Gapdh_ (Rn99999916_s1) primer/probe sets (Applied Biosystems),
with PCR conditions according to the manufacturer’s protocol. STATISTICAL ANALYSIS All data are expressed as means and standard errors of the mean (SEM). Data were analyzed using a one-way
analysis of variance (ANOVA) with treatment (repeated saline, repeated heroin, and escalated heroin) as a between-subjects factor or using a two-way repeated measure ANOVA, with group (ShA
and LgA) as the between-subjects factor and treatment (0, 15, and 30 mg/kg or 0 and 30 mg/kg) and session or time as the within-subjects factor. For elevated plus maze, data were analyzed
using a two-way ANOVA, with group (ShA and LgA) and treatment (0 and 30 mg/kg) as the between-subjects factors. Differences in PWTs across tests were analyzed using two-way ANOVA with group
(naïve, ShA, and LgA) as the between-subjects factor and treatment (0 and 30 mg/kg) as the within-subjects factor. For _TacR1_ mRNA levels, data were analyzed using a one-way ANOVA with
group (naïve, ShA, and LgA; repeated saline, repeated heroin, and escalated heroin) as the between-subjects factor. When appropriate, _post hoc_ comparisons were performed using the Fisher’s
least significance difference (LSD) test. The probability for a Type 1 error for all significance testing was set at _P_⩽0.05. RESULTS NK1R ANTAGONISM DECREASES HEROIN SELF-ADMINISTRATION
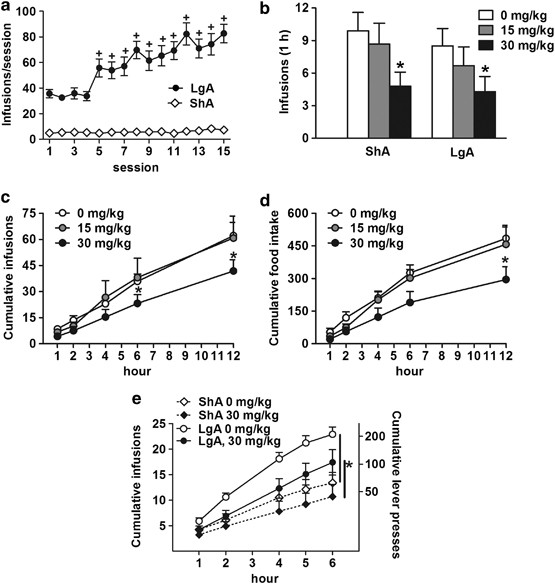
AND MOTIVATION TO OBTAIN HEROIN IN BOTH SHA AND LGA RATS ShA rats displayed stable levels of heroin self-administration (Figure 1a), whereas LgA rats displayed an escalation of intake (group
× session interaction: F(14,518)=15.8, _P_<0.0001) that became significant by session 5 (_P_<0.0001). Systemic injection of 30 mg/kg L822429 significantly decreased heroin
self-administration in the first hour under an FR1 schedule of reinforcement (Figure 1b) in both ShA and LgA rats (treatment effect: F(2,36)=7.7, _P_<0.005 followed by Fischer’s LSD _post
hoc_, _P_<0.001). The statistical analysis also revealed a time × treatment interaction (F(8, 72)=2.1, _P_<0.05) for cumulative heroin infusions in the entire 12-h session, with
self-administration significantly decreased by 30 mg/kg L822429 compared with vehicle (_P_<0.005) at 6 and 12 h (Figure 1c). Food intake during the heroin self-administration session was
also significantly affected by the treatment (F(2,36)=11.3, _P_<0.001). The highest dose of L822429 significantly decreased food intake by ShA and LgA rats (_P_<0.05; Figure 1d).
However, water intake was unaffected by the treatment, suggesting that the drug did not suppress overall motor behavior (Table 1). In the PR, LgA rats displayed a greater motivation for
heroin, measured by the number of drug infusions earned in the 6-h session, compared with ShA rats (overall group effect: F(1,18)=10.0, _P_<0.005). In all, 30 mg/kg L822429 significantly
decreased the motivation for heroin consumption in both ShA and LgA rats compared with vehicle (overall treatment effect: F(1,18)=7.9, _P_<0.05; Figure 1d). The ANOVA also revealed a
treatment _vs_ time interaction (F(5, 90)=7.0, _P_<0.0001), indicating that the drug was more effective in reducing responding later on the session when the workload for heroin
reinforcement is higher. NK1R ANTAGONISM PRODUCES AN ANXIOLYTIC-LIKE EFFECT IN SHA AND LGA RATS Anxiety-like behavior was measured using the elevated plus maze for drug-naïve, ShA, and LgA
heroin self-administering groups, shown in Figure 2. Overall group effects were detected for the % time in the open arms (F(2,55)=4.0, _P_<0.05) and % entries in the open arms (F(2,
55)=4.6, _P_<0.05). LgA rats spent less time in the open arms compared with naïve rats (_P_<0.01), and both ShA (_P_<0.05) and LgA (_P_<0.01) made fewer entries in the open arms
compared with naïve rats. Overall treatment effects were also detected for % time in the open arm (F(1, 55)=5.9, _P_<0.05; Figure 2a) and % entries in the open arms (F(1, 55)=7.4,
_P_<0.01; Figure 2b) with rats treated with the NK1R antagonist spending more time and making more entries into the open arms compared with vehicle-treated rats. No significant effects
were detected for entries into the closed arms, a measure of locomotor activity (Figure 2c). Taken together, these results indicate that ShA and (to a greater degree) LgA to heroin
self-administration increases anxiety-like behavior on the elevated plus maze. The findings also demonstrate an anxiolytic effect of L822429, as shown previously. However, there does not
appear to be any difference in response to NK1R antagonism between ShA and LgA rats. Finally, the data indicate that the effects of heroin self-administration and drug treatment that were
observed are not confounded by effects on locomotor activity. NK1R ANTAGONISM DID NOT AFFECT MECHANICAL SENSITIVITY IN SHA OR LGA RATS The results for PWT measurements (an indicator of
mechanical sensitivity) are shown in Figure 3. Two-way ANOVA revealed a significant group effect (F(2,20)=33.4, _P_<0.0001). _Post hoc_ testing showed that ShA rats displayed lower PWTs
compared with naïve rats (_P_<0.0001) and that LgA rats displayed lower PWTs compared with both ShA and naïve rats (_P_<0.001). However, L822429 (30 mg/kg) did not affect mechanical
sensitivity in any group. TACR1 MRNA EXPRESSION LEVELS ARE CHANGED IN LGA AND SHA RATS _TacR1_ mRNA levels in the PFC, NAc, BNST, AMYG, and Hipp of rats exposed repeatedly to moderate or
escalating doses of heroin in a response-contingent or -non-contingent manner are shown in Figure 4. Figure 4a shows the responding for heroin self-administration of the rats used for mRNA
analysis. As expected, ShA rats displayed stable heroin intake, whereas LgA rats displayed escalation of heroin intake (group × session interaction: F(12,132)=4.6, _P_<0.0001) that became
significant from session 5 onward (_P_<0.05). There was a main group effect on _TacR1_ mRNA levels in the PFC (F(2,21)=5.2, _P_<0.05), NAc (F(2,21)=4.0, _P_<0.05), and BNST
(F(2,21)=5.4, _P_<0.05). _Post hoc_ testing revealed that ShA rats displayed lower _TacR1_ mRNA levels in the PFC (_P_<0.05), NAc (_P_<0.01), and BNST (_P_<0.05) compared with
naïve rats. LgA rats displayed lower _TacR1_ mRNA levels in the PFC and the Hipp compared with naïve rats (_P_<0.05). A trend toward a reduction in _TacR1_ was also observed in the NAc
and BNST of LgA rats. ShA and LgA rats did not differ in expression of _TacR1_ in the brain areas studied (Figure 4b). To determine whether the observed changes in _TacR1_ gene expression
following heroin self-administration were response-related or could be produced via passive, non-contingent heroin exposure, we chronically treated rats with heroin for 18 days, either at a
constant level (repeated heroin group: 1.25 mg/kg subcutaneously, days 1–18) or via an escalating dose regimen (escalated heroin group: 1.25–20 mg/kg subcutaneously), and compared gene
expression changes relative to animals receiving chronic saline (repeated saline group). To ensure that this procedure produced heroin dependence, we tested their mechanical sensitivity
using von Frey’s filaments on day 18 just before the final injection (Figure 4c). There was a significant heroin treatment effect on mechanical sensitivity thresholds (F(2,21)=17.1,
_P_<0.0001). Repeated heroin injections significantly increased mechanical sensitivity compared with repeated saline injections (_P_<0.01), whereas injections of escalating doses of
heroin produced increased mechanical sensitivity compared with both repeated saline and repeated heroin injections (_P_<0.05). Figure 4d shows _TacR1_ mRNA levels following passive
subcutaneous injections of heroin either at a constant level (repeated heroin group: 1.25 mg/kg subcutaneously, days 1–18) or via an escalating dose regimen (escalated heroin group: 1.25–20
mg/kg subcutaneously), and compared gene expression changes relative to animals receiving chronic saline (repeated saline group). There was a main group effect on _TacR1_ mRNA levels in the
PFC (F(2,21)=13.39; _P_<0.001), NAc (F(2,21)=7.90; _P_=0.003), and Hipp (F(2, 21)=4.37; _P_<0.02). _Post hoc_ analysis revealed that _TacR1_ mRNA levels were significantly increased in
the mPFC of both repeated (_P_<0.02) and escalated (_P_<0.001) heroin groups compared with repeated saline group. Similarly, _TacR1_ mRNA levels were significantly increased in the
NAc of the repeated heroin group compared with repeated saline group (_P_<0.001). In addition, there was a significant difference in _TacR1_ expression between repeated and escalated
heroin groups. Levels of _TacR1_ mRNA in the mPFC are significantly higher in the escalated heroin group compared with the repeated heroin group (_P_<0.02). In contrast, levels of _TacR1_
mRNA in the NAc and the Hipp were significantly lower in the escalated heroin group compared with the repeated heroin group (_P_<0.02). DISCUSSION We report that the NK1R antagonist
L822429 decreased both heroin self-administration and the motivation to obtain heroin, as measured by PR responding, with no apparent difference between ShA and LgA rats. The NK1R antagonist
also produced an anxiolytic-like effect in ShA, LgA, and naïve rats alike, whereas it did not alter the mechanical hypersensitivity that developed in animals self-administering heroin.
Consistent with the behavioral results, _TacR1_ mRNA expression levels did not differ between ShA and LgA rats in stress/reward-related brain areas, but was significantly decreased in the
PFC of both ShA and LgA rats compared with heroin-naïve rats. Similarly, _TacR1_ mRNA expression was significantly decreased in the NAc and BNST of ShA rats as well as in the Hipp of LgA
rats. Furthermore, we observed a trend toward a reduction in the NAc and BNST of LgA rats. Interestingly, we also found that heroin-induced differences in _TacR1_ mRNA expression may be due
to the volitional consumption of heroin as opposed to the pharmacological effect of passive heroin exposure alone. Our present findings are in line with prior studies on the role of the
SP/NK1R system in opioid reward, but extend these in an important way. It has previously been shown that NK1R−/− mice fail to develop CPP for morphine (Murtra et al, 2000) and
self-administer lower levels of morphine than wild-type mice (Ripley et al, 2002). Because those data were obtained using constitutive knockout mice, they left unanswered a question that is
critical to assess the potential of NK1R antagonists as a possible pharmacotherapy for opioid addiction: whether compensatory developmental effects contribute to the observed phenotype. Our
present data indicate that reduction of heroin reinforcement by NK1R antagonism is not related to compensatory developmental mechanisms in the null mutants, but rather reflects a true
pharmacological effect. Our observations are consistent with the results of a recent study where pharmacological NK1R blockade attenuated the reward-potentiating effects of morphine, as
measured by reversal of the morphine reduction in ICSS thresholds (Robinson et al, 2012). However, findings of reduced self-administration need to be interpreted with caution because they
may be confounded by sedative or otherwise performance-impairing drug effects. In the present case, we believe that several observations make this unlikely. In this study, we show that the
30 mg/kg L822429 dose did not influence the total number of entries into the closed arms of the elevated plus maze, a measure of locomotor activity. Furthermore, nose-poke activity for
water, measured during heroin self-administration remained unchanged due to NK1R antagonism. Supporting the hypothesis of a specific effect of L822429 on heroin self-administration, we have
previously shown that 30 mg/kg L822429 did not influence locomotor activity, alcohol, or sucrose self-administration in Wistar rats (Schank et al, 2011). However, we found here that NK1
antagonism does inhibit responding for food at the highest dose of L822429 tested. Because the food pellets available during self-administration sessions are more palatable than their
regular chow, the suppression of responding for food following L822429 treatment might be a direct effect of the drug on suppressing food reward, which is at least partially mediated by
opioids (Barbano and Cador, 2006). Finally, the ability of L822429 to attenuate PR responding for heroin, which was most evident later on the testing session when the workload was higher,
suggests that its effects on self-administration are related to a specific motivational effect, a reduction of heroin reinforcement. We found that L822429 suppressed heroin
self-administration and motivation to obtain heroin in a similar manner in both ShA and LgA rats. This indicates that the SP/NK1R system does not contribute to the escalation of heroin
self-administration induced by extended drug access. Escalation of heroin intake is thought to reflect an important aspect of the transition from initial drug use to addiction (Ahmed and
Koob, 2005) and has been associated with brain reward dysfunction. Rats that exhibit stable heroin self-administration display lowered ICSS thresholds upon heroin administration, whereas
rats with extended access show a progressive increase in ICSS thresholds during escalation, indicating a tolerance to the rewarding effect of heroin (Kenny et al, 2006). In addition,
escalation of heroin self-administration in LgA rats produces a parallel development of mechanical hypersensitivity (Edwards et al, 2012) and anxiogenic-like effects (present results),
suggesting that a recruitment of negative emotional states such as pain and anxiety may, in part, drive excessive heroin intake. Accordingly, previous studies found an increase in
stress-induced reinstatement to heroin-seeking behavior in LgA compared with ShA rats (Ahmed et al, 2000). Given the established role of the SP/NK1R system in anxiety-like behavior (reviewed
by Ebner and Singewald (2006)), we investigated whether this system might contribute to heroin withdrawal-induced negative emotional states as modeled in LgA rats. We found an
anxiolytic-like effect of L822429, indicated by an increased exploration of the open arms of the elevated plus maze. This result is consistent with several preclinical studies showing that
genetic or pharmacological NK1R inactivation decreases anxiety-like behavior (reviewed by Ebner and Singewald (2006)). However, L822429 displayed similar anxiolytic-like effects in both ShA
and LgA rats. Furthermore, L822429 did not affect mechanical hypersensitivity (a model of hyperalgesia) observed in heroin self-administering rats. Hyperalgesia has been proposed as another
component of negative emotional states that may drive the transition to opioid addiction (Shurman et al, 2010). Taken together, these observations argue against a specific role for the
SP/NK1R system in the recruitment of negative emotionality that contributes to the escalation of heroin self-administration, although the SP/NK1R system may be engaged early on in the
neuroadaptations associated with initial opioid self-administration. Further support for this conclusion comes from our gene expression analysis, in which ShA and LgA heroin
self-administration resulted in similar _TacR1_ expression profiles. We found a decrease in _TacR1_ mRNA levels in the PFC of both ShA and LgA rats compared with heroin-naïve rats. A trend
toward a reduction in _TacR1_ was also observed in the NAc and BNST. It has been suggested that administration of opioids elicits SP release (Commons, 2010). It has also been shown that SP
release causes an increase in NK1R internalization (Kramer et al, 1998). In our study, _TacR1_ mRNA levels were measured during heroin withdrawal. Thus, although we cannot exclude the
possibility that the reduction in _TacR1_ mRNA does not reflect a reduction in protein, the decrease of _TacR1_ mRNA may reflect complementary changes in NK1R expression, potentially driven
by NK1R activation and internalization. Furthermore, our gene expression results suggest that heroin-induced changes in _TacR1_ mRNA expression are not only dependent on the pharmacological
effects of heroin but appear to be heavily dependent on response contingency. These results are in line with previous studies demonstrating differential neuroadaptations in rats receiving
heroin in a response-contingent _vs_ -non-contingent manner (Kuntz et al, 2008; Jacobs et al, 2004; Jacobs et al, 2005), and these data may have particular importance for addiction and pain
management fields, respectively. Interestingly, we also found that increases in NAc _TacR1_ mRNA following repeated (moderate dose) heroin was reduced following non-contingent,
escalated-dose heroin exposure, possibly reflecting tolerance-associated changes in the NK1 receptor system. This finding is also in accordance with a previous study implicating the NAc in
tolerance-related neuroadaptations in relation to chronic cocaine exposure (Edwards et al, 2010). Other factors may be in play regarding the differential effect of passive _vs_ operant
administration of heroin on _TacR1_ expression levels. Indeed, passive administration was performed via subcutaneous injections, whereas the heroin was infused intravenously in the
self-administration experiments. Therefore, different pharmacokinetics may contribute to the opposite effects of passive _vs_ operant heroin administration on _TacR1_ mRNA expression. In
conclusion, we have demonstrated that pharmacological NK1R blockade reduces heroin self-administration in rats. This is likely the result of its ability to attenuate acute, positively
reinforcing properties of heroin. In contrast, our findings do not provide support for a specific role of the SP/NK1R system in heroin intake escalation or potentiation of negative emotional
states that may drive the negative reinforcing properties of heroin. However, these data suggest that NK1R antagonists may have potential as pharmacotherapies for specific components of the
opioid dependence cycle. Maintenance treatment of heroin addiction with the opioid agonist methadone or the partial agonist buprenorphine is highly effective (Amato et al, 2005), but other
effective treatments are largely lacking for early-stage opioid dependence when replacement therapy may not be justified. NK1R antagonists are safe and well tolerated in humans, including
alcoholics (Kramer et al, 1998, 2004; ; Furmark et al, 2005; George et al, 2008). Taken together, we believe that these observations provide a rationale for exploring the potential of NK1R
antagonism as a first-line treatment in early-stage opioid dependence. REFERENCES * Ahmed SH, Koob GF (2005). Transition to drug addiction: a negative reinforcement model based on an
allostatic decrease in reward function. _Psychopharmacology_ 180: 473–490. Article CAS PubMed Google Scholar * Ahmed SH, Walker JR, Koob GF (2000). Persistent increase in the motivation
to take heroin in rats with a history of drug escalation. _Neuropsychopharmacology_ 22: 413–421. CAS PubMed Google Scholar * Amato L, Davoli M, Perucci A, Ferri M, Faggiano F, Mattick P
(2005). An overview of systematic reviews of the effectiveness of opiate maintenance therapies: available evidence to inform clinical practice and research. _J Subst Abuse Treat_ 28:
321–329. Article PubMed Google Scholar * Barbano MF, Cador M (2006). Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic
and opioidergic drugs. _Neuropsychopharmacology_ 31: 1371–1381. Article CAS PubMed Google Scholar * Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994). Quantitative assessment of
tactile allodynia in the rat paw. _J Neurosci Methods_ 53: 55–63. Article CAS PubMed Google Scholar * Commons KG (2010). Neuronal pathways linking substance P to drug addiction and
stress. _Brain Res_ 1314: 175–182. Article CAS PubMed Google Scholar * Dixon WJ (1980). Efficient analysis of experimental observations. _Annu Rev Pharmacol Toxicol_ 20: 441–462. Article
CAS PubMed Google Scholar * Ebner K, Muigg P, Singewald G, Singewald N (2008). Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress
circuitry. _Ann N Y Acad Sci_ 1144: 61–73. Article CAS PubMed Google Scholar * Ebner K, Rupniak NM, Saria A, Singewald N (2004). Substance P in the medial amygdala: emotional
stress-sensitive release and modulation of anxiety-related behavior in rats. _Proc Natl Acad Sci USA_ 101: 4280–4285. Article CAS PubMed PubMed Central Google Scholar * Ebner K,
Singewald N (2006). The role of substance P in stress and anxiety responses. _Amino Acids_ 31: 251–272. Article CAS PubMed Google Scholar * Edwards S, Koob GF (2010). Neurobiology of
dysregulated motivational systems in drug addiction. _Fut Neurol_ 5: 393–401. Article CAS Google Scholar * Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE _et al_
(2012). Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF receptor antagonism. _Neuropharmacology_ 62: 1142–1151. Article CAS
PubMed Google Scholar * Furmark T, Appel L, Michelgard A, Wahlstedt K, Ahs F, Zancan S _et al_ (2005). Cerebral blood flow changes after treatment of social phobia with the neurokinin-1
antagonist GR205171, citalopram, or placebo. _Biol Psychiatry_ 58: 132–142. Article CAS PubMed Google Scholar * Gadd CA, Murtra P, De Felipe C, Hunt SP (2003). Neurokinin-1
receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. _J Neurosci_ 23: 8271–8280. Article CAS PubMed PubMed Central Google Scholar *
George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C _et al_ (2008). Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. _Science_ 319: 1536–1539. Article CAS
PubMed Google Scholar * Jacobs EH, de Vries TJ, Smit AB, Schoffelmeer AN (2004). Gene transcripts selectively down-regulated in the shell of the nucleus accumbens long after heroin
self-administration are up-regulated in the core independent of response contingency. _FASEB J_ 18: 200–202. Article CAS PubMed Google Scholar * Jacobs EH, Smit AB, de Vries TJ,
Schoffelmeer AN (2005). Long-term gene expression in the nucleus accumbens following heroin administration is subregion-specific and depends on the nature of drug administration. _Addict
Biol_ 10: 91–100. Article CAS PubMed Google Scholar * Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF (2006). Conditioned withdrawal drives heroin consumption and decreases reward
sensitivity. _J Neurosci_ 26: 5894–5900. Article CAS PubMed PubMed Central Google Scholar * Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ _et al_ (1998). Distinct
mechanism for antidepressant activity by blockade of central substance P receptors. _Science_ 281: 1640–1645. Article CAS PubMed Google Scholar * Kramer MS, Winokur A, Kelsey J, Preskorn
SH, Rothschild AJ, Snavely D _et al_ (2004). Demonstration of the efficacy and safety of a novel substance P (NK1) receptor antagonist in major depression. _Neuropsychopharmacology_ 29:
385–392. Article CAS PubMed Google Scholar * Kuntz KL, Patel KM, Grigson PS, Freeman WM, Vrana KE (2008). Heroin self-administration: II. CNS gene expression following withdrawal and
cue-induced drug-seeking behavior. _Pharmacol Biochem Behav_ 90: 349–356. Article CAS PubMed PubMed Central Google Scholar * Leffler A, Ahlstedt I, Engberg S, Svensson A, Billger M,
Oberg L _et al_ (2009). Characterization of species-related differences in the pharmacology of tachykinin NK receptors 1, 2 and 3. _Biochem Pharmacol_ 77: 1522–1530. Article CAS PubMed
Google Scholar * Murtra P, Sheasby AM, Hunt SP, De Felipe C (2000). Rewarding effects of opiates are absent in mice lacking the receptor for substance P. _Nature_ 405: 180–183. Article CAS
PubMed Google Scholar * Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN (2002). Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice
lacking NK1 receptors. _Neuropharmacology_ 43: 1258–1268. Article CAS PubMed Google Scholar * Ririe DG, Eisenach JC (2006). Age-dependent responses to nerve injury-induced mechanical
allodynia. _Anesthesiology_ 104: 344–350. Article PubMed Google Scholar * Robinson JE, Fish EW, Krouse MC, Thorsell A, Heilig M, Malanga CJ (2012). Potentiation of brain stimulation
reward by morphine: effects of neurokinin-1 receptor antagonism. _Psychopharmacology (Berl)_ 220: 215–224. Article CAS Google Scholar * Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell
A, Rice KC _et al_ (2011). Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. _Psychopharmacology (Berl)_ 218:
111–119. Article CAS Google Scholar * Shurman J, Koob GF, Gutstein HB (2010). Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. _Pain Med_
11: 1092–1098. Article PubMed Google Scholar * Singewald N, Chicchi GG, Thurner CC, Tsao KL, Spetea M, Schmidhammer H _et al_ (2008). Modulation of basal and stress-induced amygdaloid
substance P release by the potent and selective NK1 receptor antagonist L-822429. _J Neurochem_ 106: 2476–2488. Article CAS PubMed Google Scholar * Thorsell A, Schank JR, Singley E, Hunt
SP, Heilig M (2010). Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. _Psychopharmacology (Berl)_ 209: 103–111. Article CAS Google Scholar * Vendruscolo
LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML _et al_ (2012). Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. _J Neurosci_ 32: 7563–7571.
Article CAS PubMed PubMed Central Google Scholar * Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF (2011). Escalation patterns of varying periods of heroin
access. _Pharmacol Biochem Behav_ 98: 570–574. Article CAS PubMed PubMed Central Google Scholar * Vendruscolo LF, Takahashi RN, Bruske GR, Ramos A (2003). Evaluation of the
anxiolytic-like effect of NKP608, a NK1-receptor antagonist, in two rat strains that differ in anxiety-related behaviors. _Psychopharmacology (Berl)_ 170: 287–293. Article CAS Google
Scholar Download references ACKNOWLEDGEMENTS We thank Dr Eric Augier for its insightful comments regarding the manuscript. This research was supported by the intramural program of the
National Institute on Alcohol Abuse and Alcoholism (MH) and the National Institute on Drug Abuse (KR). This work was also supported by grants from the National Institute on Drug Abuse
(DA004043, GFK), a Research Career Scientist Award from the Biomedical Laboratory Research and Development Program, Veterans Health Administration (GS), and by the Pearson Center for
Alcoholism and Addiction Research. This is article number 21813 from the Scripps Research Institute. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratory of Clinical and Translational
Studies, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD, USA Estelle Barbier, Nathan Juergens, Jesse Schank & Markus Heilig * Committee on the Neurobiology of Addictive
Disorders, The Scripps Research Institute, La Jolla, CA, USA Leandro F Vendruscolo, Joel E Schlosburg, Scott Edwards, Paula E Park, Kaushik K Misra & George F Koob * Drug Design and
Synthesis Section, Chemical Biology Research Branch, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, USA
Kejun Cheng & Kenner C Rice * and Department of Anesthesiology, Research Service, VA San Diego Healthcare System, University of California at San Diego School of Medicine, San Diego,
CA, USA Gery Schulteis Authors * Estelle Barbier View author publications You can also search for this author inPubMed Google Scholar * Leandro F Vendruscolo View author publications You can
also search for this author inPubMed Google Scholar * Joel E Schlosburg View author publications You can also search for this author inPubMed Google Scholar * Scott Edwards View author
publications You can also search for this author inPubMed Google Scholar * Nathan Juergens View author publications You can also search for this author inPubMed Google Scholar * Paula E Park
View author publications You can also search for this author inPubMed Google Scholar * Kaushik K Misra View author publications You can also search for this author inPubMed Google Scholar *
Kejun Cheng View author publications You can also search for this author inPubMed Google Scholar * Kenner C Rice View author publications You can also search for this author inPubMed Google
Scholar * Jesse Schank View author publications You can also search for this author inPubMed Google Scholar * Gery Schulteis View author publications You can also search for this author
inPubMed Google Scholar * George F Koob View author publications You can also search for this author inPubMed Google Scholar * Markus Heilig View author publications You can also search for
this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Estelle Barbier. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that, except for income received from
our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are
no personal financial holdings that could be perceived as constituting a potential conflict of interest. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT
SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Barbier, E., Vendruscolo, L., Schlosburg, J. _et al._ The
NK1 Receptor Antagonist L822429 Reduces Heroin Reinforcement. _Neuropsychopharmacol_ 38, 976–984 (2013). https://doi.org/10.1038/npp.2012.261 Download citation * Received: 08 June 2012 *
Accepted: 15 November 2012 * Published: 18 December 2012 * Issue Date: May 2013 * DOI: https://doi.org/10.1038/npp.2012.261 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * opioids * heroin * self-administration * substance P * neurokinin receptor