Play all audios:
ABSTRACT The coupling between DNA methylation and histone modification contributes to aberrant expression of oncogenes or tumor suppressor genes that leads to tumor development. Our previous
study demonstrated that lysine demethylase 2A (KDM2A) functions as an oncogene in breast cancer by promoting cancer stemness and angiogenesis via activation of the Notch signaling. Here, we
demonstrate that knockdown of KDM2A significantly increases the 5′-hydroxymethylcytosine (5′-hmc) level in genomic DNA and expression of tet-eleven translocation 2 (TET2) in various breast
cancer cell lines. Conversely, ectopic expression of KDM2A inhibits TET2 expression in KDM2A-depleted cells suggesting TET2 is a transcriptional repression target of KDM2A. Our results show
that KDM2A interacts with RelA to co-occupy at the TET2 gene promoter to repress transcription and depletion of RelA or KDM2A restores TET2 expression. Upregulation of TET2 in the
KDM2A-depleted cells induces the re-activation of two TET downstream tumor suppressor genes, epithelial cell adhesion molecule (EpCAM) and E-cadherin, and inhibits migration and invasion. On
the contrary, knockdown of TET2 in these cells decreases EpCAM and E-cadherin and increases cell invasiveness. More importantly, TET2 expression is negatively associated KDM2A in
triple-negative breast tumor tissues, and its expression predicts a better survival. Taken together, we demonstrate for the first time that TET2 is a direct repression target of KDM2A and
reveal a novel mechanism by which KDM2A promotes DNA methylation and breast cancer progression via the inhibition of a DNA demethylase. SIMILAR CONTENT BEING VIEWED BY OTHERS HISTONE
DEMETHYLASE KDM4C CONTROLS TUMORIGENESIS OF GLIOBLASTOMA BY EPIGENETICALLY REGULATING P53 AND C-MYC Article Open access 18 January 2021 H3K4 DEMETHYLASE KDM5B REGULATES CANCER CELL IDENTITY
AND EPIGENETIC PLASTICITY Article 19 April 2022 KMT5A-METHYLATED SNIP1 PROMOTES TRIPLE-NEGATIVE BREAST CANCER METASTASIS BY ACTIVATING YAP SIGNALING Article Open access 21 April 2022
INTRODUCTION DNA methylation and histone modifications are two major epigenetic regulatory processes that control gene expression, genomic stability, imprinting and chromosome structure.1,
2, 3 DNA methylation, the addition of the methyl group to the cytosine of the CpG dinucleotides, is mainly catalyzed by three DNA methyltransferases (DNMTs) including DNMT1, DNMT3A and
DNMT3B, and is strongly associated with gene repression. DNA methylation has been considered to be an extremely stable epigenetic marker until the identification of the tet-eleven
translocation (TET) gene family.4, 5 This family contains three members including TET1, TET2 and TET3, and the encoded proteins are Fe2+- and α-ketoglutarate-dependent dioxygenases which can
hydrolyze 5′-methylcytosine (5’-mc) to 5’-hydroxymethylcytosine (5′-hmc) and finally erase the methyl group from the CpG dinucleotides. Therefore, the TET enzymes function as DNA
demethylases which antagonize DNMT-mediated DNA methylation and gene repression. Compared to DNA methylation, histone modifications are complex and the modifications like methylation,
phosphorylation, ubiquitination, sumoylation and so on, are catalyzed by many enzymes that add or remove the functional groups on specific residues of the histone proteins dynamically to
generate the so called ‘histone code’.6, 7 The crosstalk between DNA and histone methylation in the regulation of gene transcription was firstly suggested by the observation that DNA
methylation is frequently co-existed with the methylated lysine 9 of histone H3 (H3K9), a repression histone marker.8, 9 This hypothesis was further supported by a study showing that the
mouse embryonic stem cells lack Suppressor Of Variegation 3-9 Homolog 1 (Suv39H1) and Suv39H2, the histone methyltransferases responsible for the tri-methylation of H3K9, exhibited a
significant reduction of DNA methylation.10 How the methylation of DNA and histone is co-regulated is an important issue in gene regulation. Currently, three mechanisms have been proposed
for the co-regulation. Firstly, histone methyltransferases may directly interact with DNMTs to form a functional complex and work together to coordinate DNA and histone methylation
simultaneously. For example, two H3K9 methyltransferases G9a and GLP which catalyze the mono- and di-methylation of H3K9 have been shown to interact with DNMT3A and 3B to enhance _de novo_
DNA methylation.11 Secondly, histone demethylases can also bind with DNMTs to affect epigenetic modification. Brenner _et al._ showed that the increased binding of KDM1A to DNMT1 during the
S-phase of cell cycle may play a role in the control of DNA replication.12 Third, histone demethylases may modulate the enzymatic activity or protein stability of DNMTs to alter DNA
methylation.13 Lysine demethylase 2A (KDM2A) was firstly identified as a novel Jumonji-C (JMJC) domain-containing proteins that exhibited H3K36 demethylase activity.14 Our previous study
demonstrated that KDM2A was frequently overexpressed in breast tumor tissues and this demethylase upregulated Jagged1 to activate the Notch signaling pathway to promote cancer stemness and
angiogenesis.15 Interestingly, we found that knockdown of KDM2A induced a significant increase of 5′-hmc level in genomic DNA suggesting a potential role of KDM2A in the regulation of DNA
methylation. In this study, we tried to elucidate the underlying mechanism by which KDM2A regulates DNA methylation. RESULTS AND DISCUSSION KDM2A INHIBITS THE EXPRESSION OF TET2 TO INCREASE
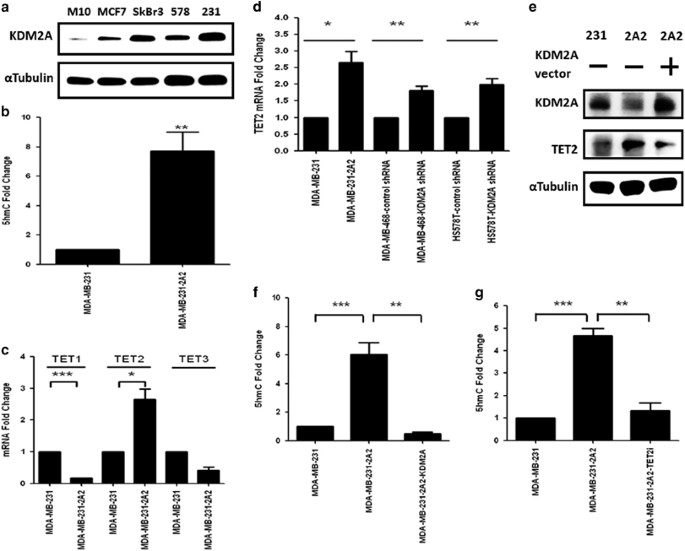
DNA METHYLATION We screened the expression of KDM2A in a panel of breast cancer cell lines and found the upregulation of this demethylase in breast cancer cells when compared to that of M10
normal mammary epithelial cells (Figure 1a). Interestingly, three triple-negative breast cancer cell lines including MDA-MB-231, Hs-578T and MDA-MB-468 exhibited the highest expression of
KDM2A (Figure 1a and data not shown). Therefore, we specifically focused on the study of KDM2A in triple-negative cells. Compared to the parental MDA-MB-231 cells, the KDM2A-depleted stable
cell line (MDA-MB-231-2A2) exhibited an eightfold increase at the 5′-hmc level in the genomic DNA (Figure 1b). Because the expression of DNMTs was not significantly changed in MDA-MB-231-2A2
cells (data not shown), we investigated the expression of TETs and found that TET2 was significantly upregulated in the KDM2A-depleted cells (Figure 1c). This is not a cell line-specific
effect because transient knockdown of KDM2A by shRNA also increased TET2 expression in the Hs-578T and MDA-MB-468 cells (Figure 1d). Ectopic expression of KDM2A reversed the upregulation of
TET2 in the MDA-MB-231-2A2 cells suggesting TET2 is a direct repression target of KDM2A (Figure 1e). In addition, the 5′-hmc level was also reduced (Figure 1f). To verify the increase of
5′-hmc in the MDA-MB-231-2A2 cells was mediated by TET2, we inhibited the expression of TET2 by siRNA and confirmed the reduction of 5′-hmc in genomic DNA (Figure 1g). These results
suggested KDM2A inhibits TET2 to increase DNA methylation. REPRESSION OF TET2 BY KDM2A IS RELA-DEPENDENT KDM2A has been shown to be a H3K36 demethylase in cells.16, 17 Mechanistic study
suggested that KDM2A utilized the zinc finger CxxC domain to recognize nonmethylated CpG dinucleotides in genomic DNA and catalyzed the demethylation of H3K36 proximal to the binding region
via its enzymatic domain.17 Because the methylation of H3K36 in the promoters implied gene activation, the demethylation of this histone marker generally caused the downregulation of gene
expression.18, 19 To confirm TET2 is a direct repression target of KDM2A, we performed chromatin immunoprecipitation (ChIP) assay to study the binding of KDM2A to the TET2 gene promoter. Our
data showed that KDM2A constitutively bound to the TET2 promoter, and the depletion of KDM2A dramatically attenuated its promoter binding that was associated with the increase of di- and
tri-methylation of H3K36 supporting KDM2A is an _in vivo_ H3K36 demethylase as reported previously (Figure 2a).16, 17 Bioinformatics prediction suggested four RelA binding sites in the TET2
promoter region (Figure 2b). Two previous evidences promoted us to study the potential role of RelA in the regulation of TET2 expression by KDM2A. Firstly, RelA is constitutively activated
in estrogen receptor-negative and triple-negative breast cancer cells.20 Secondly, a functional interaction between RelA and KDM2A has been reported recently.21, 22 We found that RelA
constitutively bound to the human TET2 gene promoter and the two proximal sites upstream of the transcription start site showed the strongest binding (Figure 2b). This is consistent with the
previous findings that RelA is constitutively activated, and binds to various gene promoters to stimulate or inhibit gene transcription in estrogen receptor-negative breast cancer cells.20
Because the most proximal RelA binding site located at the -138/-128 region overlapped with the KDM2A binding region detected in our ChIP study (Figure 2a), we hypothesized that RelA
interacted with KDM2A and co-occupied at the proximal promoter region to repress gene transcription. Indeed, knockdown of RelA in the MDA-MB-231 cells significantly reduced the binding of
KDM2A to the proximal promoter region of the TET2 gene (Figure 2c). In addition, the di- and tri-methylation of H3K36 at this region was increased. We also confirmed the depletion of RelA in
the MDA-MB-231 and Hs-578T cells restored the expression of TET2 as found in KDM2A-depleted MDA-MB-2A2 cells (Figure 2d). These data suggested that RelA and KDM2A form a repression complex
to demethylate H3K36 in the promoter region to attenuate TET2 transcription. Previous studies demonstrated that KDM2A acts as a negative regulator of RelA by demethylating the K218 and K221
residues.21, 22 However, it should be noted that six lysine residues and one arginine residue of RelA have been shown to be methylated _in vitro_ and _in vivo_.23 The biological outcome
elicited by different combinations of these methylations is more complex than originally proposed and needs further characterization. Here, we provide another model that RelA acts as an
anchor protein that constitutively binds to the TET2 promoter and may recruit KDM2A to demethylate H3K36 to attenuate TET2 expression when KDM2A is overexpressed. INCREASE OF TET2 EXPRESSION
INDUCED BY KDM2A DEPLETION PROMOTES THE RE-ACTIVATION OF THE DOWNSTREAM TARGET GENES TO SUPPRESS CELL INVASIVENESS To characterize the biological consequences of the upregulation of TET2
induced by KDM2A depletion, we investigated the expression of two reported TET target genes EpCAM and E-cadherin. In the MDA-MB-231-2A2 cells, the expression of EpCAM was significantly
increased (Figure 3a). Upregulation of EpCAM was also found in the Hs-578T and MDA-MB-468 cells transfected with the KDM2A shRNA. The enhancement of EpCAM expression was dependent on TET2
because knockdown of TET2 abolished the induction (Figure 3b). The protein level of EpCAM and E-cadherin in the MDA-MB-231-2A2 cells and the KDM2A-depleted Hs-578T cells was also increased
(Figures 3c and d). Similarly, knockdown of RelA which induced the upregulation of TET2 also increased EpCAM and E-cadherin proteins (Figure 2d). Bioinformatics prediction suggested a CpG
island located within the −79/+971 region of the human EpCAM gene (Figure 3e). Our ChIP assay demonstrated that depletion of KDM2A enhanced the binding of TET2 to this CpG island (Figure
3e), and the 5′-hmc level in this region was also increased (Figure 3f) supporting our hypothesis that KDM2A depletion upregulated TET2 expression to induce the demethylation of the EpCAM
promoter to activate its gene transcription. EpCAM and E-cadherin are involved in the control of cell–cell contact and invasiveness. We therefore studied the alteration in migration and
invasion and found that the depletion of KDM2A reduced cell migration and invasion which could be reversed by knockdown of TET2 (Figure 3g). Inhibition of EpCAM by blocking antibody also
attenuated cell invasiveness (Figure 3h). These data suggested that KDM2A depletion increases TET2 and re-activates TET2 downstream target genes to suppress cell invasiveness. TET2
EXPRESSION IS INVERSELY CORRELATED WITH KDM2A AND PREDICTS A BETTER SURVIVAL IN THE TRIPLE-NEGATIVE BREAST CANCER PATIENTS Because KDM2A was expressed at the highest level in triple-negative
breast cancer cell lines, we investigated the association of KDM2A and TET2 in 62 triple-negative breast tumor tissues. Figure 4a demonstrated the tumor tissue of a patient with strong
KDM2A staining showed very low expression of TET2. Statistical analysis showed an inverse correlation between TET2 and KDM2A in the tumor tissues (Figure 4b). In addition, downregulation of
TET2 was associated with advanced stage (Figure 4c), lymph node metastasis (Figure 4d) and high tumor grade (Figure 4e). More importantly, TET2 expression predicted a better survival in the
patients (Figure 4f). A recent study demonstrated that the decrease of global 5′-hmc was associated with poor disease-specific and disease-free survival in breast cancer patients.24 However,
the mechanism of the reduction of 5′-hmc level and TET expression was not addressed. Here, we provide the first evidence that KDM2A is an upstream inhibitor of TET2, and may modulate the
global 5′-hmc by suppressing TET2. When our study was undergoing, Borgel _et al._25 reported the potential role of KDM2A in the mediation of DNA methylation and gene silencing. The working
hypothesis proposed in the study is that KDM2A directly interacts with heterochromatin protein 1 (HP1) via its zinc finger CxxC and PHD domains to form a nucleosome binding circuit to
recruit the H3K9 lysine methyltransferases to introduce H3K9 methylation. In addition, HP1 also recruited DNMTs to deposit CpG methylation. The overall result is the simultaneous increase of
H3K9 and CpG methylation which leads to the establishment of heterochromatin. Our results establish a new model that differs from the previous model in two ways. Firstly, the major effect
of KDM2A on DNA methylation is mediated by the recruitment of DNMTs in the Borgel’s study. However, alteration of the expression of DNA demethylases and methyltransferase was not studied. We
identified TET2 is a repression target of KDM2A and is important for the modulation of DNA methylation by KDM2A. Secondly, the main histone marker investigated in the Borgel’s study is
H3K9. On the contrary, we found a global change of the 5′-hmc level and H3K36 methylation in different gene promoters after KDM2A depletion indicating KDM2A has a board impact on the
coupling of DNA and histone methylation to regulate gene expression. Collectively, this study reveals a novel mechanism by which the methylation of DNA and histone is co-regulated via
modulating the expression of TET2 by KDM2A. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES
* Cedar H, Bergman Y . Linking DNA methylation and histone modification: patterns and paradigms. _Nat Rev Genet_ 2009; 10: 295–304. Article CAS Google Scholar * Chi P, Allis CD, Wang GG
. Covalent histone modifications—miswritten, misinterpreted and mis-erased in human cancers. _Nat Rev Cancer_ 2010; 10: 457–469. Article CAS Google Scholar * Brien GL, Valerio DG,
Armstrong SA . Exploiting the epigenome to control cancer-promoting gene-expression programs. _Cancer Cell_ 2016; 29: 464–476. Article CAS Google Scholar * Ito S, D'Alessio AC,
Taranova OV, Hong K, Sowers LC, Zhang Y . Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. _Nature_ 2010; 466: 1129–1133. Article CAS
Google Scholar * Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA _et al_. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. _Science_ 2011; 333:
1300–1303. Article CAS Google Scholar * Audia JE, Campbell RM . Histone modifications and cancer. _Cold Spring Harb Perspect Biol_ 2016; 8: a019521. Article Google Scholar * Ruthenburg
AJ, Li H, Patel DJ, Allis CD . Multivalent engagement of chromatin modifications by linked binding modules. _Nat Rev Mol Cell Biol_ 2007; 8: 983–994. Article CAS Google Scholar * Lachner
M, O'Carroll D, Rea S, Mechtler K, Jenuwein T . Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. _Nature_ 2001; 410: 116–120. Article CAS Google Scholar *
Schotta G, Ebert A, Krauss V, Fischer A, Hoffmann J, Rea S _et al_. Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. _EMBO J_ 2002; 21:
1121–1131. Article CAS Google Scholar * Lehnertz B, Ueda Y, Derijck AA, Braunschweig U, Perez-Burgos L, Kubicek S _et al_. Suv39h-mediated histone H3 lysine 9 methylation directs DNA
methylation to major satellite repeats at pericentric heterochromatin. _Curr Biol_ 2003; 13: 1192–1200. Article CAS Google Scholar * Estève PO, Chin HG, Smallwood A, Feehery GR,
Gangisetty O, Karpf AR _et al_. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. _Genes Dev_ 2006; 20: 3089–3103. Article Google Scholar
* Brenner C, Luciani J, Bizet M, Ndlovu M, Josseaux E, Dedeurwaerder S _et al_. The interplay between the lysine demethylase KDM1A and DNA methyltransferases in cancer cells is cell cycle
dependent. _Oncotarget_ 2016; 7: 58939–58952. PubMed PubMed Central Google Scholar * Zhang J, Yuan B, Zhang F, Xiong L, Wu J, Pradhan S _et al_. Cyclophosphamide perturbs cytosine
methylation in Jurkat-T cells through LSD1-mediated stabilization of DNMT1 protein. _Chem Res Toxicol_ 2011; 24: 2040–2043. Article CAS Google Scholar * Tsukada Y, Fang J,
Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P _et al_. Histone demethylation by a family of JmjC domain-containing proteins. _Nature_ 2006; 439: 811–816. Article CAS Google Scholar
* Chen JY, Li CF, Chu PY, Lai YS, Chen CH, Jiang SS _et al_. Lysine demethylase 2A promotes stemness and angiogenesis of breast cancer by upregulating Jagged1. _Oncotarget_ 2016; 7:
27689–27710. PubMed PubMed Central Google Scholar * He J, Kallin EM, Tsukada Y, Zhang Y . The H3K36 demethylase Jhdm1b/Kdm2b regulates cell proliferation and senescence through
p15(Ink4b). _Nat Struct Mol Biol_ 2008; 15: 1169–1175. Article CAS Google Scholar * Blackledge NP, Zhou JC, Tolstorukov MY, Farcas AM, Park PJ, Klose RJ . CpG islands recruit a histone H3
lysine 36 demethylase. _Mol Cell_ 2010; 38: 179–190. Article CAS Google Scholar * Wagner EJ, Carpenter PB . Understanding the language of Lys36 methylation at histone H3. _Nat Rev Mol
Cell Biol_ 2012; 13: 115–126. Article CAS Google Scholar * Edmunds JW, Mahadevan LC, Clayton AL . Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36
trimethylation. _EMBO J_ 2008; 27: 406–420. Article CAS Google Scholar * Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ Jr, Sledge GW Jr . Constitutive activation of NF-kappaB during
progression of breast cancer to hormone-independent growth. _Mol Cell Biol_ 1997; 17: 3629–3639. Article CAS Google Scholar * Lu T, Jackson MW, Singhi AD, Kandel ES, Yang M, Zhang Y _et
al_. Validation-based insertional mutagenesis identifies lysine demethylase FBXL11 as a negative regulator of NFkappaB. _Proc Natl Acad Sci USA_ 2009; 106: 16339–16344. Article CAS Google
Scholar * Lu T, Jackson MW, Wang B, Yang M, Chance MR, Miyagi M _et al_. Regulation of NF-kappaB by NSD1/FBXL11-dependent reversible lysine methylation of p65. _Proc Natl Acad Sci USA_
2010; 107: 46–51. Article CAS Google Scholar * Lu T, Stark GR . NF-κB: regulation by methylation. _Cancer Res_ 2015; 75: 3692–3695. Article CAS Google Scholar * Tsai KW, Li GC, Chen
CH, Yeh MH, Huang JS, Tseng HH _et al_. Reduction of global 5-hydroxymethylcytosine is a poor prognostic factor in breast cancer patients, especially for an ER/PR-negative subtype. _Breast
Cancer Res Treat_ 2015; 153: 219–234. Article CAS Google Scholar * Borgel J, Tyl M, Schiller K, Pusztai Z, Dooley CM, Deng W _et al_. KDM2A integrates DNA and histone modification signals
through a CXXC/PHD module and direct interaction with HP1. _Nucleic Acids Res_ 2017; 45: 1114–1129. CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Drs Mei-Ren
Pan and Ming-Feng Hou for valuable discussion and Dr Y Zhang for providing the KDM2A expression vector. This study was supported by the grants 103-2320-B-400-014, 104-2320-B-400-027 and
105-2320-B-400-005 from Ministry of Science and Technology and 106-TDU-B-212-144007 from Ministry of Health and Welfare. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * National Institute of
Cancer Research, National Health Research Institutes, Tainan, Taiwan J-Y Chen, Y-S Lai & W-C Hung * Department of Pathology, Kaohsiung Medical University Hospital, Kaohsiung Medical
University, Kaohsiung, Taiwan C-W Luo & C-C Wu * Cancer Center, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan C-W Luo & W-C Hung * Graduate Institute of Medicine, College
of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan W-C Hung Authors * J-Y Chen View author publications You can also search for this author inPubMed Google Scholar * C-W Luo View
author publications You can also search for this author inPubMed Google Scholar * Y-S Lai View author publications You can also search for this author inPubMed Google Scholar * C-C Wu View
author publications You can also search for this author inPubMed Google Scholar * W-C Hung View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING
AUTHOR Correspondence to W-C Hung. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies this
paper on the Oncogenesis website SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE 1 (PDF 185 KB) RIGHTS AND PERMISSIONS _Oncogenesis_ is an open-access journal published by _Nature Publishing
Group_. This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license
holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, JY.,
Luo, CW., Lai, YS. _et al._ Lysine demethylase KDM2A inhibits TET2 to promote DNA methylation and silencing of tumor suppressor genes in breast cancer. _Oncogenesis_ 6, e369 (2017).
https://doi.org/10.1038/oncsis.2017.71 Download citation * Received: 10 June 2017 * Accepted: 26 June 2017 * Published: 07 August 2017 * Issue Date: August 2017 * DOI:
https://doi.org/10.1038/oncsis.2017.71 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative