Play all audios:
KEY POINTS * Immunologically, prostate cancer is amenable to currently available, as well as emerging, cancer immunotherapies * Tumour-associated immunosuppressive mechanisms restrict the
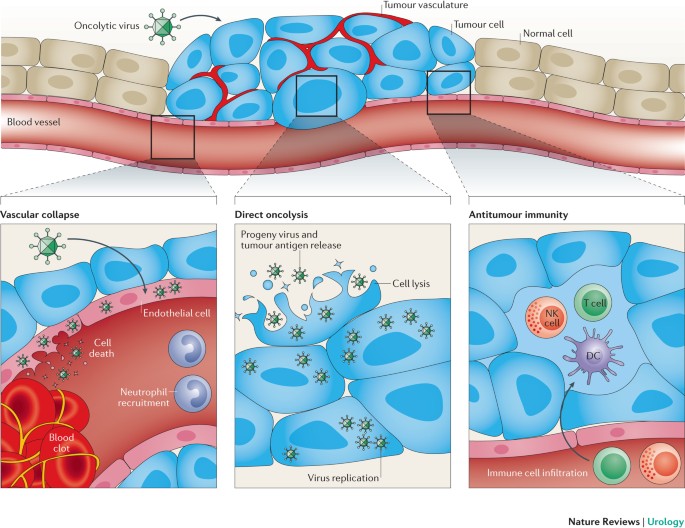
development of antitumour immune responses and are the largest barrier to the effectiveness of immunotherapies for prostate cancer * Oncolytic viruses can target prostate cancer through
three mechanisms: direct killing of tumour cells through oncolysis, destruction of the tumour vasculature, and initiation of antitumour immunity * Oncolytic viruses can override
tumour-associated immunosuppressive mechanisms and create an environment conducive to the development of antigen-specific and protective antitumour immunity * Oncolytic-virus-induced
reprogramming of the tumour microenvironment can be exploited in strategic treatment combinations to achieve improved effectiveness of otherwise subpar cancer immunotherapies, including
those based on immune checkpoint inhibitors ABSTRACT The clinical effectiveness of immunotherapies for prostate cancer remains subpar compared with that for other cancers. The goal of most
immunotherapies is the activation of immune effectors, such as T cells and natural killer cells, as the presence of these activated mediators positively correlates with patient outcomes.
Clinical evidence shows that prostate cancer is immunogenic, accessible to the immune system, and can be targeted by antitumour immune responses. However, owing to the detrimental effects of
prostate-cancer-associated immunosuppression, even the newest immunotherapeutic approaches fail to initiate the clinically desired antitumour immune reaction. Oncolytic viruses, originally
used for their preferential cancer-killing activity, are now being recognized for their ability to overturn cancer-associated immune evasion and promote otherwise absent antitumour immunity.
This oncolytic-virus-induced subversion of tumour-associated immunosuppression can potentiate the effectiveness of current immunotherapeutics, including immune checkpoint inhibitors (for
example, antibodies against programmed cell death protein 1 (PD1), programmed cell death 1 ligand 1 (PDL1), and cytotoxic T lymphocyte antigen 4 (CTLA4)) and chemotherapeutics that induce
immunogenic cell death (for example, doxorubicin and oxaliplatin). Importantly, oncolytic-virus-induced antitumour immunity targets existing prostate cancer cells and also establishes
long-term protection against future relapse. Hence, the strategic use of oncolytic viruses as monotherapies or in combination with current immunotherapies might result in the next
breakthrough in prostate cancer immunotherapy. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to
this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS USING ONCOLYTIC VIRUSES TO IGNITE THE TUMOUR IMMUNE MICROENVIRONMENT IN BLADDER CANCER Article 28 June 2021 VACCINES AS TREATMENTS FOR
PROSTATE CANCER Article 06 March 2023 REOVIRUS MUTANT JIN-3 EXHIBITS LYTIC AND IMMUNE-STIMULATORY EFFECTS IN PRECLINICAL HUMAN PROSTATE CANCER MODELS Article Open access 16 June 2021
REFERENCES * Attard, G. _ et al_. Prostate cancer. _Lancet_ 387, 70–82 (2016). Article PubMed Google Scholar * McNeel, D. G. _ et al_. The Society for Immunotherapy of Cancer consensus
statement on immunotherapy for the treatment of prostate carcinoma. _J. Immunother. Cancer._ 4, 92 (2016). Article PubMed PubMed Central Google Scholar * Maia, M. C. & Hansen, A. R.
A comprehensive review of immunotherapies in prostate cancer. _Crit. Rev. Oncol. Hematol._ 113, 292–303 (2017). Article PubMed Google Scholar * Karan, D., Holzbeierlein, J. M., Van
Veldhuizen, P. & Thrasher, J. B. Cancer immunotherapy: a paradigm shift for prostate cancer treatment. _Nat. Rev. Urol._ 9, 376–385 (2012). Article CAS PubMed Google Scholar * Yap,
T. A. _ et al_. Drug discovery in advanced prostate cancer: translating biology into therapy. _Nat. Rev. Drug Discov._ 15, 699–718 (2016). Article CAS PubMed Google Scholar * Topalian,
S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. _Cancer. Cell._ 27, 450–461 (2015). Article CAS PubMed PubMed Central
Google Scholar * Drake, C. G. Prostate cancer as a model for tumour immunotherapy. _Nat. Rev. Immunol._ 10, 580–593 (2010). Article CAS PubMed PubMed Central Google Scholar *
Kiessling, A. _ et al_. Advances in specific immunotherapy for prostate cancer. _Eur. Urol._ 53, 694–708 (2008). Article CAS PubMed Google Scholar * Silvestri, I. _ et al_. A perspective
of immunotherapy for prostate cancer. _Cancers_ 8, E64 (2016). Article CAS PubMed Google Scholar * Di Lorenzo, G., Buonerba, C. & Kantoff, P. W. Immunotherapy for the treatment of
prostate cancer. _Nat. Rev. Clin. Oncol._ 8, 551–561 (2011). Article CAS PubMed Google Scholar * Lichty, B. D., Breitbach, C. J., Stojdl, D. F. & Bell, J. C. Going viral with cancer
immunotherapy. _Nat. Rev. Cancer._ 14, 559–567 (2014). Article CAS PubMed Google Scholar * Taguchi, S., Fukuhara, H., Homma, Y. & Todo, T. Current status of clinical trials assessing
oncolytic virus therapy for urological cancers. _Int. J. Urol._ 24, 342–351 (2017). Article PubMed Google Scholar * Gujar, S., Pol, J. G., Kim, Y., Lee, P. W. & Kroemer, G. Antitumor
benefits of antiviral immunity: an underappreciated aspect of oncolytic virotherapies. _Trends Immunol._ https://doi.org/10.1016/j.it.2017.11.006 (2017). Article CAS PubMed Google
Scholar * Delwar, Z., Zhang, K., Rennie, P. S. & Jia, W. Oncolytic virotherapy for urological cancers. _Nat. Rev. Urol._ 13, 334–352 (2016). Article CAS PubMed Google Scholar *
Gravitz, L. Cancer immunotherapy. _Nature_ 504, S1 (2013). Article CAS PubMed Google Scholar * Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward
combination strategies with curative potential. _Cell_ 161, 205–214 (2015). Article CAS PubMed PubMed Central Google Scholar * Peshwa, M. V., Shi, J. D., Ruegg, C., Laus, R. & van
Schooten, W. C. Induction of prostate tumor-specific CD8+ cytotoxic T-lymphocytes in vitro using antigen-presenting cells pulsed with prostatic acid phosphatase peptide. _Prostate_ 36,
129–138 (1998). Article CAS PubMed Google Scholar * Machlenkin, A. _ et al_. Human CTL epitopes prostatic acid phosphatase-3 and six-transmembrane epithelial antigen of prostate-3 as
candidates for prostate cancer immunotherapy. _Cancer Res._ 65, 6435–6442 (2005). Article CAS PubMed Google Scholar * Johnson, L. E., Frye, T. P., Chinnasamy, N., Chinnasamy, D. &
McNeel, D. G. Plasmid DNA vaccine encoding prostatic acid phosphatase is effective in eliciting autologous antigen-specific CD8+ T cells. _Cancer Immunol. Immunother._ 56, 885–895 (2007).
Article CAS PubMed Google Scholar * Gujar, S. A., Pan, D. A., Marcato, P., Garant, K. A. & Lee, P. W. Oncolytic virus-initiated protective immunity against prostate cancer. _Mol.
Ther._ 19, 797–804 (2011). Article CAS PubMed PubMed Central Google Scholar * Pitt, J. M. _ et al_. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and
-extrinsic factors. _Immunity_ 44, 1255–1269 (2016). Article CAS PubMed Google Scholar * Barach, Y. S., Lee, J. S. & Zang, X. T cell coinhibition in prostate cancer: new immune
evasion pathways and emerging therapeutics. _Trends Mol. Med._ 17, 47–55 (2011). Article CAS PubMed PubMed Central Google Scholar * Vesely, M. D. & Schreiber, R. D. Cancer
immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. _Ann. NY Acad. Sci._ 1284, 1–5 (2013). Article CAS PubMed Google Scholar * Ostrand-Rosenberg, S., Sinha,
P., Beury, D. W. & Clements, V. K. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. _Semin. Cancer
Biol._ 22, 275–281 (2012). Article CAS PubMed PubMed Central Google Scholar * Gajewski, T. F., Schreiber, H. & Fu, Y. X. Innate and adaptive immune cells in the tumor
microenvironment. _Nat. Immunol._ 14, 1014–1022 (2013). Article CAS PubMed PubMed Central Google Scholar * van der Burg, S. H., Arens, R., Ossendorp, F., van Hall, T. & Melief, C.
J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. _Nat. Rev. Cancer._ 16, 219–233 (2016). Article CAS PubMed Google Scholar * Sharma, P. &
Allison, J. P. The future of immune checkpoint therapy. _Science_ 348, 56–61 (2015). Article CAS PubMed Google Scholar * Smyth, M. J., Ngiow, S. F., Ribas, A. & Teng, M. W.
Combination cancer immunotherapies tailored to the tumour microenvironment. _Nat. Rev. Clin. Oncol._ 13, 143–158 (2016). Article CAS PubMed Google Scholar * Modena, A. _ et al_. Immune
checkpoint inhibitors and prostate cancer: a new frontier? _Oncol. Rev._ 10, 293 (2016). Article CAS PubMed PubMed Central Google Scholar * Gujar, S. _ et al_. Multifaceted therapeutic
targeting of ovarian peritoneal carcinomatosis through virus-induced immunomodulation. _Mol. Ther._ 21, 338–347 (2013). Article CAS PubMed Google Scholar * Gujar, S. A., Marcato, P.,
Pan, D. & Lee, P. W. Reovirus virotherapy overrides tumor antigen presentation evasion and promotes protective antitumor immunity. _Mol. Cancer. Ther._ 9, 2924–2933 (2010). Article CAS
PubMed Google Scholar * Zhao, X., Chester, C., Rajasekaran, N., He, Z. & Kohrt, H. E. Strategic combinations: the future of oncolytic virotherapy with reovirus. _Mol. Cancer. Ther._
15, 767–773 (2016). Article CAS PubMed Google Scholar * Fend, L. _ et al_. Immune checkpoint blockade, immunogenic chemotherapy or IFN-alpha blockade boost the local and abscopal effects
of oncolytic virotherapy. _Cancer Res._ 77, 4146–4157 (2017). Article CAS PubMed Google Scholar * Tanoue, K. _ et al_. Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances
antitumor effects of chimeric antigen receptor T cells in solid tumors. _Cancer Res._ 77, 2040–2051 (2017). Article CAS PubMed PubMed Central Google Scholar * Montironi, R. _ et al_.
Emerging immunotargets and immunotherapies in prostate cancer. _Curr. Drug Targets_ 17, 777–782 (2016). Article CAS PubMed Google Scholar * Lu, X. _ et al_. Effective combinatorial
immunotherapy for castration-resistant prostate cancer. _Nature_ 543, 728–732 (2017). Article CAS PubMed PubMed Central Google Scholar * Gao, J. _ et al_. VISTA is an inhibitory immune
checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. _Nat. Med._ 23, 551–555 (2017). Article CAS PubMed PubMed Central Google Scholar * Zitvogel, L.,
Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. _Nat. Rev. Immunol._ 8, 59–73 (2008). Article CAS PubMed Google Scholar * Chen, D. S. &
Mellman, I. Elements of cancer immunity and the cancer-immune set point. _Nature_ 541, 321–330 (2017). Article CAS PubMed Google Scholar * Jung, S. _ et al_. In vivo depletion of CD11c+
dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. _Immunity_ 17, 211–220 (2002). Article CAS PubMed PubMed Central Google Scholar * Baas, W. _ et
al_. Immune characterization of the programmed death receptor pathway in high risk prostate cancer. _Clin. Genitourin. Cancer_ 15, 577–581 (2017). Article PubMed Google Scholar * Madan,
R. A. & Gulley, J. L. Prostate cancer: Better VISTAs ahead? Potential and pitfalls of immunotherapy. _Nat. Rev. Urol._ 14, 455–456 (2017). Article PubMed PubMed Central Google Scholar
* Sfanos, K. S. _ et al_. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. _Prostate_ 69, 1694–1703 (2009). Article CAS PubMed PubMed Central Google Scholar *
De Marzo, A. M. _ et al_. Inflammation in prostate carcinogenesis. _Nat. Rev. Cancer_ 7, 256–269 (2007). Article CAS PubMed PubMed Central Google Scholar * Taylor, B. S. _ et al_.
Integrative genomic profiling of human prostate cancer. _Cancer. Cell._ 18, 11–22 (2010). Article CAS PubMed PubMed Central Google Scholar * Tan, S. H. _ et al_. Evaluation of ERG
responsive proteome in prostate cancer. _Prostate_ 74, 70–89 (2014). Article CAS PubMed Google Scholar * Kim, J. J. _ et al_. Induction of immune responses and safety profiles in rhesus
macaques immunized with a DNA vaccine expressing human prostate specific antigen. _Oncogene_ 20, 4497–4506 (2001). Article CAS PubMed Google Scholar * Sfanos, K. S. _ et al_. Phenotypic
analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. _Clin. Cancer Res._ 14, 3254–3261 (2008). Article CAS PubMed PubMed Central Google Scholar * Lopez-Bujanda,
Z. & Drake, C. G. Myeloid-derived cells in prostate cancer progression: phenotype and prospective therapies. _J. Leukoc. Biol._ 102, 393–406 (2017). Article CAS PubMed PubMed Central
Google Scholar * Sizemore, G. M. _ et al_. Stromal PTEN inhibits the expansion of mammary epithelial stem cells through Jagged-1. _Oncogene_ 36, 2297–2308 (2017). Article CAS PubMed
Google Scholar * Kaukonen, R. _ et al_. Normal stroma suppresses cancer cell proliferation via mechanosensitive regulation of JMJD1a-mediated transcription. _Nat. Commun._ 7, 12237 (2016).
Article CAS PubMed PubMed Central Google Scholar * Turley, S. J., Cremasco, V. & Astarita, J. L. Immunological hallmarks of stromal cells in the tumour microenvironment. _Nat. Rev.
Immunol._ 15, 669–682 (2015). Article CAS PubMed Google Scholar * Kalluri, R. The biology and function of fibroblasts in cancer. _Nat. Rev. Cancer._ 16, 582–598 (2016). Article CAS
PubMed Google Scholar * Nagarsheth, N., Wicha, M. S. & Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. _Nat. Rev. Immunol._ 17, 559–572
(2017). Article CAS PubMed PubMed Central Google Scholar * Vinay, D. S. _ et al_. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. _Semin. Cancer Biol._ 35
(Suppl.), S185–S198 (2015). Article CAS PubMed Google Scholar * Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. _Nature_ 473, 298–307
(2011). Article CAS PubMed PubMed Central Google Scholar * Smith, B. A. _ et al_. A basal stem cell signature identifies aggressive prostate cancer phenotypes. _Proc. Natl Acad. Sci.
USA_ 112, E6544–E6552 (2015). Article CAS PubMed PubMed Central Google Scholar * Chauhan, A. & Anthony, L. Immune oncology and neuroendocrine tumors. _Ann. Oncol._ 28, 2322–2323
(2017). Article CAS PubMed Google Scholar * Alvarado, A. G. _ et al_. Glioblastoma cancer stem cells evade innate immune suppression of self-renewal through reduced TLR4 expression.
_Cell. Stem Cell._ 20, 450–461.e4 (2017). Article CAS PubMed PubMed Central Google Scholar * Sultan, M. _ et al_. Hide-and-seek: the interplay between cancer stem cells and the immune
system. _Carcinogenesis_ 38, 107–118 (2017). Article CAS PubMed Google Scholar * Bishop, J., Sangha, B., Gleave, M. & Zoubeidi, A. Immune evasion strategies of neuroendocrine-like
Enzalutamide resistant prostate cancer. _J. Immunother. Cancer_ 1 (Suppl. 1), P147 (2013). Article PubMed Central Google Scholar * Bronte, V. _ et al_. Boosting antitumor responses of T
lymphocytes infiltrating human prostate cancers. _J. Exp. Med._ 201, 1257–1268 (2005). Article CAS PubMed PubMed Central Google Scholar * Mercader, M. _ et al_. T cell infiltration of
the prostate induced by androgen withdrawal in patients with prostate cancer. _Proc. Natl Acad. Sci. USA_ 98, 14565–14570 (2001). Article CAS PubMed PubMed Central Google Scholar *
Healy, C. G. _ et al_. Impaired expression and function of signal-transducing zeta chains in peripheral T cells and natural killer cells in patients with prostate cancer. _Cytometry_ 32,
109–119 (1998). Article CAS PubMed Google Scholar * Ness, N. _ et al_. The prognostic role of immune checkpoint markers programmed cell death protein 1 (PD-1) and programmed death ligand
1 (PD-L1) in a large, multicenter prostate cancer cohort. _Oncotarget_ 8, 26789–26801 (2017). Article PubMed PubMed Central Google Scholar * Morse, M. D. & McNeel, D. G. T cells
localized to the androgen-deprived prostate are TH1 and TH17 biased. _Prostate_ 72, 1239–1247 (2012). Article CAS PubMed Google Scholar * Zhang, Q. _ et al_. Targeting Th17-IL-17 pathway
in prevention of micro-invasive prostate cancer in a mouse model. _Prostate_ 77, 888–899 (2017). Article CAS PubMed PubMed Central Google Scholar * Foster, B. A., Gingrich, J. R.,
Kwon, E. D., Madias, C. & Greenberg, N. M. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. _Cancer Res._
57, 3325–3330 (1997). CAS PubMed Google Scholar * Hurwitz, A. A., Foster, B. A., Allison, J. P., Greenberg, N. M. & Kwon, E. D. in _Current Protocols in Immunology_ (eds Coligan, J.
E. _ et al_.) Unit 20.5 (2001). Google Scholar * Won, H. _ et al_. TLR9 expression and secretion of LIF by prostate cancer cells stimulates accumulation and activity of polymorphonuclear
MDSCs. _J. Leukoc. Biol._ 102, 423–436 (2017). Article CAS PubMed PubMed Central Google Scholar * Leventhal, D. S. _ et al_. Dendritic cells coordinate the development and homeostasis
of organ-specific regulatory T cells. _Immunity_ 44, 847–859 (2016). Article CAS PubMed PubMed Central Google Scholar * Pasero, C. _ et al_. Inherent and tumor-driven immune tolerance
in the prostate microenvironment impairs natural killer cell antitumor activity. _Cancer Res._ 76, 2153–2165 (2016). Article CAS PubMed Google Scholar * Ammirante, M., Luo, J. L.,
Grivennikov, S., Nedospasov, S. & Karin, M. B-Cell-derived lymphotoxin promotes castration-resistant prostate cancer. _Nature_ 464, 302–305 (2010). CAS PubMed PubMed Central Google
Scholar * Shalapour, S. _ et al_. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. _Nature_ 521, 94–98 (2015). Article CAS PubMed PubMed Central Google
Scholar * Kwek, S. S., Cha, E. & Fong, L. Unmasking the immune recognition of prostate cancer with CTLA4 blockade. _Nat. Rev. Cancer._ 12, 289–297 (2012). Article CAS PubMed PubMed
Central Google Scholar * Goswami, S., Aparicio, A. & Subudhi, S. K. Immune checkpoint therapies in prostate cancer. _Cancer J._ 22, 117–120 (2016). Article CAS PubMed PubMed Central
Google Scholar * Kambayashi, T. & Laufer, T. M. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? _Nat. Rev. Immunol._ 14, 719–730
(2014). Article CAS PubMed Google Scholar * Gujar, S. A. & Lee, P. W. Oncolytic virus-mediated reversal of impaired tumor antigen presentation. _Front. Oncol._ 4, 77 (2014). Article
PubMed PubMed Central Google Scholar * Wennier, S. T., Liu, J. & McFadden, G. Bugs and drugs: oncolytic virotherapy in combination with chemotherapy. _Curr. Pharm. Biotechnol._ 13,
1817–1833 (2012). Article CAS PubMed PubMed Central Google Scholar * Marcato, P., Shmulevitz, M., Pan, D., Stoltz, D. & Lee, P. W. Ras transformation mediates reovirus oncolysis by
enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. _Mol. Ther._ 15, 1522–1530 (2007). Article CAS PubMed Google Scholar * Russell, S. J. & Peng, K. W.
Oncolytic virotherapy: a contest between apples and oranges. _Mol. Ther._ 25, 1107–1116 (2017). Article CAS PubMed PubMed Central Google Scholar * Bell, J. & McFadden, G. Viruses
for tumor therapy. _Cell. Host Microbe_ 15, 260–265 (2014). Article CAS PubMed PubMed Central Google Scholar * Parato, K. A., Lichty, B. D. & Bell, J. C. Diplomatic immunity:
turning a foe into an ally. _Curr. Opin. Mol. Ther._ 11, 13–21 (2009). CAS PubMed Google Scholar * Shmulevitz, M., Marcato, P. & Lee, P. W. Unshackling the links between reovirus
oncolysis, Ras signaling, translational control and cancer. _Oncogene_ 24, 7720–7728 (2005). Article CAS PubMed Google Scholar * Strong, J. E., Coffey, M. C., Tang, D., Sabinin, P. &
Lee, P. W. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. _EMBO J._ 17, 3351–3362 (1998). Article CAS PubMed PubMed Central Google Scholar
* Coffey, M. C., Strong, J. E., Forsyth, P. A. & Lee, P. W. Reovirus therapy of tumors with activated Ras pathway. _Science_ 282, 1332–1334 (1998). Article CAS PubMed Google Scholar
* Kirn, D. Replication-selective oncolytic adenoviruses: virotherapy aimed at genetic targets in cancer. _Oncogene_ 19, 6660–6669 (2000). Article CAS PubMed Google Scholar * Andtbacka,
R. H. _ et al_. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. _J. Clin. Oncol._ 33, 2780–2788 (2015). Article CAS PubMed Google Scholar *
Rehman, H., Silk, A. W., Kane, M. P. & Kaufman, H. L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. _J. Immunother. Cancer._
4, 53 (2016). Article PubMed PubMed Central Google Scholar * Schvartsman, G., Perez, K., Flynn, J. E., Myers, J. N. & Tawbi, H. Safe and effective administration of T-VEC in a
patient with heart transplantation and recurrent locally advanced melanoma. _J. Immunother. Cancer._ 5, 45 (2017). Article PubMed PubMed Central Google Scholar * US National Library of
Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT00769704 (2016). * Danziger, O., Shai, B., Sabo, Y., Bacharach, E. & Ehrlich, M. Combined genetic and epigenetic
interferences with interferon signaling expose prostate cancer cells to viral infection. _Oncotarget_ 7, 52115–52134 (2016). Article PubMed PubMed Central Google Scholar * DeWeese, T. L.
_ et al_. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. _Cancer
Res._ 61, 7464–7472 (2001). CAS PubMed Google Scholar * Small, E. J. _ et al_. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic
adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. _Mol. Ther._ 14, 107–117 (2006). Article CAS PubMed Google Scholar * Freytag, S. O., Barton, K. N. &
Zhang, Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. _Gene Ther._ 20, 1131–1139 (2013). Article CAS PubMed
Google Scholar * Freytag, S. O. _ et al_. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. _Mol. Ther._ 15,
636–642 (2007). Article CAS PubMed Google Scholar * Freytag, S. O. _ et al_. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of
locally recurrent prostate cancer. _Cancer Res._ 62, 4968–4976 (2002). CAS PubMed Google Scholar * Fukuhara, H., Homma, Y. & Todo, T. Oncolytic virus therapy for prostate cancer.
_Int. J. Urol._ 17, 20–30 (2010). Article CAS PubMed Google Scholar * Arulanandam, R. _ et al_. VEGF-mediated induction of PRD1-BF1/Blimp1 expression sensitizes tumor vasculature to
oncolytic virus infection. _Cancer Cell._ 28, 210–224 (2015). Article CAS PubMed Google Scholar * Ilkow, C. S. _ et al_. Reciprocal cellular cross-talk within the tumor microenvironment
promotes oncolytic virus activity. _Nat. Med._ 21, 530–536 (2015). Article CAS PubMed Google Scholar * Breitbach, C. J. _ et al_. Targeting tumor vasculature with an oncolytic virus.
_Mol. Ther._ 19, 886–894 (2011). Article CAS PubMed PubMed Central Google Scholar * Lucas, T. _ et al_. Adenoviral-mediated endothelial precursor cell delivery of soluble CD115
suppresses human prostate cancer xenograft growth in mice. _Stem Cells_ 27, 2342–2352 (2009). Article CAS PubMed PubMed Central Google Scholar * Passer, B. J. _ et al_. Combination of
vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. _Cancer Gene Ther._ 20, 17–24 (2013).
Article CAS PubMed Google Scholar * Jha, B. K., Dong, B., Nguyen, C. T., Polyakova, I. & Silverman, R. H. Suppression of antiviral innate immunity by sunitinib enhances oncolytic
virotherapy. _Mol. Ther._ 21, 1749–1757 (2013). Article CAS PubMed PubMed Central Google Scholar * Munz, C., Lunemann, J. D., Getts, M. T. & Miller, S. D. Antiviral immune
responses: triggers of or triggered by autoimmunity? _Nat. Rev. Immunol._ 9, 246–258 (2009). Article CAS PubMed PubMed Central Google Scholar * Getts, D. R., Chastain, E. M., Terry, R.
L. & Miller, S. D. Virus infection, antiviral immunity, and autoimmunity. _Immunol. Rev._ 255, 197–209 (2013). Article CAS PubMed PubMed Central Google Scholar * Thirukkumaran, C.
M. _ et al_. Oncolytic viral therapy for prostate cancer: efficacy of reovirus as a biological therapeutic. _Cancer Res._ 70, 2435–2444 (2010). Article CAS PubMed Google Scholar *
Varghese, S. _ et al_. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. _Cancer Gene Ther._
13, 253–265 (2006). Article CAS PubMed Google Scholar * Fong, L., Ruegg, C. L., Brockstedt, D., Engleman, E. G. & Laus, R. Induction of tissue-specific autoimmune prostatitis with
prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. _J. Immunol._ 159, 3113–3117 (1997). CAS PubMed Google Scholar * Kottke, T. _ et al_. Broad
antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. _Nat. Med._ 17, 854–859 (2011). Article CAS PubMed PubMed Central Google Scholar *
Castelo-Branco, P. _ et al_. Oncolytic herpes simplex virus armed with xenogeneic homologue of prostatic acid phosphatase enhances antitumor efficacy in prostate cancer. _Gene Ther._ 17,
805–810 (2010). Article CAS PubMed PubMed Central Google Scholar * Kim, Y. _ et al_. Dendritic cells in oncolytic virus-based anti-cancer therapy. _Viruses_ 7, 6506–6525 (2015). Article
CAS PubMed PubMed Central Google Scholar * Komaru, A. _ et al_. Sustained and NK/CD4+ T cell-dependent efficient prevention of lung metastasis induced by dendritic cells harboring
recombinant Sendai virus. _J. Immunol._ 183, 4211–4219 (2009). Article CAS PubMed Google Scholar * Kruslin, B., Tomas, D., Dzombeta, T., Milkovic-Perisa, M. & Ulamec, M. Inflammation
in prostatic hyperplasia and carcinoma-basic scientific approach. _Front. Oncol._ 7, 77 (2017). Article PubMed PubMed Central Google Scholar * Schenk, J. M. _ et al_. Biomarkers of
systemic inflammation and risk of incident, symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. _Am. J. Epidemiol._ 171, 571–582 (2010). Article
PubMed PubMed Central Google Scholar * Gurel, B. _ et al_. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate
cancer prevention trial. _Cancer Epidemiol. Biomarkers Prev._ 23, 847–856 (2014). Article PubMed PubMed Central Google Scholar * Hsing, A. W., Tsao, L. & Devesa, S. S. International
trends and patterns of prostate cancer incidence and mortality. _Int. J. Cancer_ 85, 60–67 (2000). Article CAS PubMed Google Scholar * Vidal, A. C. _ et al_. Racial differences in
prostate inflammation: results from the REDUCE study. _Oncotarget_ 8, 71393–71399 (2016). PubMed PubMed Central Google Scholar * Coussens, L. M. & Werb, Z. Inflammation and cancer.
_Nature_ 420, 860–867 (2002). Article CAS PubMed PubMed Central Google Scholar * Adler, H. L. _ et al_. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1
in patients with metastatic prostatic carcinoma. _J. Urol._ 161, 182–187 (1999). Article CAS PubMed Google Scholar * Yang, Y. F. _ et al_. Antitumor effects of oncolytic adenovirus
armed with PSA-IZ-CD40L fusion gene against prostate cancer. _Gene Ther._ 21, 723–731 (2014). Article CAS PubMed Google Scholar * Moussavi, M. _ et al_. Targeting and killing of
metastatic cells in the transgenic adenocarcinoma of mouse prostate model with vesicular stomatitis virus. _Mol. Ther._ 21, 842–848 (2013). Article CAS PubMed PubMed Central Google
Scholar * Seyedin, S. N. _ et al_. Strategies for combining immunotherapy with radiation for anticancer therapy. _Immunotherapy_ 7, 967–980 (2015). Article CAS PubMed Google Scholar *
Nguyen, A., Ho, L. & Wan, Y. Chemotherapy and oncolytic virotherapy: advanced tactics in the war against cancer. _Front. Oncol._ 4, 145 (2014). PubMed PubMed Central Google Scholar *
Galluzzi, L., Bravo- San Pedro, J. M., Demaria, S., Formenti, S. C. & Kroemer, G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. _Nat. Rev. Clin.
Oncol._ 14, 247–258 (2017). Article CAS PubMed Google Scholar * Galluzzi, L., Senovilla, L., Zitvogel, L. & Kroemer, G. The secret ally: immunostimulation by anticancer drugs. _Nat.
Rev. Drug Discov._ 11, 215–233 (2012). Article CAS PubMed Google Scholar * Cao, W. _ et al_. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells
requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. _Nat. Immunol._ 9, 1157–1164 (2008). Article CAS PubMed PubMed Central Google Scholar * Olagnier, D. _ et al_. Activation of
Nrf2 signaling augments vesicular stomatitis virus oncolysis via autophagy-driven suppression of antiviral immunity. _Mol. Ther._ 25, 1900–1916 (2017). Article CAS PubMed PubMed Central
Google Scholar * Garg, A. D. & Agostinis, P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. _Immunol. Rev._ 280, 126–148 (2017).
Article CAS PubMed Google Scholar * Meng, S., Xu, J., Wu, Y. & Ding, C. Targeting autophagy to enhance oncolytic virus-based cancer therapy. _Expert Opin. Biol. Ther._ 13, 863–873
(2013). Article CAS PubMed Google Scholar * Passer, B. J. _ et al_. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. _Cancer Gene
Ther._ 16, 551–560 (2009). Article CAS PubMed PubMed Central Google Scholar * Nielsen, L. L., Lipari, P., Dell, J., Gurnani, M. & Hajian, G. Adenovirus-mediated p53 gene therapy
and paclitaxel have synergistic efficacy in models of human head and neck, ovarian, prostate, and breast cancer. _Clin. Cancer Res._ 4, 835–846 (1998). CAS PubMed Google Scholar * Yu, D.
C. _ et al_. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. _Cancer Res._ 61, 517–525 (2001). CAS PubMed Google Scholar * Fehl, D. J.
& Ahmed, M. Curcumin promotes the oncoltyic capacity of vesicular stomatitis virus for the treatment of prostate cancers. _Virus Res._ 228, 14–23 (2017). Article CAS PubMed Google
Scholar * Hodzic, J., Sie, D., Vermeulen, A. & van Beusechem, V. W. Functional screening identifies human miRNAs that modulate adenovirus propagation in prostate cancer cells. _Hum.
Gene Ther._ 28, 766–780 (2017). Article CAS PubMed Google Scholar * Mansfield, D. C. _ et al_. Oncolytic vaccinia virus as a vector for therapeutic sodium iodide symporter gene therapy
in prostate cancer. _Gene Ther._ 23, 357–368 (2016). Article CAS PubMed PubMed Central Google Scholar * Trujillo, M. A., Oneal, M. J., McDonough, S., Qin, R. & Morris, J. C. A steep
radioiodine dose response scalable to humans in sodium-iodide symporter (NIS)-mediated radiovirotherapy for prostate cancer. _Cancer Gene Ther._ 19, 839–844 (2012). Article CAS PubMed
PubMed Central Google Scholar * Muthana, M. _ et al_. Macrophage delivery of an oncolytic virus abolishes tumor regrowth and metastasis after chemotherapy or irradiation. _Cancer Res._ 73,
490–495 (2013). Article CAS PubMed Google Scholar * Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. _Nat. Rev.
Immunol._ 17, 97–111 (2017). Article CAS PubMed Google Scholar * Gujar, S. A., Clements, D. & Lee, P. W. Two is better than one: Complementing oncolytic virotherapy with gemcitabine
to potentiate antitumor immune responses. _Oncoimmunology_ 3, e27622 (2014). Article PubMed PubMed Central Google Scholar * Gujar, S. A. _ et al_. Gemcitabine enhances the efficacy of
reovirus-based oncotherapy through anti-tumour immunological mechanisms. _Br. J. Cancer_ 110, 83–93 (2014). Article CAS PubMed Google Scholar * Schirrmacher, V., Bihari, A. S., Stucker,
W. & Sprenger, T. Long-term remission of prostate cancer with extensive bone metastases upon immuno- and virotherapy: a case report. _Oncol. Lett._ 8, 2403–2406 (2014). Article PubMed
PubMed Central Google Scholar * Jiang, H., Lin, J. J., Su, Z. Z., Goldstein, N. I. & Fisher, P. B. Subtraction hybridization identifies a novel melanoma differentiation associated
gene, mda-7, modulated during human melanoma differentiation, growth and progression. _Oncogene_ 11, 2477–2486 (1995). CAS PubMed Google Scholar * Sarkar, D. _ et al_. Eradication of
therapy-resistant human prostate tumors using a cancer terminator virus. _Cancer Res._ 67, 5434–5442 (2007). Article CAS PubMed Google Scholar * Sarkar, S. _ et al_. Novel therapy of
prostate cancer employing a combination of viral-based immunotherapy and a small molecule BH3 mimetic. _Oncoimmunology_ 5, e1078059 (2015). Article CAS PubMed PubMed Central Google
Scholar * Pradhan, A. K. _ et al_. mda-7/IL-24 mediates cancer cell-specific death via regulation of miR-221 and the Beclin-1 axis. _Cancer Res._ 77, 949–959 (2017). Article CAS PubMed
Google Scholar * Su, Z. Z. _ et al_. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells
overexpressing the antiapoptotic proteins bcl-xL or bcl-2. _Oncogene_ 25, 2339–2348 (2006). Article CAS PubMed Google Scholar * Lebedeva, I. V. _ et al_. Strategy for reversing
resistance to a single anticancer agent in human prostate and pancreatic carcinomas. _Proc. Natl Acad. Sci. USA_ 104, 3484–3489 (2007). Article CAS PubMed PubMed Central Google Scholar
* Fukuhara, H., Ino, Y., Kuroda, T., Martuza, R. L. & Todo, T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by
bacterial artificial chromosome-mediated system. _Cancer Res._ 65, 10663–10668 (2005). Article CAS PubMed Google Scholar * Varghese, S., Rabkin, S. D., Nielsen, P. G., Wang, W. &
Martuza, R. L. Systemic oncolytic herpes virus therapy of poorly immunogenic prostate cancer metastatic to lung. _Clin. Cancer Res._ 12, 2919–2927 (2006). Article CAS PubMed Google
Scholar * Liu, C., Hasegawa, K., Russell, S. J., Sadelain, M. & Peng, K. W. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer.
_Prostate_ 69, 1128–1141 (2009). Article CAS PubMed PubMed Central Google Scholar * Madan, R. A. _ et al_. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in
metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. _Lancet Oncol._ 13, 501–508 (2012). Article CAS PubMed PubMed Central Google Scholar * US National
Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT01867333 (2017). * US National Library of Medicine. _ClinicalTrials.gov_
https://clinicaltrials.gov/ct2/show/NCT01875250 (2017). * US National Library of Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT02861573. (2017). * Harris, K. S. &
Kerr, B. A. Prostate cancer stem cell markers drive progression, therapeutic resistance, and bone metastasis. _Stem Cells Int._ 2017, 8629234 (2017). Article CAS PubMed PubMed Central
Google Scholar * Velardi, E. _ et al_. Sex steroid blockade enhances thymopoiesis by modulating Notch signaling. _J. Exp. Med._ 211, 2341–2349 (2014). Article CAS PubMed PubMed Central
Google Scholar * Goldberg, G. L. _ et al_. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. _Transplantation_ 80, 1604–1613
(2005). Article PubMed Google Scholar * Tang, S. & Dubey, P. Opposing effects of androgen ablation on immune function in prostate cancer. _Oncoimmunology_ 1, 1220–1221 (2012). Article
PubMed PubMed Central Google Scholar * Tang, S., Moore, M. L., Grayson, J. M. & Dubey, P. Increased CD8+ T-cell function following castration and immunization is countered by
parallel expansion of regulatory T cells. _Cancer Res._ 72, 1975–1985 (2012). Article CAS PubMed PubMed Central Google Scholar * Kissick, H. T. _ et al_. Androgens alter T-cell immunity
by inhibiting T-helper 1 differentiation. _Proc. Natl Acad. Sci. USA_ 111, 9887–9892 (2014). Article CAS PubMed PubMed Central Google Scholar * Pu, Y. _ et al_. Androgen receptor
antagonists compromise T cell response against prostate cancer leading to early tumor relapse. _Sci. Transl Med._ 8, 333ra47 (2016). Article CAS PubMed Google Scholar * Antonarakis, E.
S. _ et al_. Sequencing of Sipuleucel-T and androgen deprivation therapy in men with hormone-sensitive biochemically recurrent prostate cancer: a phase II randomized trial. _Clin. Cancer
Res._ 23, 2451–2459 (2017). Article CAS PubMed Google Scholar * Gamat, M. & McNeel, D. G. Androgen deprivation and immunotherapy for the treatment of prostate cancer. _Endocr. Relat.
Cancer_ 24, T297–T310 (2017). Article CAS PubMed PubMed Central Google Scholar * Akira, S., Saitoh, T. & Kawai, T. Nucleic acids recognition by innate immunity. _Uirusu_ 62, 39–45
(2012). Article CAS PubMed Google Scholar * Alemany, R. & Cascallo, M. Oncolytic viruses from the perspective of the immune system. _Future Microbiol._ 4, 527–536 (2009). Article
CAS PubMed Google Scholar * Barker, H. E., Paget, J. T., Khan, A. A. & Harrington, K. J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. _Nat.
Rev. Cancer._ 15, 409–425 (2015). Article CAS PubMed PubMed Central Google Scholar * Murphy, J. P. _ et al_. MHC-I ligand discovery using targeted database searches of mass
spectrometry data: implications for T-cell immunotherapies. _J. Proteome Res._ 16, 1806–1816 (2017). Article CAS PubMed Google Scholar * Nielsen, M. & Andreatta, M. NetMHCpan- 3.0;
improved prediction of binding to MHC class I molecules integrating information from multiple receptor and peptide length datasets. _Genome Med._ 8, 33 (2016). Article CAS PubMed PubMed
Central Google Scholar * Croft, N. P., Purcell, A. W. & Tscharke, D. C. Quantifying epitope presentation using mass spectrometry. _Mol. Immunol._ 68, 77–80 (2015). Article CAS PubMed
Google Scholar * Yadav, M. _ et al_. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. _Nature_ 515, 572–576 (2014). Article CAS PubMed
Google Scholar * Comber, J. D. & Philip, R. MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines. _Ther. Adv. Vaccines_ 2, 77–89
(2014). Article CAS PubMed PubMed Central Google Scholar * Serganova, I. _ et al_. Enhancement of PSMA-directed CAR adoptive immunotherapy by PD-1/PD-L1 blockade. _Mol. Ther. Oncolyt._
4, 41–54 (2016). Article CAS Google Scholar * Amin Al Olama, A. _ et al_. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among
Europeans. _Hum. Mol. Genet._ 24, 5589–5602 (2015). Article CAS PubMed PubMed Central Google Scholar * Fraser, M. _ et al_. Genomic hallmarks of localized, non-indolent prostate cancer.
_Nature_ 541, 359–364 (2017). Article CAS PubMed Google Scholar * Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. _Cell_ 163, 1011–1025 (2015).
* Boutros, P. C. _ et al_. Spatial genomic heterogeneity within localized, multifocal prostate cancer. _Nat. Genet._ 47, 736–745 (2015). Article CAS PubMed Google Scholar * Chosey, L. C.
_ et al_. _Biosafety in Microbiological and Biomedical Laboratories (BMBL)_ 5th edn (U.S. Department of Health and Human Services, 2009). Google Scholar * Hoos, A., Wolchok, J. D.,
Humphrey, R. W. & Hodi, F. S. CCR 20th Anniversary Commentary: Immune-related response criteria — capturing clinical activity in immuno-oncology. _Clin. Cancer Res._ 21, 4989–4991
(2015). Article CAS PubMed Google Scholar * Freytag, S. O. _ et al_. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic
adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. _Int. J. Radiat. Oncol. Biol. Phys._ 89, 268–276 (2014). Article PubMed PubMed Central Google Scholar *
Pol, J. _ et al_. Trial Watch — Oncolytic viruses and cancer therapy. _Oncoimmunology_ 5, e1117740 (2015). Article CAS PubMed PubMed Central Google Scholar * US National Library of
Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT02555397 (2016). * [No authors listed.] UMIN-CTR Clinical Trial UMIN000010463. _University Hospital Information Network_
https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000012228&language=E (2016). * Vidal, L. _ et al_. A phase I study of
intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. _Clin. Cancer Res._ 14, 7127–7137 (2008). Article CAS PubMed Google Scholar * US National Library of
Medicine. _ClinicalTrials.gov_ https://clinicaltrials.gov/ct2/show/NCT01619813 (2016). * [No authors listed.] UMIN-CTR Clinical Trial UMIN000010840. _University Hospital Information Network_
https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000012688&language=E (2016). * Lei, N. _ et al_. An oncolytic adenovirus
expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. _Cancer Gene Ther._ 16, 33–43 (2009). Article CAS
PubMed Google Scholar * Huang, X. F. _ et al_. A broadly applicable, personalized heat shock protein-mediated oncolytic tumor vaccine. _Cancer Res._ 63, 7321–7329 (2003). CAS PubMed
Google Scholar * Li, J. L. _ et al_. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. _Gene Ther._
16, 376–382 (2009). Article CAS PubMed Google Scholar * Kaufman, H. L. _ et al_. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the
Eastern Cooperative Oncology Group. _J. Clin. Oncol._ 22, 2122–2132 (2004). Article CAS PubMed Google Scholar * Madan, R. A., Arlen, P. M., Mohebtash, M., Hodge, J. W. & Gulley, J.
L. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. _Expert Opin. Investig. Drugs_ 18, 1001–1011 (2009). Article CAS PubMed PubMed Central Google Scholar *
Fukuhara, H., Martuza, R. L., Rabkin, S. D., Ito, Y. & Todo, T. Oncolytic herpes simplex virus vector g47delta in combination with androgen ablation for the treatment of human prostate
adenocarcinoma. _Clin. Cancer Res._ 11, 7886–7890 (2005). Article CAS PubMed Google Scholar * Parato, K. A. _ et al_. The oncolytic poxvirus JX-594 selectively replicates in and destroys
cancer cells driven by genetic pathways commonly activated in cancers. _Mol. Ther._ 20, 749–758 (2012). Article CAS PubMed Google Scholar * Moussavi, M. _ et al_. Oncolysis of prostate
cancers induced by vesicular stomatitis virus in PTEN knockout mice. _Cancer Res._ 70, 1367–1376 (2010). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS S.G. and
P.L. are supported by grants from Prostate Cancer Canada, Canadian Institutes of Health Research (CIHR), Terry Fox Research Institute (TFRI), and Canadian Cancer Research Institute (CCSRI).
The authors thank Y. Kim for her help with graphics and manuscript preparation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pathology and Department of Microbiology and
Immunology, Dalhousie University, Halifax, B3H 1X5, Nova Scotia, Canada Patrick Lee & Shashi Gujar * Centre for Innovative and Collaborative Health Systems Research, IWK Health Centre,
Halifax, B3K 6R8, Nova Scotia, Canada Shashi Gujar Authors * Patrick Lee View author publications You can also search for this author inPubMed Google Scholar * Shashi Gujar View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.G. researched data for the article and wrote the manuscript. Both authors made substantial
contributions to discussion of the article content and reviewed and/or edited the manuscript before submission. CORRESPONDING AUTHORS Correspondence to Patrick Lee or Shashi Gujar. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3
POWERPOINT SLIDE FOR TABLE 1 POWERPOINT SLIDE FOR TABLE 2 GLOSSARY * Oncolytic viruses Viruses that preferentially infect and kill cancer cells ('onco' means cancer;
'lytic' means killing). * Tumour microenvironment The milieu in which the tumour resides, consisting of malignant cells and nonmalignant cells, including stromal and immune cells.
* Myeloid-derived suppressor cells (MDSCs). The heterogeneous population of myeloid cells that suppress the functions of various immune cells, especially natural killer cells and T cells,
through direct or indirect mechanisms. * Lactic acid A metabolite that has the capacity to suppress the functions of immune mediators when produced as an aberrant by-product of cancer
metabolism. * Tumour immune evasion Cancers employ various suppressive mechanisms to resist the development of antitumour immune activities and, thus, evade immune-mediated attack and
elimination. * Immune checkpoint inhibitors Agents that inhibit the interactions between immune checkpoint molecules on tumour cells and their receptors on immune cells, releasing the
inhibitory signal and enabling tumour-directed immune responses. * MHC ligands Peptides that are presented in the antigen presentation groove of major histocompatibility complex (MHC) class
I and class II molecules; this ligand-bound MHC complex is recognized by T cell receptors in a highly specific manner. * Immunologically privileged site Anatomical sites or locations, such
as the brain or eyes, that contain impaired or modified lymphatic drainage and immune surveillance are privileged against immune-response-induced collateral tissue damage. * Autoantibodies
Antibodies that are specific against an individual's own proteins and antigens. * Epithelial–mesenchymal transition A biological process through which epithelial cells lose their
characteristic cell polarity and adhesion properties and acquire a migratory and invasive phenotype similar to that of mesenchymal cells. * Cancer stem cells Cancer cells that are
pluripotent and have self-renewal capacity and, thus, possess stem-cell-like characteristics. * Neuroendocrine tumours Tumours that arise from neuroendocrine cells, which are specialized
cells that often produce hormones under neuronal control. * T cell anergy A state in which T cells remain tolerant or dysfunctional. * Immunogenic cell death A form of cell death that is
accompanied by the expression of molecules that lead to the activation of dendritic cells, which subsequently prime T cells. * Tumour-associated antigens These antigens are often
preferentially expressed on tumours but can also be found in normal tissues, whereas tumour-specific antigens are usually expressed specifically in tumours, often arising from mutations or
virally encoded oncoproteins. * Autophagy A 'self-eating' homeostatic cellular process in which damaged organelles are digested under normal physiological situations and in which,
during stress conditions, macromolecules, such as nucleic acids and amino acids, are recycled. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, P.,
Gujar, S. Potentiating prostate cancer immunotherapy with oncolytic viruses. _Nat Rev Urol_ 15, 235–250 (2018). https://doi.org/10.1038/nrurol.2018.10 Download citation * Published: 13
February 2018 * Issue Date: April 2018 * DOI: https://doi.org/10.1038/nrurol.2018.10 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative