Play all audios:
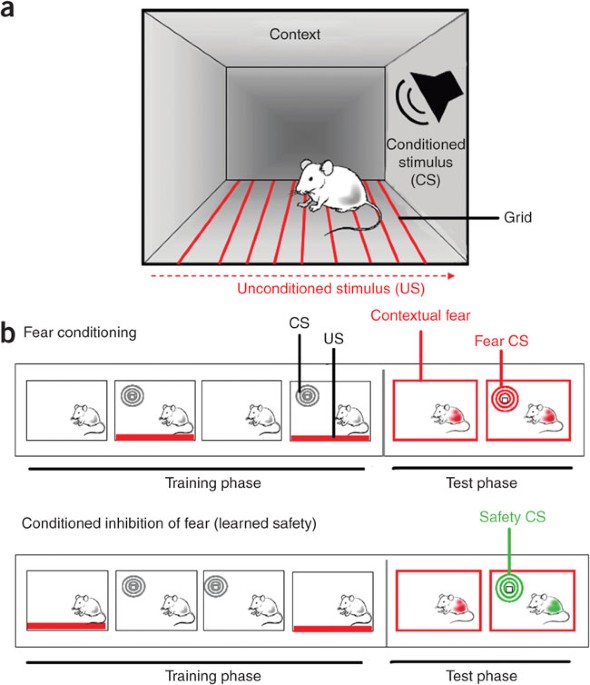
ABSTRACT Fear conditioning is one of the most widely used animal models for studying the neurobiological basis of fear and anxiety states. Conditioned inhibition of fear (or learned safety),
however, is a relatively unexplored behavioral paradigm addressing the aspect of regulation of fear, which is central to survival and mental health. Although fear conditioning is achieved
by pairing a previously neutral, conditioned stimulus (CS) with an aversive, unconditioned stimulus (US), learned safety training consists of a series of explicitly unpaired CS–US
presentations. Animals are trained for 3 d, one session per day, and learn to associate the CS with protection from the impending danger of the aversive events. The entire procedure can be
completed within 7 d. The protocol has been successfully used to study the molecular underpinnings of a behavioral intervention for depression. This paradigm complements currently used
animal tests in neuropsychiatric research addressing the dysregulation of emotional behaviors in genetic, pharmacological or environmental mouse models of human affective disorders. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal
Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices
may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support
SIMILAR CONTENT BEING VIEWED BY OTHERS DIFFERENTIAL RECRUITMENT OF BRAIN CIRCUITS DURING FEAR EXTINCTION IN NON-STRESSED COMPARED TO STRESS RESILIENT ANIMALS Article Open access 25 January
2024 REFINEMENT OF THE STRESS-ENHANCED FEAR LEARNING MODEL OF POST-TRAUMATIC STRESS DISORDER: A BEHAVIORAL AND MOLECULAR ANALYSIS Article 20 October 2022 SEROTONIN 5-HT2C RECEPTOR KNOCKOUT
IN MICE ATTENUATES FEAR RESPONSES IN CONTEXTUAL OR CUED BUT NOT COMPOUND CONTEXT-CUE FEAR CONDITIONING Article Open access 11 February 2022 REFERENCES * Davis, M. & Shi, C. The extended
amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? _Ann. NY Acad. Sci._ 877, 281–291 (1999).
Article CAS Google Scholar * LeDoux, J.E. Emotion circuits in the brain. _Annu. Rev. Neurosci._ 23, 155–184 (2000). Article CAS Google Scholar * Phelps, E.A. & LeDoux, J.E.
Contributions of the amygdala to emotion processing: from animal models to human behavior. _Neuron_ 48, 175–187 (2005). Article CAS Google Scholar * Rescorla, R.A. Conditioned inhibition
of fear resulting from negative CS–US contingencies. _J. Comp. Physiol. Psychol._ 67, 504–509 (1969). Article CAS Google Scholar * Pavlov, I.P. _Conditioned Reflexes_ (Dover, New York,
1927). * Vouimba, R.M., Garcia, R., Baudry, M. & Thompson, R.F. Potentiation of conditioned freezing following dorsomedial prefrontal cortex lesions does not interfere with fear
reduction in mice. _Behav. Neurosci._ 114, 720–724 (2000). Article CAS Google Scholar * Heldt, S.A. & Falls, W.A. Destruction of the inferior colliculus disrupts the production and
inhibition of fear conditioned to an acoustic stimulus. _Behav. Brain Res._ 144, 175–185 (2003). Article Google Scholar * Pollak, D.D. et al. An animal model of a behavioral intervention
for depression. _Neuron_ 60, 149–161 (2008). Article CAS Google Scholar * Rogan, M.T., Leon, K.S., Perez, D.L. & Kandel, E.R. Distinct neural signatures for safety and danger in the
amygdala and striatum of the mouse. _Neuron_ 46, 309–320 (2005). Article CAS Google Scholar * Watkins, L.R. et al. Reversal of spinal cord non-opiate analgesia by conditioned
anti-analgesia in the rat. _Pain_ 71, 237–247 (1997). Article CAS Google Scholar * Watkins, L.R. et al. Neurocircuitry of conditioned inhibition of analgesia: effects of amygdala, dorsal
raphe, ventral medullary, and spinal cord lesions on antianalgesia in the rat. _Behav. Neurosci._ 112, 360–378 (1998). Article CAS Google Scholar * Dinsmoor, J.A. Stimuli inevitably
generated by behavior that avoids electric shock are inherently reinforcing. _J. Exp. Anal. Behav._ 75, 311–333 (2001). Article CAS Google Scholar * Rescorla, R.A. Establishment of a
positive reinforcer through contrast with shock. _J. Comp. Physiol. Psychol._ 67, 260–263 (1969). Article CAS Google Scholar * Santarelli, L. et al. Genetic and pharmacological disruption
of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. _Proc. Natl Acad. Sci. USA_ 98, 1912–1917 (2001). Article CAS Google Scholar *
Sahay, A. & Hen, R. Adult hippocampal neurogenesis in depression. _Nat. Neurosci._ 10, 1110–1115 (2007). Article CAS Google Scholar * Maren, S. The amygdala, synaptic plasticity, and
fear memory. _Ann. NY Acad. Sci._ 985, 106–113 (2003). Article Google Scholar * LaBar, K.S. & Cabeza, R. Cognitive neuroscience of emotional memory. _Nat. Rev. Neurosci._ 7, 54–64
(2006). Article CAS Google Scholar * Maren, S. & Quirk, G.J. Neuronal signalling of fear memory. _Nat. Rev. Neurosci._ 5, 844–852 (2004). Article CAS Google Scholar * Anderson,
A.K. et al. Dissociated neural representations of intensity and valence in human olfaction. _Nat. Neurosci._ 6, 196–202 (2003). Article CAS Google Scholar * Weiner, I. & Arad, M.
Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. _Behav. Brain Res._ 204, 369–386 (2009). Article CAS Google Scholar *
Swerdlow, N.R., Weber, M., Qu, Y., Light, G.A. & Braff, D.L. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. _Psychopharmacology (Berl)_
199, 331–388 (2008). Article CAS Google Scholar * van Gaalen, M.M. & Steckler, T. Behavioural analysis of four mouse strains in an anxiety test battery. _Behav. Brain Res._ 115,
95–106 (2000). Article CAS Google Scholar * Brinks, V., de Kloet, E.R. & Oitzl, M.S. Strain specific fear behaviour and glucocorticoid response to aversive events: modelling PTSD in
mice. _Prog. Brain Res._ 167, 257–261 (2008). Article CAS Google Scholar * Schweizer, M.C., Henniger, M.S. & Sillaber, I. Chronic mild stress (CMS) in mice: of anhedonia,
'anomalous anxiolysis' and activity. _PLoS One_ 4, e4326 (2009). Article Google Scholar * Milner, L.C. & Crabbe, J.C. Three murine anxiety models: results from multiple
inbred strain comparisons. _Genes Brain Behav._ 7, 496–505 (2008). Article CAS Google Scholar * Holmes, A., Wrenn, C.C., Harris, A.P., Thayer, K.E. & Crawley, J.N. Behavioral profiles
of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. _Genes Brain Behav._ 1, 55–69 (2002). Article CAS Google Scholar * Griebel, G., Belzung,
C., Perrault, G. & Sanger, D.J. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. _Psychopharmacology (Berl)_ 148, 164–170
(2000). Article CAS Google Scholar * Trullas, R. & Skolnick, P. Differences in fear motivated behaviors among inbred mouse strains. _Psychopharmacology (Berl)_ 111, 323–331 (1993).
Article CAS Google Scholar * Schaefer, T.L., Vorhees, C.V. & Williams, M.T. Mouse plasmacytoma-expressed transcript 1 knock out induced 5-HT disruption results in a lack of cognitive
deficits and an anxiety phenotype complicated by hypoactivity and defensiveness. _Neuroscience_ 164, 1431–1443 (2009). Article CAS Google Scholar * Bortolato, M., Godar, S.C., Davarian,
S., Chen, K. & Shih, J.C. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. _Neuropsychopharmacology_ 34, 2746–2757 (2009). Article CAS
Google Scholar * Welch, J.M. et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. _Nature_ 448, 894–900 (2007). Article CAS Google Scholar *
Klemenhagen, K.C., Gordon, J.A., David, D.J., Hen, R. & Gross, C.T. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. _Neuropsychopharmacology_ 31, 101–111
(2006). Article CAS Google Scholar * Shumyatsky, G.P. et al. stathmin, a gene enriched in the amygdala, controls both learned and innate fear. _Cell_ 123, 697–709 (2005). Article CAS
Google Scholar * Gross, C. et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. _Nature_ 416, 396–400 (2002). Article CAS Google
Scholar * Rupprecht, R. et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. _Science_ 325, 490–493 (2009). Article CAS Google Scholar
* Yokoyama, F. et al. Anxiolytic-like profiles of histamine H3 receptor agonists in animal models of anxiety: a comparative study with antidepressants and benzodiazepine anxiolytic.
_Psychopharmacology (Berl)_ 205, 177–187 (2009). Article CAS Google Scholar * Hovatta, I. et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. _Nature_ 438, 662–666
(2005). Article CAS Google Scholar * Rudolph, U. et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. _Nature_ 401, 796–800 (1999). Article
CAS Google Scholar * Rutten, K. et al. Enhanced long-term potentiation and impaired learning in phosphodiesterase 4D-knockout (PDE4D) mice. _Eur. J. Neurosci._ 28, 625–632 (2008). Article
Google Scholar * Matzel, L.D., Babiarz, J., Townsend, D.A., Grossman, H.C. & Grumet, M. Neuronal cell adhesion molecule deletion induces a cognitive and behavioral phenotype
reflective of impulsivity. _Genes Brain Behav._ 7, 470–480 (2008). Article CAS Google Scholar * Reijmers, L.G. et al. A mutant mouse with a highly specific contextual fear-conditioning
deficit found in an _N_-ethyl-_N_-nitrosourea (ENU) mutagenesis screen. _Learn Mem._ 13, 143–149 (2006). Article CAS Google Scholar * Chiba, S., Nishiyama, T., Yoshikawa, M. & Yamada,
Y. The antinociceptive effects of midazolam on three different types of nociception in mice. _J. Pharmacol. Sci._ 109, 71–77 (2009). Article CAS Google Scholar * Noble, F., Benturquia,
N., Bilkei-Gorzo, A., Zimmer, A. & Roques, B.P. Use of preproenkephalin knockout mice and selective inhibitors of enkephalinases to investigate the role of enkephalins in various
behaviours. _Psychopharmacology (Berl)_ 196, 327–335 (2008). Article CAS Google Scholar * Kabbaj, M., Devine, D.P., Savage, V.R. & Akil, H. Neurobiological correlates of individual
differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. _J. Neurosci._ 20, 6983–6988 (2000). Article CAS Google Scholar * Joels, M., Pu,
Z., Wiegert, O., Oitzl, M.S. & Krugers, H.J. Learning under stress: how does it work? _Trends Cogn. Sci._ 10, 152–158 (2006). Article Google Scholar * Sandi, C. & Pinelo-Nava, M.T.
Stress and memory: behavioral effects and neurobiological mechanisms. _Neural. Plast._ 2007, 78970 (2007). Article Google Scholar * Luksys, G., Gerstner, W. & Sandi, C. Stress,
genotype and norepinephrine in the prediction of mouse behavior using reinforcement learning. _Nat. Neurosci._ 12, 1180–1186 (2009). Article CAS Google Scholar * Lu, A. et al. Conditional
mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. _Mol. Psychiatry_ 13, 1028–1042 (2008). Article CAS Google Scholar * Tschenett,
A. et al. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. _Eur. J. Neurosci._ 18, 143–148 (2003). Article Google Scholar * Campbell, T., Lin, S.,
DeVries, C. & Lambert, K. Coping strategies in male and female rats exposed to multiple stressors. _Physiol. Behav._ 78, 495–504 (2003). Article CAS Google Scholar * Steiner, M.A. et
al. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. _Psychoneuroendocrinology_ 33, 54–67
(2008). Article CAS Google Scholar * Bowers, S.L., Bilbo, S.D., Dhabhar, F.S. & Nelson, R.J. Stressor-specific alterations in corticosterone and immune responses in mice. _Brain
Behav. Immun._ 22, 105–113 (2008). Article CAS Google Scholar * Yang, R.J. et al. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and
fear learning. _Neuropsychopharmacology_ 33, 2595–2604 (2008). Article CAS Google Scholar * Deacon, R.M. Housing, husbandry and handling of rodents for behavioral experiments. _Nat.
Protoc._ 1, 936–946 (2006). Article Google Scholar * Crabbe, J.C., Wahlsten, D. & Dudek, B.C. Genetics of mouse behavior: interactions with laboratory environment. _Science_ 284,
1670–1672 (1999). Article CAS Google Scholar * Chesler, E.J., Wilson, S.G., Lariviere, W.R., Rodriguez-Zas, S.L. & Mogil, J.S. Influences of laboratory environment on behavior. _Nat.
Neurosci._ 5, 1101–1102 (2002). Article CAS Google Scholar * Oxenkrug, G.F. Genetic and hormonal regulation of tryptophan kynurenine metabolism: implications for vascular cognitive
impairment, major depressive disorder, and aging. _Ann. NY Acad. Sci._ 1122, 35–49 (2007). Article CAS Google Scholar * Pollak, D.D. et al. A translational bridge between mouse and human
models of learned safety. _Ann. Med._ 42, 127–134 (2010). Article Google Scholar * Uyttenhove, C. et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation
by indoleamine 2,3-dioxygenase. _Nat. Med._ 9, 1269–1274 (2003). Article CAS Google Scholar Download references AUTHOR INFORMATION Author notes * Daniela D Pollak and Francisco J Monje:
These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Physiology, Center for Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria
Daniela D Pollak & Francisco J Monje * Department of Pediatrics, Division of Pediatric Neuroscience, Medical University of Vienna, Vienna, Austria Gert Lubec Authors * Daniela D Pollak
View author publications You can also search for this author inPubMed Google Scholar * Francisco J Monje View author publications You can also search for this author inPubMed Google Scholar
* Gert Lubec View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.D.P. jointly conceived the study with F.J.M.; D.D.P. conducted behavioral
experiments; D.D.P. and F.J.M. interpreted and analyzed the data; G.L. and D.D.P. wrote the paper. CORRESPONDING AUTHOR Correspondence to Daniela D Pollak. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pollak, D., Monje, F. & Lubec, G. The
learned safety paradigm as a mouse model for neuropsychiatric research. _Nat Protoc_ 5, 954–962 (2010). https://doi.org/10.1038/nprot.2010.64 Download citation * Published: 29 April 2010 *
Issue Date: May 2010 * DOI: https://doi.org/10.1038/nprot.2010.64 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative