Play all audios:
ABSTRACT Partial liquid ventilation (PLV) has been shown to improve gas exchange in paralyzed animals and in humans with lung disease. This study tests the hypothesis that PLV combined with
respiratory mechanical unloading results in stable ventilation and gas exchange in spontaneously breathing animals. Ten adult anesthetized, intubated, and spontaneously breathing rabbits
received ventilatory support by respiratory mechanical unloading (FiO2 1.0). Minute ventilation, respiratory rate, esophageal pressure, heart rate, and arterial blood pressure were recorded
continuously during gas ventilation for 1 h. Next, 30 mL/kg of perfluorocarbon was instilled into the endotracheal tube. Thereafter, data were recorded again for 1 h (PLV). Arterial blood
gases were obtained at the end of each period. Variability of recorded data was assessed by calculating coefficients of variation using data obtained each minute. Compared with gas
ventilation, minute ventilation was larger during PLV (275 ± 93 _versus_ 368 ± 89 mL/kg/min.;_p_ < 0.01). This was because of a higher respiratory rate during PLV (58 ± 23 _versus_ 74 ±
18 breaths/min;_p_ < 0.05), while tidal volume was similar. Compared with gas ventilation, PaO2 was lower during PLV (61.31 ± 5.32 _versus_ 47.35 ± 8.38 kPa;_p_ < 0.05). PaCO2, peak
esophageal pressure deflections, heart rate, mean arterial blood pressure, and coefficients of variation for minute ventilation, tidal volume, respiratory rate, and peak esophageal pressure
were not significantly different between modes. Compliance was decreased and resistance and work of breathing were increased during PLV. We conclude that stable ventilation and gas exchange
may be achieved during PLV combined with mechanical unloading in spontaneously breathing animals without lung disease. SIMILAR CONTENT BEING VIEWED BY OTHERS SYNCHRONIZED AND PROPORTIONAL
SUB-DIAPHRAGMATIC UNLOADING IN AN ANIMAL MODEL OF RESPIRATORY DISTRESS Article 08 August 2022 EVALUATION OF LUNG VOLUMES AND GAS EXCHANGE IN SURFACTANT-DEFICIENT RABBITS BETWEEN VARIABLE AND
FIXED SERVO PRESSURES DURING HIGH-FREQUENCY JET VENTILATION Article 25 November 2023 REDUCING LUNG LIQUID VOLUME IN FETAL LAMBS DECREASES VENTRICULAR CONSTRAINT Article 27 January 2021 MAIN
Neonates undergoing mechanical ventilation often have vigorous spontaneous respiratory activity. It has been shown that synchronizing ventilator cycles to spontaneous breathing by
patient-triggered ventilation or respiratory mechanical unloading may improve gas exchange (1, 2) and reduce ventilator pressure requirements (2, 3). Potential disadvantages of neuromuscular
blockade in ventilated neonates include atrophy of respiratory muscles, reduced cardiac output and blood pressure (4), and lower functional residual capacity (5). PLV, also known as
perfluorocarbon-associated gas exchange, initially described by Fuhrman _et al._ (6), uses conventional gas tidal breathing into a lung partially filled with perfluorochemical fluids. PLV
has been shown to decrease alveolar surface tension, resulting in alveolar recruitment, which may improve lung mechanics (7–9) and reduce ventilation-perfusion mismatch, resulting in
improved gas exchange (10, 11). Using various animal models of lung disease, it has been shown that PLV may result in improved gas exchange and pulmonary mechanics (8, 9, 12–19) and cause
less barotrauma as assessed by lung histology (15). However, PLV has been tested almost exclusively in pharmacologically paralyzed subjects and not during preserved spontaneous respiratory
activity. Most recently, it has been shown that synchronized mechanical ventilation is feasible in spontaneously breathing piglets with healthy lungs during PLV (20). This study is the first
step of a number of ongoing studies evaluating the feasibility of spontaneous breathing assisted by respiratory mechanical unloading during PLV in animals without and with different lung
diseases. The aim of these studies is to explore physiologic advantages of preserved spontaneous ventilation during PLV. Respiratory mechanical unloading, also called proportional assist
ventilation, is a recently developed mode of ventilatory support (21–23) that adjusts its support in proportion to the patient's effort. During respiratory mechanical unloading, there
is no fixed target airflow, volume, or Paw. However, the Paw applied is in proportion to the patient's airflow and to the inspired tidal volume at any point in time within each
respiratory cycle. Thus, the depth, timing, and pressure profile of each ventilator breath is controlled by the patient (21, 22). This ventilatory mode was chosen because it provides
potential advantages compared with other assisted modes of ventilation, such as improved patient comfort, preservation of the subject's own reflex and control mechanisms, and a lower
peak as well as mean Paw to maintain ventilation (2, 23). Combination of PLV and respiratory mechanical unloading may be useful to improve and to compensate for decreased lung compliance in
subjects with lung disease and during the weaning process from PLV. Respiratory mechanical unloading is highly dependent on the patient's own respiratory control. The focus was to study
the interaction between the animals and the ventilator. Because respiratory control mechanisms may be affected by lung diseases, we used animals with healthy lungs as a first step before
studying animals with lung disease. We hypothesized that respiratory mechanical unloading, when combined with PLV, would result in stable ventilation and gas exchange in spontaneously
breathing animals. METHODS This animal research protocol was approved by the Animal Care Committee of the government agencies of Baden-Wuerttemberg. The study was designed as a cohort study
using each animal as its own control. The animals were initially supported with respiratory unloading and then with unloading combined with PLV. VENTILATOR. A Stephanie infant ventilator
(Stephan Medizintechnik GmbH, Gackenbach, Germany) was used throughout the study. In addition to conventional ventilation, this ventilator provides negative ventilator resistance and
elastance, also called resistive and elastic unloading. This device has been described in detail elsewhere (21, 24–26). It is a servocontrolled system with a pneumotachograph (dead space,
0.6 mL) placed between the endotracheal tube connector and the ventilator circuit. The system continuously receives the flow signal of the animal's spontaneous breathing from the
pneumotachograph. This signal is processed by microcomputer using special algorithms to control a rapid valve that determines the Paw applied at the endotracheal tube. The pressure applied
per unit airflow determines the degree of resistive unloading (Kr). The pressure applied per unit of inspired volume determines the degree of elastic unloading (Ke). Thus, the delivered Paw
is a weighted sum of the resistive and the elastic components at any point in time during a spontaneous breathing cycle MATH where V˙ is flow and V is volume. ANIMAL PREPARATION. Ten female
adult New Zealand White rabbits with a body weight of 3063 ± 149 g (mean ± SD) were given 0.2 mg/kg atropine and anesthetized with ketamine (15–40 mg/kg) and xylazine (1.5–4 mg/kg) i.v.
After supine positioning, animals were intubated using a 3.0 or 3.5 mm cuffed endotracheal tube, and the cuff was inflated to prevent leaks. A rectal temperature probe (Siemens Sirecust 302,
Erlangen, Germany) was placed, and a core temperature of 38 to 39.5°C was maintained using a heating blanket and an overhead warmer (Babytherm 8000, Draeger, Luebeck, Germany). Anesthesia
was maintained with a continuous infusion of ketamine (50–130 mg/kg/h). The dose was adjusted to maintain anesthesia deep enough to prevent spontaneous movements other than respiration.
During the surgical instrumentation, the animals were placed on volume-controlled, synchronized intermittent positive-pressure ventilation with the following settings: assist/control mode;
FiO2, 0.21–0.4; tidal volume, 10 mL/kg; PEEP, 0.4–0.6 kPa; inspiratory time, 0.4 s; and minimum respiratory rate, 15–20/min, which, in case of poor respiratory effort, was adjusted to
maintain normoventilation (PaCO2, 4.7–6.0 kPa). This ventilator mode ensured adequate ventilation in case respiratory effort would be impaired secondary to deep anesthesia. Dextrose 5% with
35 mmol/L Na, 18 mmol/L K, and 1 U/mL heparin was administered at 5 mL/kg/h into a peripheral vein. A 3.5F arterial femoral line was inserted for continuous blood pressure monitoring and
sampling for blood gas analyses and was continuously flushed with heparinized (1 U/mL) normal saline solution at a rate of 3 mL/h. Airflow was measured using the pneumotachograph of the
ventilator. Tidal volume was calculated by integrating flow. Paw was measured at the connector of the endotracheal tube, and Pe was measured through a fluid-filled 5F feeding tube with its
tip placed into the distal esophagus. All pressure transducers (Sorenson Transpac tranducers, Abbott Critical Care Systems, North Chicago, IL) were calibrated using a water manometer.
Immediately before data acquisition, correct placement of the esophageal tube was checked by performing end-inspiratory airway occlusions and comparing Paw and Pe. A ΔPe/ΔPaw ratio of 1.00 ±
0.05 was accepted (27). Otherwise, the esophageal catheter was repositioned until correct placement was confirmed. The catheter was continuously flushed with water (3 mL/h) to avoid bubble
formation. Arterial Hb oxygen saturation (SpO2) was measured transcutaneously with a Nellcor N 200 pulse oximeter (Nellcor Inc., Hayward, CA). All signals were digitized at a frequency of
100 Hz and recorded simultaneously using a data acquisition system (DATAQ Instruments, Inc., Akron, OH). PROTOCOL. After instrumentation, animals were switched to the unloading mode of
ventilation using the same PEEP and an FiO2 of 1.0. Resistive unloading was adjusted to compensate approximately for the expected resistance of the endotracheal tube, which was estimated by
measuring peak airflow and by using previously published data (28). Elastic unloading was adjusted to maintain a PaCO2 within the target range. After allowing the animal 30 min to adjust to
the new mode of ventilation, data were recorded for 60 min, and arterial blood gases were measured at the end of this GV period. Next, 30 mL/kg of prewarmed (38°C) PFC (Rimar 101, Miteni,
Italy) was instilled continuously at a rate of 1 mL/kg/min into the endotracheal tube without disconnecting the ventilator. The degree of resistive unloading was increased during the
administration of the PFC to compensate for increased airway resistance caused by the presence of liquid in the airways during filling. The degree of elastic unloading was readjusted to
maintain a PaCO2 within the target range. Specifically, the degree of elastic unloading was increased if respiratory rate increased during liquid filling by >50% or increased to >100
breaths/min, because preliminary experience has shown that this increase would be very likely to be associated with CO2 retention. The degree of elastic unloading was decreased if tidal
volume was >10 mL/kg, because it has been our experience that this would be associated with low respiratory rates, low PCO2, and apnea in these animals without lung disease. Arterial
blood gases were used to confirm PaCO2 values being within the target range. The filling condition was ascertained by disconnecting the animal from the ventilator and performing a slight
thoracic compression to observe a meniscus at the endotracheal tube. Immediately after filling, data were recorded again for 60 min, and arterial blood gases were measured at the end of this
PLV period. No refill was performed during the PLV period. Sequence randomization was not feasible because complete removal of the liquid from the lung would not have been possible within
the time frame of the study. DATA ANALYSIS. Primary outcome measures for the comparison of the two ventilatory modes (GV _versus_ PLV) were V˙E and its variability. Variability of V˙E was
measured as the coefficient of variation, calculated as the SD of V˙E data, obtained from 1-min intervals, divided by the mean V˙E for each animal. Secondary outcome measures were tidal
volume as obtained by integration of the expiratory flow signal from all breaths, and respiratory rate as measured using flow and Pe traces. The means and coefficients of variation of data
obtained from 1-min intervals of these variables were compared between both ventilation modes (GV _versus_ PLV). Peak Pe deflections were measured from 20 randomly distributed breaths from
each recording period to calculate mean peak Pe and coefficients of variation. Mean Paw was calculated as the integral of Paw divided by the recording time. Lung compliance and airway
resistance were calculated by a program based on the equation of motion (29). Work of breathing of the lung was calculated as the area given by the integral of inspiratory transpulmonary
pressure over volume. Transpulmonary pressure was defined as Paw minus Pe. The power of breathing was calculated as work of breathing over time. Heart rate and mean ABP were measured from
the ABP trace using the complete recording periods. Arterial blood gases were drawn at the end of each ventilation mode. The time with SpO2 <85% was measured as the percentage of the
complete recording period. STATISTICS. Two-tailed paired _t_ tests were used to compare matched data. If data were not normally distributed, Wilcoxon signed rank tests were used instead. We
considered _p_ < 0.05 to indicate statistical significance. Values are expressed as mean ± SD or as median and range. RESULTS Instillation of PFC was well tolerated in all animals,
without apparent adverse respiratory or hemodynamic consequences except for ventilation problems in one animal, secondary to an inadvertent overfill with liquid, which were abolished
immediately by increasing PEEP transiently from 0.6 to 0.8 kPa. Coughing was induced in three animals during filling but disappeared immediately after an additional i.v. injection of 5–30 mg
of ketamine. The ketamine dose was similar during both ventilatory conditions (72 ± 17 _versus_ 77 ± 20 mg/kg/h; GV _versus_ PLV). The degree of resistive unloading was similar during both
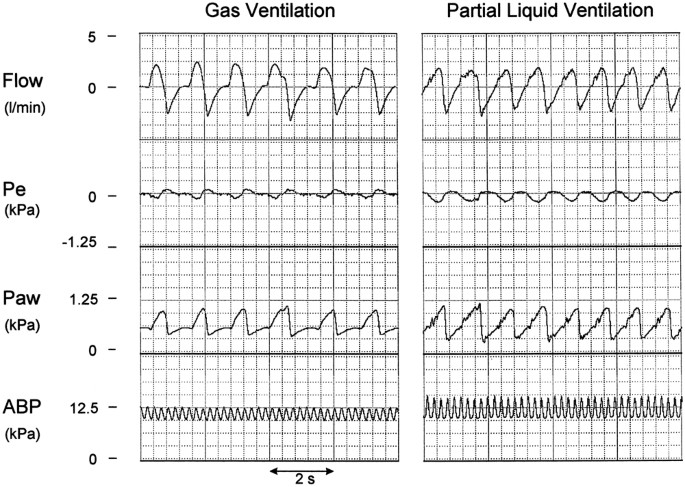
ventilatory conditions (2.5 [1.0–3.7] kPa/L/s), and the degree of elastic unloading was 0.01 (0–0.02) _versus_ 0.02 (0.01–0.03) kPa/mL during GV _versus_ PLV. Figure 1 shows representative
traces of airflow, Pe, Paw, and ABP of one of the animals during both ventilatory conditions. A decrease in Pe was followed instantaneously by an increase in Paw, indicating that patient
effort and ventilator pressure were in phase during both GV and PLV. Table 1 shows the effects of filling the lung with liquid on ventilatory and hemodynamic variables. Compared with GV, V˙E
was significantly larger during PLV, whereas there was no difference in tidal volume. Mean respiratory rate was also significantly higher during PLV. Therefore, the larger V˙E during PLV
was entirely caused by the higher respiratory rate. Mean Paw was slightly higher during PLV, indicating more ventilatory support during PLV. Lung compliance was decreased, whereas airway
resistance and work and power of breathing were increased during PLV compared with GV. There were no statistically significant differences between the means of peak Pe deflections, heart
rate, and mean ABP between ventilation modes. Variability data expressed as coefficients of variation for V˙E, tidal volume, respiratory rate, and peak Pe deflections for both ventilatory
conditions (GV _versus_ PLV) are shown in Table 2. Coefficients of variation were similar for all variables, suggesting no difference in variability of these variables between both
ventilatory conditions. Arterial blood gas values, drawn at the end of each ventilation mode, are shown in Table 3. There were no differences for pH, base excess, and PaCO2, but PaO2 was
higher during GV than during PLV. SpO2 was always between 99% and 100% during both ventilation modes in all animals. DISCUSSION The principal finding of this study is that ventilation and
gas exchange are maintained in spontaneously breathing rabbits with normal lungs partially filled with PFC. V˙E was larger during PLV _versus_ GV, whereas tidal volume remained unchanged.
Despite increased V˙E during PLV, PaCO2 values were unchanged after transition to PLV in our animals. This observation is consistent with findings from other investigators, who found
increased PaCO2 values after transition from GV to PLV in piglets with healthy lungs when V˙E was controlled for by using volume-controlled ventilation (30). Because PaCO2 remained unchanged
in our study, only changes in ventilation-perfusion mismatch, an increased arterial-alveolar CO2 gradient, or an increased metabolic rate during PLV can explain the difference in V˙E. The
latter seems to be unlikely, because, at least for total liquid ventilation it has been shown that oxygen consumption and CO2 production do not change during transition from gas to liquid
ventilation (31). However, there is evidence of ventilation-perfusion heterogeneity and diffusion limitation in animals with healthy lungs during PLV (32–34). Mates _et al._ (32) found that
O2 and CO2 exchange is impaired in proportion to the volume of added PFC fluid into the lung of normal piglets. This may entirely explain the higher respiratory rate and V˙E observed in our
study and also the higher mean Paw because of the inherent coupling of ventilatory support and patient effort of our ventilator system. Although any increase in ventilatory support over time
potentially may promote ventilator-associated lung injury, the magnitude of the observed difference in mean Paw is probably not clinically significant. The decreased lung compliance and
increased resistance during PLV is explained by the high density and viscosity of the PFC in comparison to gas. Similar changes in pulmonary mechanics have been observed during
volume-controlled mechanical ventilation after intratracheal PFC administration in healthy animals and therefore do not seem to be specific for the type of ventilatory support used in our
study (35). The changes in pulmonary mechanics resulted in increased work of breathing. Power of breathing increased even more, because respiratory rate increased as well to maintain PaCO2.
Improvement of lung mechanics resulting in a decreased work of breathing can be expected only in subjects with severe pulmonary disease in which PLV may lead to significant recruitment of
alveoli. In this case, a lower degree of ventilatory support, resulting in a lower mean Paw, may be expected during PLV compared with GV. The decreased PaO2 during PLV is consistent with
results of other studies (6, 30, 32, 33, 35) and may be explained by impaired diffusion, ventilation-perfusion heterogeneity, and shunt in animals with healthy lungs. Because animals were
ventilated with an FiO2 of 1.0 and had no lung disease, we expected a PaO2 of >40 kPa with an SpO2 of 99–100% throughout the study, provided ventilation was stable. Inasmuch as
ventilation was stable during both ventilation modes, episodes of hypoxemia did not occur. Heart rate and ABP were unchanged after transition from GV to PLV, which is consistent with
observations of other investigators (6, 14, 30, 35, 36). Shaffer and Moskowitz (37) have performed early experiments in dogs with a demand-controlled device using the Pe of the animal as the
respiratory input signal to control pumps and valves of a total liquid-ventilation system. Although these animal experiments demonstrated that this system can provide adequate control of
gas exchange, there was a tendency toward progressive hypercarbia over time. Most recently, Bendel-Stenzel _et al._ (20, 38) have published studies in which they assessed the dynamics of
spontaneous breathing during patient-triggered PLV in piglets without lung disease and with ARDS, showing basically that spontaneous breathing and triggering a synchronized infant ventilator
is feasible with a liquid-filled lung. As we found in our study, these authors also showed that the animals were able to self-regulate their respiratory rate and minute ventilation to
maintain physiologic blood gases during all tested ventilatory modes such as assist/control, regular IMV, and synchronized IMV, with assist/control being the most efficient mode in terms of
CO2 elimination. The unloading mode of ventilation used in our study is to some extent comparable with the assist/control mode of patient-triggered ventilation, because every spontaneous
breath is supported by the ventilator system. However, the major difference is that during the unloading mode, the pressure profile within each ventilator breath is in proportion to the
volume and flow produced by the subject's own spontaneous breathing at any point in time of each breath. In the studies mentioned above, the animals triggered ventilator breaths, which
were characterized by a preset peak pressure and inspiratory time. However, currently no studies are available to determine whether any of the ventilatory modes such as mechanical unloading,
IMV, synchronized IMV, assist/control, or pressure support ventilation have any long-term physiologic or clinical advantages in spontaneously breathing animals with a partially
liquid-filled lung. All changes observed between the ventilatory modes in our study may, at least in part, be related to sequence effects. However, this is unlikely, inasmuch as the observed
changes can easily be explained on the basis of known physiologic mechanisms that typically occur during PLV. Carryover effects are also unlikely, as the filling procedure allowed a washout
period of as long as 30 min between modes. Furthermore, the main purpose of the study was to demonstrate stability of the target variables. In conclusion, PLV combined with respiratory
mechanical unloading achieved stable ventilation and gas exchange in spontaneously breathing animals without lung disease. Therefore, spontaneous breathing with a partially liquid-filled
lung seems to be feasible, allowing avoidance of paralysis, which is associated with side effects. However, PLV combined with respiratory mechanical unloading resulted in impaired lung
mechanics and increased work of breathing in animals without lung disease. Therefore, there is no direct clinical application for this mode of ventilation in most subjects without severe
lung disease. However, our findings may be of clinical relevance for future applications of liquid ventilation such as pulmonary administration of drugs (39, 40) or serving as a carrier for
gene targeting of bronchial or alveolar cells (41). Further studies should clarify whether PLV combined with respiratory mechanical unloading may help to recruit lung volume and improve gas
exchange in spontaneously breathing animals with lung disease, and whether or not spontaneous breathing has circulatory or other advantages compared with controlled mechanical ventilation in
paralyzed subjects with a partially liquid-filled lung. ABBREVIATIONS * ABP: arterial blood pressure * GV: gas ventilation * IMV: intermittent mandatory ventilation * Paw: airway pressure *
Pe: esophageal pressure * PEEP: positive end-expiratory pressure * PFC: perfluorocarbon chemical * PLV: partial liquid ventilation * FiO2: fraction of inspired oxygen * SpO2: arterial
oxygen saturation as measured by pulse oximetry * V˙E: expiratory minute ventilation REFERENCES * Cleary JP, Bernstein G, Mannino FL, Heldt GP 1995 Improved oxygenation during synchronized
intermittent mandatory ventilation in neonates with respiratory distress syndrome: a randomized crossover study. _J Pediatr_ 126: 407–411 CAS PubMed Google Scholar * Schulze A, Gerhardt
T, Musante G, Schaller P, Claure N, Everett R, Gomez-Martin O, Bancalari E 1999 Proportional assist ventilation in low birth weight infants with acute respiratory disease: a comparison to
assist/control and conventional mechanical ventilation. _J Pediatr_ 135: 339–344 CAS PubMed Google Scholar * Hummler H, Gerhardt T, Gonzalez A, Claure N, Everett R, Bancalari E 1996
Influence of different methods of synchronized mechanical ventilation on ventilation, gas exchange, patient effort, and blood pressure fluctuations in premature neonates. _Pediatr Pulmonol_
22: 305–313 CAS PubMed Google Scholar * Runkle B, Bancalari E 1984 Acute cardiopulmonary effects of pancuronium bromide in mechanically ventilated newborn infants. _J Pediatr_ 104:
614–617 CAS PubMed Google Scholar * Miller J, Law AB, Parker RA, Sundell H, Silberberg AR, Cotton RB 1994 Effects of morphine and pancuronium on lung volume and oxygenation in premature
infants with hyaline membrane disease. _J Pediatr_ 125: 97–103 CAS PubMed Google Scholar * Fuhrman BP, Paczan PR, De Francisis M 1991 Perfluorocarbon-associated gas exchange. _Crit Care
Med_ 19: 712–722 CAS PubMed Google Scholar * Tarczy-Hornoch P, Hildebrandt J, Standaert TA, Lamm W, Jackson JC 1998 Surfactant replacement increases compliance in premature lamb lungs
during partial liquid ventilation _in situ_. _J Appl Physiol_ 84: 1316–1322 CAS PubMed Google Scholar * Leach CL, Holm B, Morin FC III, Fuhrman BP, Papo MC, Steinhorn D, Hernan LJ 1995
Partial liquid ventilation in premature lambs with respiratory distress syndrome: efficacy and compatibility with exogenous surfactant. _J Pediatr_ 126: 412–420 CAS PubMed Google Scholar
* Leach C, Fuhrman B, Morin F III, Raht M 1993 Perfluorocarbon-associated gas exchange (partial liquid ventilation) in respiratory distress syndrome: a prospective, randomized, controlled
study. _Crit Care Med_ 21: 1270–1278 CAS PubMed Google Scholar * Doctor A, Ibla J, Grenier BM, Thompson JE, Lillehei CW, Arnold JH 1998 Pulmonary blood flow distribution during partial
liquid ventilation. _J Appl Physiol_ 84: 1540–1550 CAS PubMed Google Scholar * Gauger PG, Overbeck MC, Koeppe RA, Shulkin BL, Hrycko JN, Weber ED, Hirschl RB 1997 Distribution of
pulmonary blood flow and total lung water during partial liquid ventilation in acute lung injury. _Surgery_ 122: 313–332 CAS PubMed Google Scholar * Foust R III, Tran NN, Cox C, Miller
TF, Greenspan JS, Wolfson MR, Shaffer TH 1996 Liquid assisted ventilation: an alternative ventilatory strategy for acute meconium aspiration injury. _Pediatr Pulmonol_ 21: 316–322 PubMed
Google Scholar * Shaffer TH, Lowe CA, Bhutani VK, Douglas PR 1984 Liquid ventilation: effects on pulmonary function in distressed meconium-stained lambs. _Pediatr Res_ 18: 48–52 Google
Scholar * Tütüncü AS, Faithfull NS, Lachmann B 1993 Intratracheal perfluorocarbon administration combined with artificial ventilation in experimental respiratory distress syndrome:
dose-dependent improvement of gas exchange. _Crit Care Med_ 21: 962–969 PubMed Google Scholar * Tütüncü A, Faithful N, Lachmann B 1993 Comparison of ventilatory support with intratracheal
perfluorocarbon administration and conventional mechanical ventilation in animals with acute respiratory failure. _Am Rev Respir Dis_ 148: 785–792 PubMed Google Scholar * Hirschl RB,
Overbeck MC, Parent A, Hernandez R, Schwartz S, Dosanjh A, Johnson K, Bartlett RH 1994 Liquid ventilation provides uniform distribution of perfluorocarbon in the setting of respiratory
failure. _Surgery_ 116: 159–168 CAS PubMed Google Scholar * Curtis SE, Peek JT, Kelly DR 1993 Partial liquid breathing with perflubron improves arterial oxygenation in acute canine lung
injury. _J Appl Physiol_ 75: 2696–2702 CAS PubMed Google Scholar * Hirschl RB, Tooley R, Parent AC, Johnson K, Bartlett RH 1995 Improvement of gas exchange, pulmonary function and lung
injury with partial liquid ventilation: a study model in a setting of severe respiratory failure. _Chest_ 108: 500–508 CAS PubMed Google Scholar * Nesti FD, Fuhrman BP, Steinhorn DM, Papo
MC, Hernan LJ, Duffy LC, Fisher JE, Leach CL, Paczan PR, Burak BA 1994 Perfluorocarbon-associated gas exchange in gastric aspiration. _Crit Care Med_ 22: 1445–1452 CAS PubMed Google
Scholar * Bendel-Stenzel EM, Mrozek JD, Bing D, Meyers PA, Connett JE, Mammel MC 1998 Dynamics of spontaneous breathing during patient-triggered partial liquid ventilation. _Pediatr
Pulmonol_ 26: 319–325 CAS PubMed Google Scholar * Schaller P, Schulze A 1991 A ventilator generating a positive or negative internal compliance. _Upsala J Med Sci_ 96: 219–234 CAS PubMed
Google Scholar * Younes M 1992 Proportional assist ventilation, a new approach to ventilatory support: theory. _Am Rev Respir Dis_ 145: 114–120 CAS PubMed Google Scholar * Younes M,
Puddy A, Roberts D, Ligut RB, Quesada A, Taylor K, Oppenheimer L, Cramp H 1992 Proportional assist ventilation: results of an initial clinical trial. _Am Rev Respir Dis_ 145: 121–129 CAS
PubMed Google Scholar * Schulze A, Schaller P, Gehrhardt B, Mädler HJ, Gmyrek D 1990 An infant ventilator technique for resistive unloading during spontaneous breathing: results in a
rabbit model of airway obstruction. _Pediatr Res_ 28: 79–82 CAS PubMed Google Scholar * Schulze A, Schaller P, Jonzon A, Sedin G 1993 Assisted mechanical ventilation using elastic
unloading: a study in cats with normal and injured lungs. _Pediatr Res_ 34: 600–605 CAS PubMed Google Scholar * Schulze A, Schaller P, Töpfer A, Kirpalani H 1993 Resistive and elastic
unloading to assist spontaneous breathing does not change functional residual capacity. _Pediatr Pulmonol_ 16: 170–176 CAS PubMed Google Scholar * Coates A, Stocks J, Gerhardt T 1996
Esophageal manometry. In: Stocks J, Sly PD, Tepper RS, Morgan WJ (eds) _Infant Respiratory Function Testing_. Wiley-Liss, New York pp 241–258 Google Scholar * Wall MA 1980 Infant
endotracheal tube resistance: effects of changing length, diameter, and gas density. _Crit Care Med_ 8: 38–40 CAS PubMed Google Scholar * Neto GS, Gerhardt T, Silberberg A, Claure N,
Duara S, Bancalari E 1992 Nonlinear pressure/volume relationship and measurements of lung mechanics in infants. _Pediatr Pulmonol_ 12: 146–152 Google Scholar * Hernan LJ, Fuhrman BP, Papo
MC, Steinhorn DM, Leach CL, Salman N, Paczan PR, Kahn B 1995 Cardiorespiratory effects of perfluorocarbon-associated gas exchange at reduced oxygen concentrations. _Crit Care Med_ 23:
553–559 CAS PubMed Google Scholar * Hirschl RB, Grover B, McCracken M, Wolfson MR, Shaffer TH, Bartlett RH 1993 Oxygen consumption and carbon dioxide production during liquid ventilation.
_J Pediatr Surg_ 28: 513–519 CAS PubMed Google Scholar * Mates EA, Jackson JC, Hildebrandt J, Truog WE, Standaert TA, Hlastala MP 1994 Respiratory gas exchange and inert gas retention
during partial liquid ventilation. In: Hogan MC, Mathieu-Costello O, Pode DC, Wagner PD (eds). Oxygen Transport to Tissue XVI, Plenum Press, New York, pp 427–435 Google Scholar * Mates EA,
Hildebrandt J, Jackson JC, Tarczy-Hornoch P, Hlastala MP 1997 Shunt and ventilation-perfusion distribution during partial liquid ventilation in healthy piglets. _J Appl Physiol_ 82: 933–942
CAS PubMed Google Scholar * Mates EA, Tarczy-Hornoch P, Hildebrandt J, Jackson JC, Hlastala MP 1996 Negative slope of exhaled CO2 profile: implications for ventilation heterogeneity
during partial liquid ventilation. _Adv Exp Med Biol_ 388: 585–597 CAS PubMed Google Scholar * Tütüncü AS, Houmes RJM, Bos JAH, Wollmer P, Lachmann B 1996 Evaluation of lung function
after intratracheal perfluorocarbon administration in healthy animals. _Crit Care Med_ 24: 274–279 PubMed Google Scholar * Houmes RJM, Verbrugge SJC, Hendrik ER, Lachmann B 1995
Hemodynamic effects of partial liquid ventilation with perfluorocarbon in acute lung injury. _Intensive Care Med_ 21: 966–972 CAS PubMed Google Scholar * Shaffer TH, Moskowitz GD 1974
Demand-controlled liquid ventilation of the lungs. _J Appl Physiol_ 36: 208–213 CAS PubMed Google Scholar * Bendel-Stenzel EM, Bing DR, Meyers PA, Connett JE, Mammel MC 1999 Synchronized
gas and partial liquid ventilation in lung-injured animals: improved gas exchange with decreased effort. _Pediatr Pulmonol_ 27: 242–250 CAS PubMed Google Scholar * Wolfson MR, Greenspan
JS, Shaffer TH 1996 Pulmonary administration of vasoactive substances by perfluorochemical ventilation. _Pediatrics_ 97: 449–455 CAS PubMed Google Scholar * Zelinka MA, Wolfson MR,
Calligaro I, Rubenstein SD, Greenspan JS, Shaffer TH 1997 A comparison of intratracheal and intravenous administration of gentamicin during liquid ventilation. _Eur J Pediatr_ 156: 401–404
CAS PubMed Google Scholar * Lisby DA, Ballard PL, Fox WW, Wolfson MR, Shaffer TH, Gonzales LW 1997 Enhanced distribution of adenovirus-mediated gene transfer to lung parenchyma by
perfluorochemical liquid. _Hum Gene Ther_ 8: 919–928 CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Prof. B. Jilge for generously supporting this study
and Dr. B. Kuhnt for her expert technical assistance. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Neonatology and Pediatric Critical Care, Children's Hospital, University
of Ulm, Ulm, 89070 Helmut D Hummler, Frank Pohlandt & Ulrich Thome * Division of Neonatology, Women's Hospital Grosshadern, Ludwig-Maximilians-University, Munich, 81377, Germany
Andreas Schulze Authors * Helmut D Hummler View author publications You can also search for this author inPubMed Google Scholar * Andreas Schulze View author publications You can also search
for this author inPubMed Google Scholar * Frank Pohlandt View author publications You can also search for this author inPubMed Google Scholar * Ulrich Thome View author publications You can
also search for this author inPubMed Google Scholar ADDITIONAL INFORMATION Supported by a grant from: Deutsche Forschungsgemeinschaft (DFG HU 793/1–1). Presented at the Annual Meeting of
the European Society of Pediatric Research, Sept. 13–17, 1998, Belfast, Northern Ireland. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hummler, H.,
Schulze, A., Pohlandt, F. _et al._ Dynamics of Breathing during Partial Liquid Ventilation in Spontaneously Breathing Rabbits Supported by Elastic and Resistive Unloading. _Pediatr Res_ 47,
392–397 (2000). https://doi.org/10.1203/00006450-200003000-00018 Download citation * Received: 18 February 1999 * Accepted: 29 October 1999 * Issue Date: 01 March 2000 * DOI:
https://doi.org/10.1203/00006450-200003000-00018 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative