Play all audios:
ABSTRACT Eleven open-chest piglets were studied to examine the effects of vascular engorgement on partitioned airway and lung tissue mechanics, and to investigate the role of vagal
denervation on lung function during engorgement. Alveolar pressure was measured using alveolar capsules. Pulmonary elastance(EL) and resistance (RL), airway (Raw), and tissue(_V_ti)
resistance were calculated during mechanical ventilation. Acute fluid administration with polygeline (10 mL/kg boluses up to a total of 30 mL/kg) resulted in an increase of RL with increases
in both Raw and _V_ti. _V_ti rose 2-3-fold in comparison with Raw. To increase left atrial pressure, a balloon catheter was inserted into the left atrium of the heart and inflated with
different volumes. The responses of EL, RL, Raw and _V_ti were comparable to those with acute fluid load. Alterations in pulmonary mechanics were closely correlated to mean pulmonary artery
blood pressure. In another six animals the effect of vagotomy on vascular engorgement was studied. Vagotomy did not alter baseline airway or tissue mechanics. Furthermore, vagotomy did not
influence the change of pulmonary mechanics to fluid load or increase of left atrial pressure. Our data indicate that in young piglets vascular engorgement causes alterations in airway and
lung tissue mechanics that are not dependent on vagal influences. SIMILAR CONTENT BEING VIEWED BY OTHERS SYNCHRONIZED AND PROPORTIONAL SUB-DIAPHRAGMATIC UNLOADING IN AN ANIMAL MODEL OF
RESPIRATORY DISTRESS Article 08 August 2022 SLOWING LUNG DEFLATION BY INCREASING THE EXPIRATORY RESISTANCE ENHANCES FRC IN PRETERM RABBITS Article 08 July 2024 REDUCING LUNG LIQUID VOLUME IN
FETAL LAMBS DECREASES VENTRICULAR CONSTRAINT Article 27 January 2021 MAIN Increasing evidence suggests that the traditional concept of changes in airway caliber and/or changes in the lung
parenchyma as the determinants for alterations in lung function needs to be reconsidered. In recent years, the potential roles of the bronchial and pulmonary circulations in altering lung
function and in modifying the response of the lung to agonists have gained increasing attention. Experiments have been carried out both in animals and humans to evaluate the relationship
between the airway vasculature and pulmonary mechanics. In dogs, it has been found that both bronchial(1) and pulmonary blood flow(2) affected recovery time constants from i.v. challenge
with histamine to a substantial and similar degree. Ishii _et al._(3) reported that an increase in left atrial pressure caused increased resistance of both the central and peripheral airways
measured by a retrograde catheter technique. Also Hogg _et al._(4) observed an increase in peripheral Raw immediately after elevating left atrial pressure. It has been suggested recently
that the resulting changes in lung function are due to reflex bronchoconstriction of the small airways(5) and can at least in part be prevented by vagotomy(3). However, the functional
integrity of cholinergic efferent innervation is controversial in the newborn, and there is evidence that efferent vagal control differs between juvenile and adult animals(6). We have
described a technique for partitioning the pulmonary response to different agents into airway and parenchymal components(7, 8). This has been shown to be useful in the evaluation of the
sites of action of different agents such as histamine, methacholine, and hypertonic saline in altering lung function. The present study was undertaken to determine whether vagal influences
contribute to changes in lung function due to vascular engorgement in a juvenile animal model. Furthermore, we aimed to determine the pulmonary sites of action of different mechanisms
elevating PAP. Two different techniques for producing acute vascular engorgement, namely acute fluid loading and increasing left atrial pressure by a inflatable balloon were used. These two
techniques could conceivably have differential effects on the pulmonary and bronchial circulations and thus have differential effects on airway and parenchymal mechanics. Physiologic data
were obtained from 11 piglets during vascular engorgement. METHODS _ANIMAL PREPARATION_. Our study was approved by the Institutional Animal Ethics Committee. Eleven piglets (weight mean, 7.1
± 0.4 kg; age, 3.9 ± 0.2 wk) were studied. Anesthesia was induced with halothane and maintained with fentanyl (1 μg/kg/h i.v.) and pentobarbitone sodium (10 mg/kg i.v.). The piglets
underwent a tracheotomy, were intubated, and mechanically ventilated (Harvard Apparatus model 708) with a tidal volume of 10 mL/kg body weight. Paralysis was obtained with pancuronium
bromide (0.2 mg/kg i.v.). After insertion of central venous and femoral arterial catheters the chest was widely opened by midline sternotomy. Blood pressure and heart rate were monitored
continuously. Once set, tidal volume and ventilator frequency were kept constant throughout the study. Catheters were inserted into the pulmonary artery and the left atrium of the heart, the
former to measure pulmonary artery blood pressure continuously and the latter to elevate the left atrial pressure when inflated. The atrial catheter consisted of a self-made latex balloon,
capable of being inflated with up to 10 mL of fluid or air, connected to a modified 20-gauge venous cannula (Insyte, Becton Dickinson, Sandy, UT). _MEASURING AT THE AIRWAY OPENING_. Pao was
measured with a piezoresistive pressure transducer (Endevco, San Juan Capistrano, CA, 8510 B-2). Flow was measured with a pneumotachograph (Hans Rudolph Inc., Kansas City, MO). _ALVEOLAR
CAPSULE TECHNIQUE_. The alveolar capsule technique(9) was used to measure PA in the open-chest piglets. Three small plastic capsules were glued to the pleural surface with cyanoacrylate glue
(Loctite Corp., Ontario, Canada, 41621). The visceral pleura was punctured four times to a depth of approximately 0.5 mm with a 19-gauge needle to bring the underlying alveoli into
communication with the capsule chamber. A piezoresistive pressure transducer (Endevco 8507C-2) was lodged into each capsule to measure PA. The capsules were glued to the right middle and
left upper lobe. _MEASUREMENT OF RESPIRATORY MECHANICS_. _Mechanical ventilation_. Respiratory mechanics were calculated from the Pao,_V'_ (flow), and _V_ (volume) signals using a
multilinear regression implementation of the equation of motion of a single compartment model of the respiratory system, as follows: equation 1 where EL represents the elastance of the lung,
RL represents the resistance of the lung and PAee represents the Pa at end-expiration(10). We have found that EL frequently varies with volume during mechanical ventilation(11). We
therefore allow EL to vary during ventilation by including a volume-dependent term _i.e.:_ equation 2 where E1L represents the volume-independent component of elastance and E2L represents
the volume-dependent component. The tissue contribution to energy dissipation (_V_ti) during regular mechanical ventilation was calculated using theequation with equation 4 where E1ti is the
volume-independent and E2ti is the volume-dependent component of tissue elastance. RL, E1L, E2L, _V_ti, E1ti, and E2ti were calculated by multiple linear regression fitted to 30-s data
epochs containing periods of regular mechanical ventilation. Data were acceptable if the multiple linear regression correlation coefficient was >0.97. Raw was then calculated by
subtracting _V_ti from RL, _i.e._equation 5 _Signal conditioning_. All signals were amplified (PR signal conditioner, Endevco 4423), low pass filtered (Frequency Devices, Haverhill, MA, 902
LPF, 8 pole Bessel, Fc = 10 Hz) and recorded by a 12-bit analog-to-digital converter at a rate of 100 Hz. The data were stored on a computer for analysis. _EXPERIMENTAL PROTOCOL_. _Baseline
measurements_. After surgical preparation, a stable ventilation pattern was established with a tidal volume of 10 mL/kg. Three discrete 30-s data epochs were then recorded for calculation of
baseline RL, _V_ti, and EL, as described above. _Acute fluid overload_. Five piglets received a volume of 10 mL/kg polygeline (Haemaccel®) via the central venous line within 5 min. After
the end of the infusion three separate 30-s data epochs were collected. Subsequently, 10 mL/kg blood was removed within 5 min using the femoral artery catheter, and data were collected after
this procedure. Next, an amount of 20 mL/kg polygeline was infused and subsequently 10 mL/kg blood removed. This was repeated once. These procedures resulted in the following fluid balance:
0 mL/kg, +10 mL/kg, 0 mL/kg, +20 mL/kg, +10 mL/kg, +30 mL/kg, +20 mL/kg. _Inflation of the left atrial balloon_. The left atrial balloon was inflated with isotonic saline in 2-mL steps to
establish a dose-response curve. Between the different steps the balloon was deflated and measurements were recollected. This procedure was repeated until a maximal elevation in mean PAP was
seen without significant depression of systemic blood pressure. Acute fluid load or inflation of the left atrial balloon were carried out in random order. _Effects of bilateral vagotomy_.
Six additional piglets were investigated to study the influence of vagotomy on changes in airway and tissue mechanics. Three pigs underwent an acute fluid load with 20 mL/kg polygeline and
subsequent removal of the same amount of blood. After bilateral vagotomy, ensuing the baseline measurements, the fluid load was repeated. In another three animals a balloon catheter was
inflated and deflated before and after the vagi were cut as above. _STATISTICAL ANALYSIS_. Multivariate ANOVA with repeated measures using Excel 5.0 was performed to test for a significant
change of respiratory mechanics during the three different experimental procedures in comparison with baseline measurements. Two-way ANOVA with repeated measures (each test comparing only
two treatment conditions) was used to locate significant differences between treatment conditions. Using a Bonferroni correction for multiple comparisons these tests were performed at the
0.002 and 0.005 level of significance for the fluid load and balloon technique, respectively, to preserve an overall type I error rate of 5%. ANOVA without repeated measures was used for
comparison of mean PAP. For the effects of vagotomy, power tests were performed to determine the sensitivity to detect a type II error. All data are given as mean ± SD. RESULTS _ACUTE FLUID
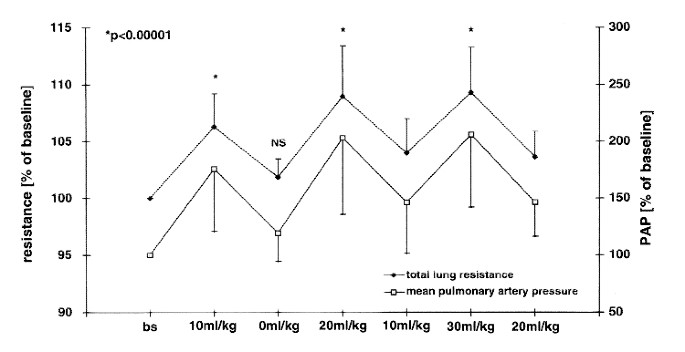
LOAD_. Initial total RL was 1.59 ± 0.29 kPa·L-1 ·s. The changes of RL and concomitant mean PAP during acute fluid administration are shown in Fig. 1. After rapid administration of 10 mL/kg
polygeline and subsequent withdrawal of an equal amount of blood, resistance measurements returned almost to baseline in all five animals without statistically significant increases in RL,
Raw, or _V_ti (Figs. 1 and 2). Mean PAP rose from 3.03 ± 0.60 kPa (22.7 ± 4.5 mm Hg) to maximal 5.65 ± 1.12 kPa (42.4 ± 8.4 mm Hg). During administration of 30 mL/kg an attenuation of the
pulmonary pressure occurred. Resulting changes in Raw and _V_ti in comparison with baseline are given in Fig. 2. Raw and _V_ti increased also significantly from 10 to 20 mL/kg and from 20 to
30 mL/kg (_p_ < 0.002). The baseline ratio of _V_ti/RL was 33.2% and did not change significantly during fluid overload. The relative changes of _V_ti during fluid administration were
2-3-fold larger than changes in Raw (_p_ = 0.03 for 10 mL/kg and 30 mL/kg,_p_ = 0.08 for 20 mL/kg). Total EL increased in response to fluid administration by maximal 30.9± 11.4% compared
with baseline (_p_ < 0.00001). Increases between 10 and 20 mL/kg and 20 to 30 mL/kg were also statistically significant(_p_ < 0.00002). The magnitude of the response to fluid overload
differed between animals but exhibited a similar pattern in each piglet with only very small intraindividual variations. Both the response of mean PAP as well as resistance and elastance
parameters was attenuated at higher levels of fluid administration. _ATRIAL BALLOON TECHNIQUE_. Fig. 3 summarizes the increase of RL and the elevation of mean PAP after inflation of the
atrial balloon with increasing volumes. The changes of _V_ti and Raw with different levels of PAP are shown in Fig. 4. The relative contribution of both compartments to the increase in total
RL was not significantly different from each other(_p_ = 0.69 for ΔPAP = 8 mm Hg, _p_ = 0.31 forΔPAP = 17 mm Hg). The baseline ratio of _V_ti/RL was 27.7% and did not change significantly
with different degrees of elevation of left atrial pressure. EL increased by maximal 23.0 ± 16.3% (_p_< 0.00001) during inflation of the balloon. The correlation between changes in mean
PAP and total RL,_V_ti, and Raw was very close and similar with both methods used to cause vascular engorgement (_r_ > 0.90, _p_< 0.002). During acute fluid load, each increase of the
mean PAP by 10 mm Hg was associated with an increase in total RL by 4.7 ± 0.5%. When PAP was increased by elevating left atrial pressure, by means of the balloon, the increase in RL per 10
mm Hg increase in PAP was 3.7 ± 0.8%, respectively. _EFFECTS OF VAGOTOMY_. Baseline total RL and _V_ti resistance before and after vagotomy are shown in Table 1. Vagotomy did not change
significantly these parameters in any of the studied animals. Furthermore, no significant effect of vagotomy was found in the effects of acute fluid load or elevation of left atrial pressure
on pulmonary mechanics (Table 2). Based on these data, the risk of a type II error was calculated. There was a 80% power of detecting at least a 7% change in baseline RL and at least a 12%
change in response to vascular engorgement before and after vagotomy. DISCUSSION The results of the present study have shown that: _1_) vascular engorgement produces largely reversible
increases in resistance of both the airway and the parenchymal compartments of the lung with predominance of the tissue resistive properties as well as increases in EL; _2_) vagotomy does
not influence basal bronchomotor tone nor changes in airway or lung _V_ti caused by vascular engorgement; and _3_) the primary site of action within the lung does not differ whether vascular
engorgement is induced by acute fluid loading or increasing left atrial pressure. Increasing evidence exists that the vasculature within the lung has a larger impact on pulmonary mechanics
than assumed previously. However, the underlying mechanisms are still not clearly defined. Several investigators have reported that elevated left atrial pressure in patients with heart
disease can produce obstructive symptoms(12, 13), and airway wall edema has been described in congestive heart failure(14). In patients with acute postoperative pulmonary hypertension,
respiratory system resistance is increased and compliance decreased(15). Gilbert _et al._(16) administered 2 L of isotonic saline i.v. in normal and asthmatic subjects and reported profound
decrements in forced expiratory volume in 1 s. Few studies have been performed to investigate the role of influences of the nervous system on bronchial tone during vascular engorgement.
Ishii _et al._(3) elevated left atrial pressure in vagotomized dogs to exclude the effects of vagal reflex on the increase in resistance. They observed an almost complete reversibility of
the changes in resistance of the central and peripheral airways within the first 30 min after elevation of left atrial pressure, whereas after this period increases in peripheral Raw were
not completely reversible. This supports the assumption that the initial changes in pulmonary mechanics are not influenced by vagal responses. Other authors, however, have found, that vagal
stimulation has an effect on central airways and an even more pronounced effect on peripheral airways(17, 18). Wagner and Mitzner(5) recently found that vascular engorgement decreased
bronchial luminal area only in the presence of airway smooth muscle tone, suggesting a reflex bronchoconstriction in response to engorgement. However, most of these studies were performed in
adult animals. It has been shown that cholinergic efferent innervation develops after birth and, unlike adult pigs, vagotomy in newborn piglets does not decrease repiratory system
compliance and resistance(6). Pérez Fontán demonstrated that vagal bronchomotor tone is absent in developing piglets at least to the age of 6 wk(19). The data of our present study support
and extend these findings by demonstrating that vascular engorgement in newborn piglets does not act on lung function via vagally mediated bronchoconstriction. In our experiment, the animals
were anesthetized initially with halothane, and anesthesia was maintained with pentobarbitone and fentanyl. Pancuronium bromide was used for muscle relaxation. These agents are commonly
used in animal studies of respiratory function but may themselves change pulmonary mechanics(20–22). Pancuronium is known to enhance the increase in pulmonary resistance due to vagal nerve
stimulation, probably via block of prejunctional muscarinic receptors that physiologically inhibit vagally mediated increases in pulmonary resistance(22). We cannot completely rule out that
effects of the above agents may have influenced the results of our study, especially in enhancing the changes in pulmonary mechanics. However, the response of pulmonary resistance to
vascular engorgement was not altered by vagotomy, which suggests that vagal stimulation is not responsible for the effects of vascular engorgement, and pancuronium should be expected to
enhance any differences if present. In our fluid experiment we used polygeline rather than saline because we wanted to decrease the possibility of extravasation of fluid into the
interstitium and alveoli. For hemodynamic reasons we could not extract the same amount of fluid as administered within a short period of time. However, we found that each single step of
fluid administration was largely reversible, indicating that interstitial edema was unlikely to be responsible for the alterations in resistance and elastance. In addition, one may have
expected changes due to edema to be progressive and increasing throughout the study. This thesis is supported by the fact that both the changes in elastance and resistance induced by
elevation of left atrial pressure with the inflation of the balloon were completely reversible. These findings are compatible with observations of Wetzel _et al._(23). The authors
investigated the change in airway cross-sectional area in piglets after intravascular volume expansion with 30 mL/kg Ringer's lactate by high resolution computed tomography and found a
decreased internal cross-sectional area in both large (2-5 mm) and small (0.75-2 mm) airways. These changes were rapidly reversed by intravascular volume reduction suggesting a reversible
bronchial mucosal vascular engorgement rather than edema as the potential cause of increased Raw. On a morphologic level, the bronchial circulation would be expected to be the principal site
of vasodilation when hyperemia occurs in the conducting peripheral airways, whereas the pulmonary circulation would be expected to be involved in hyperemia of the alveolar tissue. An
important aim in our study was to partition RL into its airway and tissue components using the alveolar capsule technique(9). The relative alteration in _V_ti exceeded Raw 2-3-fold with both
methods used to cause vascular engorgement, but the relationship between _V_ti/RL did not change throughout the experiments. This indicates that both tissue and airway compartments (and
thus, bronchial and pulmonary circulations) are affected. This is consistent with work of Kelly _et al._(1, 2) who concluded that both vascular beds affect the recovery time from i.v.
histamine challenge in the lung periphery. There is still controversy in the literature regarding whether pulmonary artery blood flow or mean PAP is the major determining factor for changes
in respiratory mechanics. Some authors observed an effect on pulmonary mechanics only when both PAP and pulmonary artery blood flow were elevated(24). In animal models some investigators
even failed to detect any changes in respiratory function after experimental changes in PAP(25, 26). In children undergoing cardiopulmonary bypass we have previously reported that
respiratory mechanics were correlated with pulmonary blood flow but not PAP(27). Griffen _et al._(28), however, reported a decrease in dynamic compliance with increasing PAP alone. Bancalari
_et al._(29), studying infants with increased and decreased pulmonary artery blood flow, suggested that PAP rather than flow is the primary factor affecting compliance in humans with
left-to-right shunts. The results of the present study are consistent with this conclusion. The two different techniques we used to cause vascular engorgement have opposing effects on the
pulmonary blood flow. Elevation of left atrial pressure diminishes the blood flow, whereas acute fluid administration increases it. Despite different blood flows we have found that the
responses of both the airway and the tissue compartments to vascular engorgement were closely correlated to mean PAP. We observed an attenuation of the response of both PAP and pulmonary
mechanics to increasing fluid volumes. The former is very likely due to the autoregulatory response of the heart, the Frank-Starling mechanism. With increasing venous return and elevated
end-diastolic volume the cardiac output will also increase, thus compensating a further elevation of pulmonary venous and PAP. If we assume the PAP to be the driving force for changes in
lung mechanics, the attenuation of RL and elastance in response to larger fluid volumes may be attributed to the above mechanism. In summary, we found that in 4-wk-old piglets cholinergic
innervation does not play a role in changes in airway and lung tissue mechanics caused by vascular engorgement. Both techniques we used (acute fluid administration and increasing left atrial
pressure by a balloon catheter) cause similar increases in both Raw and _V_ti with and without vagi intact. The alterations in pulmonary mechanics were closely associated with changes in
pulmonary artery blood pressure. This model provides the opportunity to gain further valuable insight into the role of the vasculature altering lung mechanics. ABBREVIATIONS * EL: lung
elastance * Pa: alveolar pressure * Paee: end-expiratory alveolar pressure * Pao: airway opening pressure * PAP: pulmonary artery pressure * Raw: airway resistance * RL: lung resistance *
_V_ : gas volume * _V'_ : gas flow * _V_ ti : tissue resistance REFERENCES * Kelly L, Kolbe J, Mitzner W, Spannhake EW, Bromberger-Barnea B, Menkes H 1986 Bronchial blood flow affects
recovery from constriction in dog lung periphery. _J Appl Physiol_ 60: 1954–1959. Article CAS Google Scholar * Kelly LJ, Mitzner W, Spannhake EW, Bromberger-Barnea B, Menkes HA 1986
Pulmonary blood flow affects recovery from bronchoconstriction in dog lung periphery. _J Appl Physiol_ 60: 1554–1560. Article CAS Google Scholar * Ishii M, Matsumoto N, Fuyuki T, Hida W,
Ichinose M, Inoue H, Takishima T 1985 Effects of hemodynamic edema formation on peripheral vs. central airway mechanics. _J Appl Physiol_ 59: 1578–1584. Article CAS Google Scholar * Hogg
JC, Agarawal JB, Gardiner AJS, Palmer WH, Macklem PT 1972 Distribution of airway resistance with developing pulmonary edema in dogs. _J Appl Physiol_ 32: 20–24. Article CAS Google Scholar
* Wagner EM, Mitzner W 1996 Effects of bronchial vascular engorgement on airway dimensions. _J Appl Physiol_ 81: 293–301. Article CAS Google Scholar * Clement MG, Mortola JP, Albertini
M, Aguggini G 1986 Effects of vagotomy on respiratory mechanics in newborn and adult pigs. _J Appl Physiol_ 60: 1992–1999. Article CAS Google Scholar * Sly PD, Lanteri CJ 1990
Differential responses of the airways and pulmonary tissues to inhaled histamine in young dogs. _J Appl Physiol_ 68: 1562–1567. Article CAS Google Scholar * Sly PD, Lanteri CJ 1991
Partitioning of pulmonary responses to inhaled metacholine in puppies. _J Appl Physiol_ 71: 886–891. Article CAS Google Scholar * Fredberg JJ, Keefe DH, Glass GM, Castile RG, Frantz ID
1984 Alveolar pressure nonhomogeneity during small-amplitude high-frequency oscillation. _J Appl Physiol_ 57: 788–800. Article CAS Google Scholar * Nicolai T, Lanteri CJ, Freezer N, Sly
PD 1991 Non-invasive determination of alveolar pressure during mechanical ventilation. _Eur Respir J_ 4: 1275–1283. CAS PubMed Google Scholar * Lanteri CJ, Willett K, Kano S, Jobe A,
Ikegami I, Polk D, Newnham J, Kohan R, Kelly R, Sly PD 1994 Changes in lung mechanics over the first hour of life in preterm lambs: the effects of intra-uterine steroid treatment. _Am J
Respir Crit Care Med_ 150: 759–765. Article CAS Google Scholar * Saxton GA Jr, Rabinowitz M, Dexter L, Haynes F 1965 The relationship of pulmonary compliance to pulmonary vascular
pressures in patients with heart disease. _J Clin Invest_ 35: 611–613. Article Google Scholar * Tattersfield AE, McNicol MW, Sillett RW 1972 Relationship between hemodynamic and
respiratory function in patients with myocardial infarction and left ventricular failure. _Clin Sci_ 42: 751–768. Article CAS Google Scholar * Don C, Johnson R 1977 The nature and
significance of peribronchial cuffing in pulmonary edema. _Radiology_ 125: 577–582. Article CAS Google Scholar * Schindler MB, Bohn DJ, Bryan AC, Cutz E, Rabinovitch M 1995 Increased
respiratory system resistance and bronchial smooth muscle hypertrophy in children with acute postoperative pulmonary hypertension. _Am J Respir Crit Care Med_ 152: 1347–1352. Article CAS
Google Scholar * Gilbert IA, Winslow CJ, Lenner KA, Nelson JA, McFadden ER Jr 1993 Vascular volume expansion and thermally induced asthma. _Eur Respir J_ 6: 189–197. CAS PubMed Google
Scholar * Benson MK, Graf PD 1977 Bronchial reactivity: interaction between vagal stimulation and inhaled histamine. _J Appl Physiol_ 43: 643–647. Article CAS Google Scholar * Hoppin FG
Jr, Green M, Morgan MS 1978 Relationship of central and peripheral airway resistance to lung volume in dogs. _J Appl Physiol_ 44: 728–737. Article Google Scholar * Pérez Fontán JJ, Ray AO
1991 Vagal control of central and peripheral pulmonary resistance in developing piglets. _J Appl Physiol_ 70: 1617–1626. Article Google Scholar * Joyner MJ, Warner DO, Rehder K 1992
Halothane changes the relationships between lung resistance and lung volume. _Anesthesiology_ 76: 229–235. Article CAS Google Scholar * Advenier C, Boissier JR, Ho S, Mallard B, Ruff F
1978 The effects of pentobarbitone and urethane on pulmonary airway resistance in guinea-pigs and their interactions with drugs. _Br J Pharmacol_ 64: 519–525. Article CAS Google Scholar *
Vettermann J, Beck KC, Lindahl SG, Brichant JF, Rehder K 1988 Actions of enflurane, isoflurane, vecuronium, atracurium, and pancuronium on pulmonary resistance in dogs. _Anesthesiology_ 69:
688–695. Article CAS Google Scholar * Wetzel RC, Herold CJ, Zerhouni EA, Robotham JL 1993 Intravascular volume loading reversibly decreases airway cross-sectional area. _Chest_ 103:
865–870. Article CAS Google Scholar * Howlett G 1972 Lung mechanics in normal infants and infants with congenital heart disease. _Arch Dis Child_ 47: 707–715. Article CAS Google Scholar
* Csete ME, Abraham WM, Wanner A 1990 Vasomotion influences airflow in peripheral airways. _Am Rev Respir Dis_ 141: 1409–1413. Article CAS Google Scholar * Tang GJ, Freed AN 1994 The
role of submucosal oedema in increased peripheral airway resistance by intravenous volume loading. _Eur Respir J_ 7: 311–7. Article CAS Google Scholar * Lanteri CJ, Kano S, Duncan A W,
Sly PD 1995 Changes in respiratory mechanics in children undergoing cardiopulmonary bypass. _Am J Respir Crit Care Med_ 152: 1893–1900. Article CAS Google Scholar * Griffen AJ, Ferrara
JD, Lax JO, Cassels DE 1972 Pulmonary compliance: an index of cardiovascular status in infancy. _Am J Dis Child_ 123: 89–95. Article Google Scholar * Bancalari E, Jesse MJ, Gelband H,
Garcia O 1977 Lung mechanics in congenital heart disease with increased and decreased pulmonary flow. _J Pediatr_ 90: 192–195. Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS The authors thank Dr. Nigel Dore for his assistance and Lyle Gurrin for his advice concerning the statistical analysis of the data. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Division of Clinical Sciences, Princess Margaret Hospital for Children, Perth, Western Australia Torsten Uhlig, Ernst Eber & Peter D Sly * Department of Respiratory
Medicine, Princess Margaret Hospital for Children, Perth, Western Australia Johannes H Wildhaber Authors * Torsten Uhlig View author publications You can also search for this author inPubMed
Google Scholar * Johannes H Wildhaber View author publications You can also search for this author inPubMed Google Scholar * Ernst Eber View author publications You can also search for this
author inPubMed Google Scholar * Peter D Sly View author publications You can also search for this author inPubMed Google Scholar ADDITIONAL INFORMATION Supported, in part by research
grants from the Deutsche Forschungsgemeinschaft (DFG), Germany and the NH&MRC, Australia. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Uhlig, T.,
Wildhaber, J., Eber, E. _et al._ Vagal Reflex Is Not Responsible for Changes in Airway and Lung Tissue Mechanics Due to Vascular Engorgement in Young Piglets. _Pediatr Res_ 42, 533–538
(1997). https://doi.org/10.1203/00006450-199710000-00019 Download citation * Received: 12 December 1996 * Accepted: 11 May 1997 * Issue Date: 01 October 1997 * DOI:
https://doi.org/10.1203/00006450-199710000-00019 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative