Play all audios:
Fatty acid-binding proteins (FABPs) are versatile proteins that can modulate lipid fluxes, trafficking, signalling and metabolism
Fatty acid-binding protein, adipocyte (FABP4) regulates metabolic and inflammatory pathways, and in mouse models its inhibition can improve type 2 diabetes mellitus and atherosclerosis
FABP4 is actively secreted by adipocytes and its levels are increased in obesity; in humans, elevated circulating FABP4 levels are associated with obesity, metabolic disease and cardiac
dysfunction
Circulating FABP4 is secreted through a vesicular pathway and has pleiotropic roles that include the stimulation of hepatic glucose production
Targeting FABP4 offers a novel therapeutic approach for the treatment of many metabolic diseases
The signalling components of hormonal FABP4 and determinants of FABP-mediated functions in the context of specific lipid or other cargo are issues that must be addressed in future research
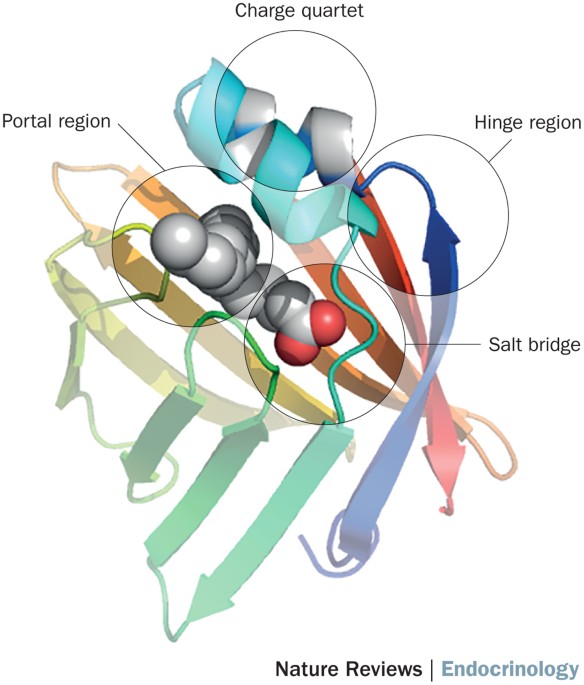
Intracellular and extracellular interactions with proteins enables the functional and mechanistic diversity of lipids. Fatty acid-binding proteins (FABPs) were originally described as
intracellular proteins that can affect lipid fluxes, metabolism and signalling within cells. As the functions of this protein family have been further elucidated, it has become evident that
they are critical mediators of metabolism and inflammatory processes, both locally and systemically, and therefore are potential therapeutic targets for immunometabolic diseases. In
particular, genetic deficiency and small molecule-mediated inhibition of FABP4 (also known as aP2) and FABP5 can potently improve glucose homeostasis and reduce atherosclerosis in mouse
models. Further research has shown that in addition to their intracellular roles, some FABPs are found outside the cells, and FABP4 undergoes regulated, vesicular secretion. The circulating
form of FABP4 has crucial hormonal functions in systemic metabolism. In this Review we discuss the roles and regulation of both intracellular and extracellular FABP actions, highlighting new
insights that might direct drug discovery efforts and opportunities for management of chronic metabolic diseases.
The authors thank members of the Hotamisligil and Bernlohr laboratories for helpful discussions. We thank A. P. Arruda for assistance in generating the initial figures, and K. Claiborn for
critical reading and editing of the manuscript. The Hotamisligil laboratory is supported in this area by research funding from the NIH (grant number DK064360) and a sponsored research
agreement with Union Chimique Belge. The Bernlohr laboratory is supported by the NIH (grant number DK053189).
Department of Genetics and Complex Diseases and Sabri Ülker Center, Harvard T.H. Chan School of Public Health, 677 Huntington Avenue, Boston, 02115, MA, USA
Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota, 321 Church Street SE, Minneapolis, 55455, MN, USA
Both authors researched data for the article, discussed the content, and wrote, reviewed and edited the manuscript before submission.
G.S.H. receives research funding under a sponsored agreement with Union Chimique Belge. D.A.B. declares no competing interests.
The association of circulating FABP4 with different human diseases.This figure is an adaptation of Figure 3 in the main text, but includes a complete list of references for the association
of circulating FABP4 with different human diseases. Abbreviations: FABP4, fatty acid binding protein 4; NAFLD, nonalcoholic fatty-liver disease. (PDF 96 kb)
Anyone you share the following link with will be able to read this content: