Play all audios:
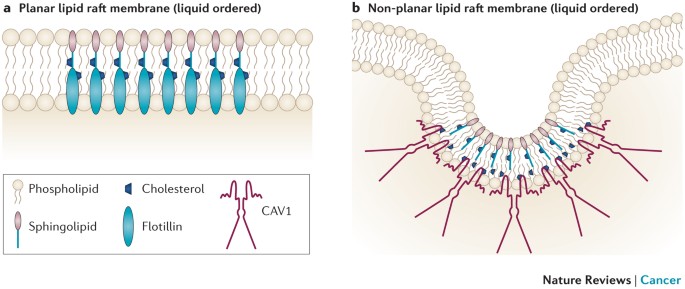
KEY POINTS * Lipid rafts are cell membrane microdomains that are enriched for cholesterol and signalling proteins. Lipid rafts can have a planar or a non-planar configuration. Caveolae are a
subset of lipid rafts that are invaginated, non-planar structures. Caveolins are the main integral membrane proteins of caveolae and are required for their formation. * Caveolin 1 (CAV1) is
a key regulator of cell signalling. The caveolin scaffolding domain binds to many divergent signalling molecules and modulates their activity. In many of these instances CAV1 represses
signalling cascades and its downregulation leads to signalling activation. For example, the activity of endothelial nitric oxide synthase (eNOS), G proteins, SRC family tyrosine kinases and
members of the RAS family are all repressed by binding to CAV1. Loss of _CAV1_ frequently leads to the activation of signalling cascades, with tumorigenic effects such as increased cell
motility and proliferation. * Alterations in caveolae have a strong cancer-specific prognostic value. Three caveolar components have all been shown to be reduced or absent in the tumour
stroma of high-risk cancer patients. These caveolar biomarkers are CAV1, cavin 1 and CD36. * Loss of CAV1 expression in the tumour microenvironment is consistently associated with poor
clinical outcomes in a wide variety of cancers, including breast, prostate, pancreatic, oesophageal and gastric carcinomas, as well as melanomas. By contrast, there is no universal pattern
of CAV1 expression in epithelial cancer cells that is associated with clinical outcome. * Alterations in caveolae in the tumour microenvironment promote paracrine tumour growth via
myofibroblast differentiation, transforming growth factor-β (TGFβ) activation, oxidative stress, autophagy and catabolism, as well as premature senescence. * Altered caveolae in the tumour
microenvironment induce tumour metabolic heterogeneity. The loss of CAV1 generates a catabolic tumour microenvironment that is characterized by increased glycolysis and the generation of
L-lactate, ketone bodies and free amino acids. Conversely, cancer cells have increased oxidative metabolism (OXPHOS) and resistance to apoptosis, when there is a loss of CAV1 in the tumour
microenvironment. ABSTRACT It has been over 20 years since the discovery that caveolar lipid rafts function as signalling organelles. Lipid rafts create plasma membrane heterogeneity, and
caveolae are the most extensively studied subset of lipid rafts. A newly emerging paradigm is that changes in caveolae also generate tumour metabolic heterogeneity. Altered caveolae create a
catabolic tumour microenvironment, which supports oxidative mitochondrial metabolism in cancer cells and which contributes to dismal survival rates for cancer patients. In this Review, we
discuss the role of caveolae in tumour progression, with a special emphasis on their metabolic and cell signalling effects, and their capacity to transform the tumour microenvironment.
Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this
journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now
Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS CAVEOLIN-1 MEDIATES THE UTILIZATION OF EXTRACELLULAR PROTEINS FOR SURVIVAL IN REFRACTORY GASTRIC CANCER Article Open access 02 November 2023 A
NOVEL TRPM7/_O_-GLCNAC AXIS MEDIATES TUMOUR CELL MOTILITY AND METASTASIS BY STABILISING C-MYC AND CAVEOLIN-1 IN LUNG CARCINOMA Article Open access 20 July 2020 CAVEOLIN-1 SUPPRESSES TUMOR
FORMATION THROUGH THE INHIBITION OF THE UNFOLDED PROTEIN RESPONSE Article Open access 03 August 2020 REFERENCES * Singer, S. J. & Nicolson, G. L. The fluid mosaic model of the structure
of cell membranes. _Science_ 175, 720–731 (1972). Article CAS PubMed Google Scholar * Lingwood, D. & Simons, K. Lipid rafts as a membrane-organizing principle. _Science_ 327, 46–50
(2010). Article CAS PubMed Google Scholar * Holopainen, J. M., Subramanian, M. & Kinnunen, P. K. Sphingomyelinase induces lipid microdomain formation in a fluid
phosphatidylcholine/sphingomyelin membrane. _Biochemistry_ 37, 17562–17570 (1998). Article CAS PubMed Google Scholar * Veatch, S. L. & Keller, S. L. Organization in lipid membranes
containing cholesterol. _Phys. Rev. Lett._ 89, 268101 (2002). Article CAS PubMed Google Scholar * Rothberg, K. G. et al. Caveolin, a protein component of caveolae membrane coats. _Cell_
68, 673–682 (1992). Article CAS PubMed Google Scholar * Simons, K. & Ikonen, E. Functional rafts in cell membranes. _Nature_ 387, 569–572 (1997). Article CAS PubMed Google Scholar
* Parton, R. G. & Simons, K. The multiple faces of caveolae. _Nature Rev. Mol. Cell Biol._ 8, 185–194 (2007). Article CAS Google Scholar * Pike, L. J. Rafts defined: a report on the
Keystone Symposium on lipid rafts and cell function. _J. Lipid Res._ 47, 1597–1598 (2006). Article CAS PubMed Google Scholar * Parton, R. G. & del Pozo, M. A. Caveolae as plasma
membrane sensors, protectors and organizers. _Nature Rev. Mol. Cell Biol._ 14, 98–112 (2013). Article CAS Google Scholar * Stuermer, C. A. The reggie/flotillin connection to growth.
_Trends Cell Biol._ 20, 6–13 (2010). Article CAS PubMed Google Scholar * Brown, D. A. & London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. _J.
Biol. Chem._ 275, 17221–17224 (2000). Article CAS PubMed Google Scholar * Brown, D. A. & Rose, J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains
during transport to the apical cell surface. _Cell_ 68, 533–544 (1992). Article CAS PubMed Google Scholar * Lisanti, M. P., Scherer, P. E., Tang, Z. & Sargiacomo, M. Caveolae,
caveolin and caveolin-rich membrane domains: a signalling hypothesis. _Trends Cell Biol._ 4, 231–235 (1994). THIS IS THE FIRST PAPER TO PROPOSE THE HYPOTHESIS THAT CAVEOLAE REPRESENT
SIGNALLING MICRODOMAINS AT THE PLASMA MEMBRANE. Article CAS PubMed Google Scholar * Garcia-Cardena, G., Oh, P., Liu, J., Schnitzer, J. E. & Sessa, W. C. Targeting of nitric oxide
synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. _Proc. Natl Acad. Sci. USA_ 93, 6448–6453 (1996). Article CAS PubMed Google Scholar *
Galbiati, F. et al. The dually acylated NH2-terminal domain of gi1α is sufficient to target a green fluorescent protein reporter to caveolin-enriched plasma membrane domains. Palmitoylation
of caveolin-1 is required for the recognition of dually acylated g-protein α subunits _in vivo_. _J. Biol. Chem._ 274, 5843–5850 (1999). Article CAS PubMed Google Scholar * Tao, N.,
Wagner, S. J. & Lublin, D. M. CD36 is palmitoylated on both N- and C-terminal cytoplasmic tails. _J. Biol. Chem._ 271, 22315–22320 (1996). Article CAS PubMed Google Scholar *
Dietzen, D. J., Hastings, W. R. & Lublin, D. M. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. _J.
Biol. Chem._ 270, 6838–6842 (1995). Article CAS PubMed Google Scholar * Palade, G. E. Fine structure of blood capillaries. _J. Appl. Phys._ 24, 1424–1436 (1953). Google Scholar *
Yamada, E. The fine structure of the gall bladder epithelium of the mouse. _J. Biophys. Biochem. Cytol._ 1, 445–458 (1955). Article CAS PubMed PubMed Central Google Scholar * Okamoto,
T., Schlegel, A., Scherer, P. E. & Lisanti, M. P. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. _J. Biol. Chem._
273, 5419–5422 (1998). Article CAS PubMed Google Scholar * Owen, D. M., Magenau, A., Williamson, D. & Gaus, K. The lipid raft hypothesis revisited—new insights on raft composition
and function from super-resolution fluorescence microscopy. _Bioessays_ 34, 739–747 (2012). Article CAS PubMed Google Scholar * Schlormann, W. et al. The shape of caveolae is omega-like
after glutaraldehyde fixation and cup-like after cryofixation. _Histochem. Cell Biol._ 133, 223–228 (2010). Article CAS PubMed Google Scholar * Sotgia, F. et al. Caveolin-1 and cancer
metabolism in the tumor microenvironment: markers, models, and mechanisms. _Annu. Rev. Pathol._ 7, 423–467 (2012). Article CAS PubMed Google Scholar * Lajoie, P. & Nabi, I. R.
Regulation of raft-dependent endocytosis. _J. Cell. Mol. Med._ 11, 644–653 (2007). Article CAS PubMed PubMed Central Google Scholar * Parat, M. O., Anand-Apte, B. & Fox, P. L.
Differential caveolin-1 polarization in endothelial cells during migration in two and three dimensions. _Mol. Biol. Cell_ 14, 3156–3168 (2003). Article CAS PubMed PubMed Central Google
Scholar * Murata, M. et al. VIP21/caveolin is a cholesterol-binding protein. _Proc. Natl Acad. Sci. USA_ 92, 10339–10343 (1995). THIS IS THE FIRST PAPER TO DEMONSTRATE THAT CAV1 IS A
CHOLESTEROL-BINDING PROTEIN. Article CAS PubMed Google Scholar * Sargiacomo, M. et al. Oligomeric structure of caveolin: implications for caveolae membrane organization. _Proc. Natl
Acad. Sci. USA_ 92, 9407–9411 (1995). Article CAS PubMed Google Scholar * Couet, J., Li, S., Okamoto, T., Ikezu, T. & Lisanti, M. P. Identification of peptide and protein ligands for
the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. _J. Biol. Chem._ 272, 6525–6533 (1997). Article CAS PubMed Google Scholar
* Li, S., Couet, J. & Lisanti, M. P. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates
the auto-activation of Src tyrosine kinases. _J. Biol. Chem._ 271, 29182–29190 (1996). Article CAS PubMed PubMed Central Google Scholar * Drab, M. et al. Loss of caveolae, vascular
dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. _Science_ 293, 2449–2452 (2001). Article CAS PubMed Google Scholar * Razani, B. et al. Caveolin-1 null mice are
viable but show evidence of hyperproliferative and vascular abnormalities. _J. Biol. Chem._ 276, 38121–38138 (2001). REFERENCES 30 AND 31 ARE THE FIRST PAPERS TO DESCRIBE THE GENERATION AND
INITIAL PHENOTYPIC CHARACTERIZATION OF _CAV1_ -KNOCKOUT MOUSE MODELS. Article CAS PubMed Google Scholar * Fra, A. M., Williamson, E., Simons, K. & Parton, R. G. De novo formation of
caveolae in lymphocytes by expression of VIP21-caveolin. _Proc. Natl Acad. Sci. USA_ 92, 8655–8659 (1995). Article CAS PubMed Google Scholar * Vinten, J. et al. A 60-kDa protein abundant
in adipocyte caveolae. _Cell Tissue Res._ 305, 99–106 (2001). Article CAS PubMed Google Scholar * Voldstedlund, M., Thuneberg, L., Tranum-Jensen, J., Vinten, J. & Christensen, E. I.
Caveolae, caveolin and cav-p60 in smooth muscle and renin-producing cells in the rat kidney. _Acta Physiol. Scand._ 179, 179–188 (2003). Article CAS PubMed Google Scholar * Hill, M. M.
et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. _Cell_ 132, 113–124 (2008). THIS IS THE FIRST PAPER TO SHOW THAT CAVIN IS REQUIRED FOR
CAVEOLAE FORMATION. Article CAS PubMed PubMed Central Google Scholar * Bastiani, M. et al. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. _J. Cell Biol._
185, 1259–1273 (2009). Article CAS PubMed PubMed Central Google Scholar * Fairn, G. D. et al. High-resolution mapping reveals topologically distinct cellular pools of
phosphatidylserine. _J. Cell Biol._ 194, 257–275 (2011). Article CAS PubMed PubMed Central Google Scholar * Hansen, C. G., Shvets, E., Howard, G., Riento, K. & Nichols, B. J.
Deletion of cavin genes reveals tissue-specific mechanisms for morphogenesis of endothelial caveolae. _Nature Commun._ 4, 1831 (2013). Article CAS Google Scholar * Shastry, S. et al.
Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. _Am. J. Med. Genet. A_ 152A, 2245–2253 (2010). Article CAS PubMed PubMed Central
Google Scholar * Rajab, A. et al. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN
mutations. _PLoS Genet._ 6, e1000874 (2010). Article CAS PubMed PubMed Central Google Scholar * Bai, L. et al. Down-regulation of the cavin family proteins in breast cancer. _J. Cell
Biochem._ 113, 322–328 (2012). Article CAS PubMed Google Scholar * Zochbauer-Muller, S. et al. Expression of the candidate tumor suppressor gene hSRBC is frequently lost in primary lung
cancers with and without DNA methylation. _Oncogene_ 24, 6249–6255 (2005). Article CAS PubMed Google Scholar * Souto, R. P. et al. Immunopurification and characterization of rat
adipocyte caveolae suggest their dissociation from insulin signaling. _J. Biol. Chem._ 278, 18321–18329 (2003). Article CAS PubMed Google Scholar * Daumke, O. et al. Architectural and
mechanistic insights into an EHD ATPase involved in membrane remodelling. _Nature_ 449, 923–927 (2007). Article CAS PubMed Google Scholar * Moren, B. et al. EHD2 regulates caveolar
dynamics via ATP-driven targeting and oligomerization. _Mol. Biol. Cell_ 23, 1316–1329 (2012). Article CAS PubMed PubMed Central Google Scholar * Senju, Y., Itoh, Y., Takano, K.,
Hamada, S. & Suetsugu, S. Essential role of PACSIN2/syndapin-II in caveolae membrane sculpting. _J. Cell Sci._ 124, 2032–2040 (2011). Article CAS PubMed Google Scholar * Hansen, C.
G., Howard, G. & Nichols, B. J. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. _J. Cell Sci._ 124, 2777–2785 (2011). Article CAS PubMed Google Scholar *
Parton, R. G., Way, M., Zorzi, N. & Stang, E. Caveolin-3 associates with developing T-tubules during muscle differentiation. _J. Cell Biol._ 136, 137–154 (1997). Article CAS PubMed
PubMed Central Google Scholar * Parton, R. G., Joggerst, B. & Simons, K. Regulated internalization of caveolae. _J. Cell Biol._ 127, 1199–1215 (1994). Article CAS PubMed Google
Scholar * Boucrot, E., Howes, M. T., Kirchhausen, T. & Parton, R. G. Redistribution of caveolae during mitosis. _J. Cell Sci._ 124, 1965–1972 (2011). Article CAS PubMed PubMed
Central Google Scholar * del Pozo, M. A. et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. _Nature Cell Biol._ 7, 901–908 (2005). Article CAS PubMed
Google Scholar * Furuchi, T. & Anderson, R. G. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK). _J. Biol. Chem._ 273, 21099–21104
(1998). Article CAS PubMed Google Scholar * Fielding, C. J., Bist, A. & Fielding, P. E. Caveolin mRNA levels are up-regulated by free cholesterol and down-regulated by oxysterols in
fibroblast monolayers. _Proc. Natl Acad. Sci. USA_ 94, 3753–3758 (1997). Article CAS PubMed Google Scholar * Feron, O., Dessy, C., Moniotte, S., Desager, J. P. & Balligand, J. L.
Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. _J. Clin. Invest._ 103, 897–905 (1999). Article CAS
PubMed PubMed Central Google Scholar * Frank, P. G. et al. Caveolin-1 and regulation of cellular cholesterol homeostasis. _Am. J. Physiol. Heart Circ. Physiol._ 291, H677–H686 (2006).
Article CAS PubMed Google Scholar * Bosch, M. et al. Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. _Curr. Biol._ 21, 681–686
(2011). Article CAS PubMed PubMed Central Google Scholar * Martinez-Outschoorn, U. E., Lisanti, M. P. & Sotgia, F. Catabolic cancer-associated fibroblasts transfer energy and
biomass to anabolic cancer cells, fueling tumor growth. _Semin. Cancer Biol._ 25, 47–60 (2014). Article CAS PubMed Google Scholar * Witkiewicz, A. K. et al. An absence of stromal
caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. _Am. J. Pathol._ 174, 2023–2034 (2009). Article CAS PubMed PubMed Central Google
Scholar * Sloan, E. K. et al. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. _Am. J. Pathol._ 174, 2035–2043 (2009). REFERENCES 58 AND 59 ARE THE FIRST PAPERS TO
DEMONSTRATE THAT LOSS OF STROMAL CAV1 EXPRESSION IS ASSOCIATED WITH POOR CLINICAL OUTCOME IN PATIENTS WITH BREAST CANCER. Article CAS PubMed PubMed Central Google Scholar * Simpkins, S.
A., Hanby, A. M., Holliday, D. L. & Speirs, V. Clinical and functional significance of loss of caveolin-1 expression in breast cancer-associated fibroblasts. _J. Pathol._ 227, 490–498
(2012). Article CAS PubMed Google Scholar * Sotgia, F. et al. Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor
microenvironment. _Breast Cancer Res._ 13, 213 (2011). Article PubMed PubMed Central Google Scholar * Trimmer, C. et al. Caveolin-1 and mitochondrial SOD2 (MnSOD) function as tumor
suppressors in the stromal microenvironment: a new genetically tractable model for human cancer associated fibroblasts. _Cancer Biol. Ther._ 11, 383–394 (2011). Article CAS PubMed PubMed
Central Google Scholar * Williams, T. M. et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis _in vivo_. Role of Cav-1 in cell
invasiveness and matrix metalloproteinase (MMP-2/9) secretion. _J. Biol. Chem._ 279, 51630–51646 (2004). Article CAS PubMed Google Scholar * Witkiewicz, A. K. et al. Using the “reverse
Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. _Cell Cycle_ 11, 1108–1117 (2012). Article CAS
PubMed PubMed Central Google Scholar * Goetz, J. G. et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. _Cell_ 146, 148–163
(2011). Article CAS PubMed PubMed Central Google Scholar * Ayala, G. et al. Loss of caveolin-1 in prostate cancer stroma correlates with reduced relapse-free survival and is
functionally relevant to tumour progression. _J. Pathol._ 231, 77–87 (2013). Article CAS PubMed PubMed Central Google Scholar * Giatromanolaki, A., Koukourakis, M. I., Koutsopoulos, A.,
Mendrinos, S. & Sivridis, E. The metabolic interactions between tumor cells and tumor-associated stroma (TAS) in prostatic cancer. _Cancer Biol. Ther._ 13, 1284–1289 (2012). Article
PubMed PubMed Central Google Scholar * Di Vizio, D. et al. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation.
_Cell Cycle_ 8, 2420–2424 (2009). Article CAS PubMed PubMed Central Google Scholar * Jia, Y. et al. Down-regulation of stromal caveolin-1 expression in esophageal squamous cell
carcinoma: a potent predictor of lymph node metastases, early tumor recurrence, and poor prognosis. _Ann. Surg. Oncol._ 21, 329–336 (2014). Article PubMed Google Scholar * Zhao, X. et al.
Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. _PLoS ONE_ 8, e59102 (2013). Article CAS PubMed PubMed Central Google Scholar * Wu, K.
N. et al. Loss of stromal caveolin-1 expression in malignant melanoma metastases predicts poor survival. _Cell Cycle_ 10, 4250–4255 (2011). Article CAS PubMed Google Scholar * Chen, D.
& Che, G. Value of caveolin-1 in cancer progression and prognosis: Emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (Review). _Oncol.
Lett._ 8, 1409–1421 (2014). Article CAS PubMed PubMed Central Google Scholar * Savage, K. et al. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic
breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and _in situ_ hybridization analysis. _Clin. Cancer Res._ 13, 90–101 (2007). Article CAS PubMed Google Scholar *
Yang, G., Truong, L. D., Wheeler, T. M. & Thompson, T. C. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. _Cancer Res._ 59, 5719–5723
(1999). CAS PubMed Google Scholar * Thompson, T. C., Timme, T. L., Li, L. & Goltsov, A. Caveolin-1, a metastasis-related gene that promotes cell survival in prostate cancer.
_Apoptosis_ 4, 233–237 (1999). Article CAS PubMed Google Scholar * Sunaga, N. et al. Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell
lung cancer. _Cancer Res._ 64, 4277–4285 (2004). Article CAS PubMed Google Scholar * Felicetti, F. et al. Caveolin-1 tumor-promoting role in human melanoma. _Int. J. Cancer_ 125,
1514–1522 (2009). Article CAS PubMed PubMed Central Google Scholar * Zhang, H. et al. Restoration of caveolin-1 expression suppresses growth and metastasis of head and neck squamous
cell carcinoma. _Br. J. Cancer_ 99, 1684–1694 (2008). Article CAS PubMed PubMed Central Google Scholar * Murakami, S. et al. Caveolin-I overexpression is a favourable prognostic factor
for patients with extrahepatic bile duct carcinoma. _Br. J. Cancer_ 88, 1234–1238 (2003). CAS PubMed Google Scholar * Bender, F. C., Reymond, M. A., Bron, C. & Quest, A. F. Caveolin-1
levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. _Cancer Res._ 60, 5870–5878 (2000). CAS
PubMed Google Scholar * Wiechen, K. et al. Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. _Am. J. Pathol._ 159, 1635–1643 (2001).
Article CAS PubMed PubMed Central Google Scholar * Lin, M. I., Yu, J., Murata, T. & Sessa, W. C. Caveolin-1-deficient mice have increased tumor microvascular permeability,
angiogenesis, and growth. _Cancer Res._ 67, 2849–2856 (2007). Article CAS PubMed Google Scholar * Friedrich, T. et al. Deficiency of caveolin-1 in Apcmin/+ mice promotes colorectal
tumorigenesis. _Carcinogenesis_ 34, 2109–2118 (2013). Article CAS PubMed Google Scholar * Capozza, F. et al. Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal
hyperplasia and tumor formation. _Am. J. Pathol._ 162, 2029–2039 (2003). Article CAS PubMed PubMed Central Google Scholar * Capozza, F. et al. Genetic ablation of Cav1 differentially
affects melanoma tumor growth and metastasis in mice: role of Cav1 in Shh heterotypic signaling and transendothelial migration. _Cancer Res._ 72, 2262–2274 (2012). Article CAS PubMed
PubMed Central Google Scholar * Mercier, I. et al. Caveolin-1 and accelerated host aging in the breast tumor microenvironment: chemoprevention with rapamycin, an mTOR inhibitor and
anti-aging drug. _Am. J. Pathol._ 181, 278–293 (2012). Article CAS PubMed PubMed Central Google Scholar * Moon, H. et al. PTRF/cavin-1 neutralizes non-caveolar caveolin-1 microdomains
in prostate cancer. _Oncogene_ 33, 3561–3570 (2013). THIS PAPER SUGGESTS THAT LOSS OF STROMAL CAVIN 1 EXPRESSION IS ASSOCIATED WITH POOR OUTCOME IN PATIENTS WITH PROSTATE CANCER. Article
CAS PubMed Google Scholar * DeFilippis, R. A. et al. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. _Cancer Discov._ 2,
826–839 (2012). THIS PAPER INDICATES THAT LOSS OF STROMAL CD36 EXPRESSION IS ASSOCIATED WITH AGGRESSIVE BREAST CANCER. Article CAS PubMed PubMed Central Google Scholar * Frank, P. G. et
al. Stabilization of caveolin-1 by cellular cholesterol and scavenger receptor class B type I. _Biochemistry_ 41, 11931–11940 (2002). Article CAS PubMed Google Scholar * Garcia-Cardena,
G., Fan, R., Stern, D. F., Liu, J. & Sessa, W. C. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. _J. Biol. Chem._ 271,
27237–27240 (1996). Article CAS PubMed Google Scholar * Galbiati, F. et al. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44
MAP kinase cascade. _EMBO J._ 17, 6633–6648 (1998). Article CAS PubMed PubMed Central Google Scholar * Couet, J., Sargiacomo, M. & Lisanti, M. P. Interaction of a receptor tyrosine
kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. _J. Biol. Chem._ 272, 30429–30438 (1997). Article CAS PubMed Google
Scholar * Song, K. S. et al. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae
microdomains. _J. Biol. Chem._ 271, 9690–9697 (1996). Article CAS PubMed Google Scholar * Garcia-Cardena, G. et al. Dissecting the interaction between nitric oxide synthase (NOS) and
caveolin. Functional significance of the nos caveolin binding domain _in vivo_. _J. Biol. Chem._ 272, 25437–25440 (1997). Article CAS PubMed Google Scholar * Ariotti, N. et al. Caveolae
regulate the nanoscale organization of the plasma membrane to remotely control Ras signaling. _J. Cell Biol._ 204, 777–792 (2014). Article CAS PubMed PubMed Central Google Scholar *
Lee, S. W., Reimer, C. L., Oh, P., Campbell, D. B. & Schnitzer, J. E. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. _Oncogene_ 16, 1391–1397
(1998). Article CAS PubMed Google Scholar * Galbiati, F. et al. Caveolin-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via a
p53/p21(WAF1/Cip1)-dependent mechanism. _Mol. Biol. Cell_ 12, 2229–2244 (2001). Article CAS PubMed PubMed Central Google Scholar * Hulit, J. et al. The cyclin D1 gene is
transcriptionally repressed by caveolin-1. _J. Biol. Chem._ 275, 21203–21209 (2000). Article CAS PubMed Google Scholar * Koleske, A. J., Baltimore, D. & Lisanti, M. P. Reduction of
caveolin and caveolae in oncogenically transformed cells. _Proc. Natl Acad. Sci. USA_ 92, 1381–1385 (1995). Article CAS PubMed Google Scholar * Engelman, J. A., Zhang, X. L., Razani, B.,
Pestell, R. G. & Lisanti, M. P. p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression. Activation of Ras-MAP kinase and protein kinase a
signaling cascades transcriptionally down-regulates caveolin-1 promoter activity. _J. Biol. Chem._ 274, 32333–32341 (1999). Article CAS PubMed Google Scholar * Sherif, Z. A. &
Sultan, A. S. Divergent control of Cav-1 expression in non-cancerous Li-Fraumeni syndrome and human cancer cell lines. _Cancer Biol. Ther._ 14, 29–38 (2013). Article CAS PubMed PubMed
Central Google Scholar * Hayashi, K. et al. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. _Cancer Res._ 61, 2361–2364 (2001). CAS PubMed Google Scholar *
Lee, H. et al. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1−/− null mice show mammary
epithelial cell hyperplasia. _Am. J. Pathol._ 161, 1357–1369 (2002). Article CAS PubMed PubMed Central Google Scholar * Bonuccelli, G. et al. Caveolin-1 (P132L), a common breast cancer
mutation, confers mammary cell invasiveness and defines a novel stem cell/metastasis-associated gene signature. _Am. J. Pathol._ 174, 1650–1662 (2009). Article CAS PubMed PubMed Central
Google Scholar * Patani, N. et al. Non-existence of caveolin-1 gene mutations in human breast cancer. _Breast Cancer Res. Treat._ 131, 307–310 (2012). Article CAS PubMed Google Scholar
* Joshi, B. et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. _Cancer Res._ 68, 8210–8220 (2008). Article CAS
PubMed Google Scholar * Samarakoon, R. et al. Redox-induced Src kinase and caveolin-1 signaling in TGFβ1-initiated SMAD2/3 activation and PAI-1 expression. _PLoS ONE_ 6, e22896 (2011).
Article CAS PubMed PubMed Central Google Scholar * Zhuang, L., Lin, J., Lu, M. L., Solomon, K. R. & Freeman, M. R. Cholesterol-rich lipid rafts mediate akt-regulated survival in
prostate cancer cells. _Cancer Res._ 62, 2227–2231 (2002). CAS PubMed Google Scholar * Xia, H. et al. Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. _Am. J.
Pathol._ 176, 2626–2637 (2010). Article CAS PubMed PubMed Central Google Scholar * Trimboli, A. J. et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. _Nature_
461, 1084–1091 (2009). Article CAS PubMed PubMed Central Google Scholar * Sumitomo, M. et al. Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN.
_Cancer Cell_ 5, 67–78 (2004). Article CAS PubMed Google Scholar * Midgley, A. C. et al. Transforming growth factor-β1 (TGFβ1)-stimulated fibroblast to myofibroblast differentiation is
mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. _J. Biol. Chem._ 288, 14824–14838 (2013). Article CAS PubMed
PubMed Central Google Scholar * Calon, A., Tauriello, D. V. & Batlle, E. TGFβ in CAF-mediated tumor growth and metastasis. _Semin. Cancer Biol._ 25, 15–22 (2014). Article CAS PubMed
Google Scholar * Kojima, Y. et al. Autocrine TGFβ and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. _Proc. Natl
Acad. Sci. USA_ 107, 20009–20014 (2010). Article PubMed Google Scholar * Guido, C. et al. Metabolic reprogramming of cancer-associated fibroblasts by TGFβ drives tumor growth: connecting
TGFβ signaling with “Warburg-like” cancer metabolism and L-lactate production. _Cell Cycle_ 11, 3019–3035 (2012). Article CAS PubMed PubMed Central Google Scholar * Rosenthal, E. et al.
Elevated expression of TGFβ1 in head and neck cancer-associated fibroblasts. _Mol. Carcinog._ 40, 116–121 (2004). Article CAS PubMed Google Scholar * Calon, A. et al. Dependency of
colorectal cancer on a TGFβ-driven program in stromal cells for metastasis initiation. _Cancer Cell_ 22, 571–584 (2012). Article CAS PubMed PubMed Central Google Scholar * Ting, H. J.
et al. Silibinin prevents prostate cancer cell-mediated differentiation of naive fibroblasts into cancer-associated fibroblast phenotype by targeting TGF β2. _Mol. Carcinog._
http://dx.doi.org/10.1002/mc.22135 (2015). * Razani, B. et al. Caveolin-1 regulates transforming growth factor TGFβ/SMAD signaling through an interaction with the TGFβ type I receptor. _J.
Biol. Chem._ 276, 6727–6738 (2001). Article CAS PubMed Google Scholar * Stuelten, C. H. et al. Breast cancer cells induce stromal fibroblasts to express MMP9 via secretion of TNFα and
TGFβ. _J. Cell Sci._ 118, 2143–2153 (2005). Article CAS PubMed Google Scholar * Yu, Q. & Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates
TGFβ and promotes tumor invasion and angiogenesis. _Genes Dev._ 14, 163–176 (2000). PubMed PubMed Central Google Scholar * Grubisha, M. J., Cifuentes, M. E., Hammes, S. R. & Defranco,
D. B. A local paracrine and endocrine network involving TGFβ, Cox-2, ROS, and estrogen receptor β influences reactive stromal cell regulation of prostate cancer cell motility. _Mol.
Endocrinol._ 26, 940–954 (2012). Article CAS PubMed PubMed Central Google Scholar * Toullec, A. et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading.
_EMBO Mol. Med._ 2, 211–230 (2010). Article CAS PubMed PubMed Central Google Scholar * Bailey, K. M. & Liu, J. Caveolin-1 up-regulation during epithelial to mesenchymal transition
is mediated by focal adhesion kinase. _J. Biol. Chem._ 283, 13714–13724 (2008). Article CAS PubMed PubMed Central Google Scholar * Li, L. et al. Caveolin-1 promotes autoregulatory,
Akt-mediated induction of cancer-promoting growth factors in prostate cancer cells. _Mol. Cancer Res._ 7, 1781–1791 (2009). Article CAS PubMed Google Scholar * Meyer, C. et al. Distinct
dedifferentiation processes affect caveolin-1 expression in hepatocytes. _Cell Commun. Signal_ 11, 6 (2013). Article CAS PubMed PubMed Central Google Scholar * Asterholm, I. W., Mundy,
D. I., Weng, J., Anderson, R. G. & Scherer, P. E. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. _Cell. Metab._ 15, 171–185 (2012).
Article CAS PubMed PubMed Central Google Scholar * Martinez-Outschoorn, U. E. et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1
induction and NFκB activation in the tumor stromal microenvironment. _Cell Cycle_ 9, 3515–3533 (2010). Article CAS PubMed PubMed Central Google Scholar * Chiavarina, B. et al. HIF1-α
functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: Autophagy drives compartment-specific oncogenesis. _Cell Cycle_ 9, 3534–3551
(2010). Article CAS PubMed PubMed Central Google Scholar * Shaul, P. W. et al. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. _J. Biol. Chem._ 271,
6518–6522 (1996). Article CAS PubMed Google Scholar * Ju, H., Zou, R., Venema, V. J. & Venema, R. C. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits
synthase activity. _J. Biol. Chem._ 272, 18522–18525 (1997). Article CAS PubMed Google Scholar * Brouet, A. et al. Antitumor effects of _in vivo_ caveolin gene delivery are associated
with the inhibition of the proangiogenic and vasodilatory effects of nitric oxide. _Faseb J._ 19, 602–604 (2005). Article CAS PubMed Google Scholar * Augsten, M. et al. Cancer-associated
fibroblasts expressing CXCL14 rely upon Nos1-derived nitric oxide signaling for their tumor supporting properties. _Cancer Res._ 74, 2999–3010 (2014). Article CAS PubMed Google Scholar
* Bolanos, J. P., Peuchen, S., Heales, S. J., Land, J. M. & Clark, J. B. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. _J. Neurochem._
63, 910–916 (1994). Article CAS PubMed Google Scholar * Riobo, N. A. et al. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation.
_Biochem. J._ 359, 139–145 (2001). Article CAS PubMed PubMed Central Google Scholar * Moncada, S. & Erusalimsky, J. D. Does nitric oxide modulate mitochondrial energy generation and
apoptosis? _Nature Rev. Mol. Cell Biol._ 3, 214–220 (2002). Article CAS Google Scholar * Xu, W., Liu, L., Charles, I. G. & Moncada, S. Nitric oxide induces coupling of mitochondrial
signalling with the endoplasmic reticulum stress response. _Nature Cell Biol._ 6, 1129–1134 (2004). Article CAS PubMed Google Scholar * Galkin, A. & Moncada, S. S-nitrosation of
mitochondrial complex I depends on its structural conformation. _J. Biol. Chem._ 282, 37448–37453 (2007). Article CAS PubMed Google Scholar * Peterson, T. E. et al. Opposing effects of
reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. _Circ. Res._ 85, 29–37 (1999). Article CAS PubMed Google Scholar * Parat, M.
O., Stachowicz, R. Z. & Fox, P. L. Oxidative stress inhibits caveolin-1 palmitoylation and trafficking in endothelial cells. _Biochem. J._ 361, 681–688 (2002). Article CAS PubMed
PubMed Central Google Scholar * Whitaker-Menezes, D. et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated
fibroblasts. _Cell Cycle_ 10, 1772–1783 (2011). Article CAS PubMed PubMed Central Google Scholar * Whitaker-Menezes, D. et al. Hyperactivation of oxidative mitochondrial metabolism in
epithelial cancer cells _in situ_: visualizing the therapeutic effects of metformin in tumor tissue. _Cell Cycle_ 10, 4047–4064 (2011). Article CAS PubMed PubMed Central Google Scholar
* Erez, N., Truitt, M., Olson, P., Arron, S. T. & Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an
NF-κB-dependent manner. _Cancer Cell_ 17, 135–147 (2010). Article CAS PubMed Google Scholar * Rius, J. et al. NF-κB links innate immunity to the hypoxic response through transcriptional
regulation of HIF-1α. _Nature_ 453, 807–811 (2008). Article CAS PubMed PubMed Central Google Scholar * Subramaniam, K. S. et al. Cancer-associated fibroblasts promote proliferation of
endometrial cancer cells. _PLoS ONE_ 8, e68923 (2013). Article CAS PubMed PubMed Central Google Scholar * Vicent, S. et al. Cross-species functional analysis of cancer-associated
fibroblasts identifies a critical role for CLCF1 and IL-6 in non-small cell lung cancer _in vivo_. _Cancer Res._ 72, 5744–5756 (2012). Article CAS PubMed Google Scholar * Erez, N.,
Glanz, S., Raz, Y., Avivi, C. & Barshack, I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. _Biochem. Biophys. Res. Commun._ 437,
397–402 (2013). Article CAS PubMed Google Scholar * Martinez-Outschoorn, U. E. et al. Cytokine production and inflammation drive autophagy in the tumor microenvironment: role of stromal
caveolin-1 as a key regulator. _Cell Cycle_ 10, 1784–1793 (2011). Article CAS PubMed PubMed Central Google Scholar * Sotgia, F. et al. Caveolin-1−/− null mammary stromal fibroblasts
share characteristics with human breast cancer-associated fibroblasts. _Am. J. Pathol._ 174, 746–761 (2009). Article CAS PubMed PubMed Central Google Scholar * Park, D. S. et al.
Caveolin-1 null−/− mice show dramatic reductions in life span. _Biochemistry_ 42, 15124–15131 (2003). Article CAS PubMed Google Scholar * Le Lay, S. et al. The lipoatrophic caveolin-1
deficient mouse model reveals autophagy in mature adipocytes. _Autophagy_ 6, 754–763 (2010). Article CAS PubMed Google Scholar * Chaudhri, V. K. et al. Metabolic alterations in lung
cancer-associated fibroblasts correlated with increased glycolytic metabolism of the tumor. _Mol. Cancer Res._ 11, 579–592 (2013). Article CAS PubMed PubMed Central Google Scholar *
Crighton, D. et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. _Cell_ 126, 121–134 (2006). Article CAS PubMed Google Scholar * Yang, G. et al. The chemokine
growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. _Proc. Natl Acad. Sci. USA_ 103, 16472–16477 (2006). Article CAS
PubMed Google Scholar * Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. _Cell_ 120, 513–522 (2005). Article CAS PubMed Google
Scholar * Paradis, V. et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. _Hum. Pathol._ 32, 327–332 (2001). Article CAS PubMed Google
Scholar * Sun, Y. et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. _Nature Med._ 18, 1359–1368 (2012). Article CAS
PubMed Google Scholar * Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between
cancer and aging. _Proc. Natl Acad. Sci. USA_ 98, 12072–12077 (2001). Article CAS PubMed Google Scholar * Bavik, C. et al. The gene expression program of prostate fibroblast senescence
modulates neoplastic epithelial cell proliferation through paracrine mechanisms. _Cancer Res._ 66, 794–802 (2006). Article CAS PubMed Google Scholar * Dimmer, K. S., Friedrich, B., Lang,
F., Deitmer, J. W. & Broer, S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. _Biochem. J._ 350, 219–227 (2000).
Article CAS PubMed PubMed Central Google Scholar * Ullah, M. S., Davies, A. J. & Halestrap, A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by
hypoxia through a HIF-1α-dependent mechanism. _J. Biol. Chem._ 281, 9030–9037 (2006). Article CAS PubMed Google Scholar * Martins, D. et al. Loss of caveolin-1 and gain of MCT4
expression in the tumor stroma: key events in the progression from an _in situ_ to an invasive breast carcinoma. _Cell Cycle_ 12, 2684–2690 (2013). Article CAS PubMed PubMed Central
Google Scholar * Cowell, C. F. et al. Progression from ductal carcinoma _in situ_ to invasive breast cancer: revisited. _Mol. Oncol._ 7, 859–869 (2013). Article PubMed PubMed Central
Google Scholar * Pavlides, S. et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. _Cell Cycle_ 8, 3984–4001 (2009). THIS IS THE
FIRST PAPER TO DEMONSTRATE THAT AEROBIC GLYCOLYSIS (THE WARBURG EFFECT) OCCURS IN CAFS AND THE TUMOUR STROMA. Article CAS PubMed Google Scholar * Shiroto, T. et al. Caveolin-1 is a
critical determinant of autophagy, metabolic switching, and oxidative stress in vascular endothelium. _PLoS ONE_ 9, e87871 (2014). Article CAS PubMed PubMed Central Google Scholar *
Martinez-Outschoorn, U. E. et al. Ketone bodies and two-compartment tumor metabolism: stromal ketone production fuels mitochondrial biogenesis in epithelial cancer cells. _Cell Cycle_ 11,
3956–3963 (2012). Article CAS PubMed PubMed Central Google Scholar * Fiaschi, T. et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma
interplay. _Cancer Res._ 72, 5130–5140 (2012). Article CAS PubMed Google Scholar * Brauer, H. A. et al. Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast
cancer. _Clin. Cancer Res._ 19, 571–585 (2013). Article CAS PubMed Google Scholar * Larsson, N. G. et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and
embryogenesis in mice. _Nature Genet._ 18, 231–236 (1998). Article CAS PubMed Google Scholar * Bonuccelli, G. et al. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that
epithelial cancer cells use oxidative mitochondrial metabolism. _Cell Cycle_ 9, 3506–3514 (2010). Article CAS PubMed PubMed Central Google Scholar * Vegran, F., Boidot, R., Michiels,
C., Sonveaux, P. & Feron, O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. _Cancer Res._ 71,
2550–2560 (2011). Article CAS PubMed Google Scholar * Ramanathan, A., Wang, C. & Schreiber, S. L. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic
measurements. _Proc. Natl Acad. Sci. USA_ 102, 5992–5997 (2005). Article CAS PubMed Google Scholar * Chiavarina, B. et al. Pyruvate kinase expression (PKM1 and PKM2) in cancer-associated
fibroblasts drives stromal nutrient production and tumor growth. _Cancer Biol. Ther._ 12, 1101–1113 (2011). Article CAS PubMed PubMed Central Google Scholar * Sanita, P. et al.
Tumor-stroma metabolic relationship based on lactate shuttle can sustain prostate cancer progression. _BMC Cancer_ 14, 154 (2014). Article CAS PubMed PubMed Central Google Scholar *
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. _Nature Med._ 17, 1498–1503 (2011). Article CAS PubMed Google Scholar * Kim,
H. M., Kim do, H., Jung, W. H. & Koo, J. S. Metabolic phenotypes in primary unknown metastatic carcinoma. _J. Transl. Med._ 12, 2 (2014). Article CAS PubMed PubMed Central Google
Scholar * Pellerin, L. & Magistretti, P. J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. _Proc. Natl
Acad. Sci. USA_ 91, 10625–10629 (1994). Article CAS PubMed Google Scholar * Kasischke, K. A., Vishwasrao, H. D., Fisher, P. J., Zipfel, W. R. & Webb, W. W. Neural activity triggers
neuronal oxidative metabolism followed by astrocytic glycolysis. _Science_ 305, 99–103 (2004). Article CAS PubMed Google Scholar * Wallace, D. C. Mitochondria and cancer. _Nature Rev.
Cancer_ 12, 685–698 (2012). Article CAS Google Scholar * Birsoy, K. et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. _Nature_ 508, 108–112
(2014). Article CAS PubMed PubMed Central Google Scholar * Zhang, X. et al. Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised
microenvironments. _Nature Commun._ 5, 3295 (2014). Article CAS Google Scholar * Ni Chonghaile, T. et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic
chemotherapy. _Science_ 334, 1129–1133 (2011). Article CAS PubMed Google Scholar * Lee, H. et al. Palmitoylation of caveolin-1 at a single site (Cys-156) controls its coupling to the
c-Src tyrosine kinase: targeting of dually acylated molecules (GPI-linked, transmembrane, or cytoplasmic) to caveolae effectively uncouples c-Src and caveolin-1 (TYR-14). _J. Biol. Chem._
276, 35150–35158 (2001). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors apologize that they were unable to cite many primary references owing to space
limitations. U.E.M.-O. was supported, in part, by funding from the US National Cancer Institute of the National Institutes of Health under Award Number K08 CA175193-01A1. M.P.L. and F.S.
were supported, in part, by funding from the European Union (ERC Advanced Grant), Breakthrough Breast Cancer and the Manchester Cancer Research Centre (MCRC). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Medical Oncology, Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, 19107, Pennsylvania, USA Ubaldo E. Martinez-Outschoorn * Breakthrough
Breast Cancer Research Unit, Institute of Cancer Sciences, University of Manchester, Manchester, M20 4BX, UK Federica Sotgia & Michael P. Lisanti * Manchester Centre for Cellular
Metabolism (MCCM), University of Manchester, M20 4BX, Manchester, UK Federica Sotgia & Michael P. Lisanti Authors * Ubaldo E. Martinez-Outschoorn View author publications You can also
search for this author inPubMed Google Scholar * Federica Sotgia View author publications You can also search for this author inPubMed Google Scholar * Michael P. Lisanti View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Federica Sotgia or Michael P. Lisanti. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing financial interests. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR
FIG. 4 POWERPOINT SLIDE FOR FIG. 5 POWERPOINT SLIDE FOR FIG. 6 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Martinez-Outschoorn, U., Sotgia, F. &
Lisanti, M. Caveolae and signalling in cancer. _Nat Rev Cancer_ 15, 225–237 (2015). https://doi.org/10.1038/nrc3915 Download citation * Published: 24 March 2015 * Issue Date: April 2015 *
DOI: https://doi.org/10.1038/nrc3915 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative