Play all audios:
ABSTRACT Perinatal asphyxia is a major cause of immediate and postponed brain injury in the newborn. We hypothesized that resuscitation with 100% O2 compared with ambient air is detrimental
to the cerebral tissue. We assessed cerebral injury in newborn piglets that underwent global hypoxia and subsequent resuscitation with 21 or 100% O2 by extracellular glycerol, matrix
metalloproteinase (MMP) expression levels, and oxidative stress. Extracellular glycerol was sampled by cerebral microdialysis. MMP levels were analyzed in cerebral tissue by gelatin
zymography, broad matrix degrading capacity, and real-time PCR. Total endogenous antioxidant capacity was measured by the oxygen radical absorbance capacity assay. Extracellular glycerol
increased 50% after resuscitation with 100% O2 compared with 21% O2. Total MMP activity was doubled in resuscitated animals at endpoint compared with baseline (_p_ = 0.018), and the MMP-2
activity was significantly increased in piglets that were resuscitated with 21% O2 (_p_ = 0.003) and 100% O2 (_p_ = 0.001) compared with baseline. MMP-2 mRNA level was 100% increased in
piglets that were resuscitated with 100% O2 as compared with 21% O2 (_p_ < 0.05). Oxygen radical absorbance capacity values in piglets that were resuscitated with 100% O2 were
considerably reduced compared with both baseline (_p_ = 0.001) and piglets that were resuscitated with 21% O2 (_p_ = 0.001). In conclusion, our data show increased MMP-2 activity at both
gene and protein levels, accompanied with cerebral leakage of glycerol, presumably triggered by augmented oxidative stress. Our findings suggest that resuscitation of asphyxiated piglets
with 100% O2 is detrimental to the piglet brain compared with resuscitation with 21% O2. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFECT OF DIMETHYL FUMARATE ON MITOCHONDRIAL METABOLISM IN A
PEDIATRIC PORCINE MODEL OF ASPHYXIA-INDUCED IN-HOSPITAL CARDIAC ARREST Article Open access 15 June 2024 HYDROGEN GAS INHALATION AMELIORATES GLOMERULAR ENLARGEMENT AFTER HYPOXIC-ISCHEMIC
INSULT IN ASPHYXIATED PIGLET MODEL Article Open access 11 January 2025 BIO-PHYSIOLOGICAL SUSCEPTIBILITY OF THE BRAIN, HEART, AND LUNGS TO SYSTEMIC ISCHEMIA REPERFUSION AND HYPEROXIA-INDUCED
INJURY IN POST-CARDIAC ARREST RATS Article Open access 28 February 2023 MAIN Traditionally, asphyxiated newborn infants have been resuscitated using 100% oxygen in the delivery room (1).
However, clinical trials have shown that room air is as efficient as pure oxygen in securing the survival of asphyxiated newborn infants (2–5). Therefore there is an ongoing debate whether
to use ambient air or 100% O2 in neonatal resuscitation (6–9). Hyperoxia followed by reoxygenation is thought to increase the production of reactive oxygen species and disrupt the
antioxidant mechanisms (10). Elevated oxidative stress can directly influence the cell cycle and additionally alter a number of significant cell functions, such as signal transduction, DNA
and RNA synthesis, protein synthesis, and enzyme activation (11,12). Furthermore, hypoxic-ischemic injury and reactive oxygen species are found to trigger inflammation in the immature brain
(13). Interstitial glycerol is a sensitive and reliable marker of cell damage in experimental cerebral ischemia (14–16). Degradation of membrane phospholipids is a well-known phenomenon in
acute brain injuries and is thought to underlie the disturbance of vital cellular membrane functions. Matrix metalloproteinases (MMPs) are a family of >20 zinc- and calcium-dependent
endopeptidases that are involved in the remodeling of the extracellular matrix (ECM) in a variety of physiologic and pathologic conditions (17). Most MMPs are secreted as proenzymes and are
activated by cleavage of their propeptide to lower molecular weight active forms (18). MMPs can degrade many components of the ECM, and enzyme activities are strictly regulated (19). Tissue
inhibitors of MMP and interactions with surrounding ECM molecules tightly regulate proenzyme activation and enzyme activities. Uncontrolled expression of MMPs can result in nervous tissue
injury and inflammation (20,21). Several studies have documented the up-regulation of MMPs and their activation in animal models of focal and global cerebral ischemia (22–27) and in humans
(28). In the brain, MMP-2 and MMP-9 are synthesized primarily by reactive astrocytes and microglia (27,29). MMP-2 production and secretion are enhanced after prolonged hypoxia in cell
culture, whereas reoxygenation further increases the level of MMP-2 (30). MMP-2 and -9 have been shown to degrade components of the cerebral basal lamina after ischemia (24), causing a
disruption of the blood-brain barrier (31). In the pathologic condition of ischemia/reperfusion, digestion of the endothelial basal lamina is reported to occur as early as 2 h after ischemia
(26). In term newborn brain, cerebral oxygen demands are increased in areas of active neural development such as the basal ganglia and thalamus (32). Corpus striatum becomes selectively
vulnerable in the hypoxic term piglet model. In a piglet model of hypoxic-ischemic brain injury in newborns, striatal neuron necrosis evolves rapidly (33). We have investigated changes in
corpus striatum in a global hypoxia piglet model, imitating birth asphyxia, as in several of our earlier studies (34,35). Especially the cerebral ischemia after a hypoxia-induced myocardial
failure is most harmful (36) because permitting the mean arterial blood pressure (MABP) to fall to 15 mm Hg also induces cerebral perfusion disturbances. The main aim of this study was to
explore whether resuscitation with 100% O2 compared with ambient air increases the acute cerebral damage after global hypoxia in a piglet model. Birth asphyxia is often associated with
hypercapnia, and Pco2 influences cerebral blood flow (37,38). Therefore, we also investigated effects of different Pco2 levels. METHODS APPROVAL. The Norwegian Council for Animal Research
approved the experimental protocol. The animals were cared for and handled in accordance with the European Guidelines for Use of Experimental Animals. SURGICAL PREPARATION AND ANESTHESIA
Sixty-nine Landrace piglets (12–36 h old) were transported (1 h) from a local farmer on the day of the experiment. Anesthesia was induced by Halothane 4% (Fluthane ZENECA); an ear vein was
cannulated, halothane was disconnected, and the piglets were given pentobarbital sodium 20 mg/kg and Fentanyl 50 μg/kg intravenously as a bolus injection. Anesthesia was maintained by a
continuous infusion of Fentanyl (50 μg · kg−1 · h−1) and Midazolam (0.25 mg · kg−1 · h−1; IVAC P2000 infusion pump). When necessary, a bolus of Fentanyl (10 μg) or Midazolam (1 mg) was
added. A continuous i.v. infusion (saline 0.7% and glucose 1.25%, 20 mL · kg−1 · h−1) was given throughout the experiment. Tracheotomy was performed, and a pressure-controlled ventilator
(Babylog 8000+; Drägerwerk, Lübeck, Germany) ventilated the piglets at a rate of 30 breaths/min. Normoventilation [arterial carbon dioxide tension (Paco2) 4.5–6.0 kPa] and a tidal volume
10–15 mL/kg were achieved by adjusting the peak inspiratory pressure or ventilatory rate. During surgery, stabilization, and hypoxia, ventilatory rate was 30–40 respirations/min. Inspiratory
time of 0.4 s and positive end-expiratory pressure of 4 cm H2O were kept constant throughout the experiment. Inspired fraction of O2 and end-tidal CO2 were monitored continuously (Datex
Normocap Oxy; Datex, Helsinki, Finland). The right femoral artery was cannulated with polyethylene catheters (Portex PE-50, inner diameter 0.58 mm; Portex Ltd Hythe, Kent, UK). Rectal
temperature was maintained between 38 and 40°C with a heating blanket and a radiant heating lamp. One hour of stabilization was allowed after surgery. At the end of the experiment, the
piglets were given an overdose of 150 mg/kg pentobarbital intravenously. EXPERIMENTAL PROTOCOL. Hypoxemia was achieved by ventilation with a gas mixture of 8% O2 in N2 (AGA, Oslo, Norway),
until either MABP reached 15 mm Hg or base excess reached −20 mM. For imitating birth asphyxia, CO2 was added during hypoxemia aiming at a Paco2 of 8.0–9.5 kPa. Before start of
resuscitation, the piglets were block randomized into six different groups by drawing lots. Resuscitation was performed with either ambient air (group A) or 100% O2 (group B). Nine piglets,
referred to as baseline piglets, were controls that went through surgery and 1 h of stabilization. We divided each main group that was resuscitated with 100% (A) or 21% (B) oxygen in three
subgroups ventilated with low (A1 and B 1), normal (A2 and B2), or high (A3 and B3) CO2 level during resuscitation. The piglets in group 1 (A1, _n_ = 10; B1, _n_ = 10) were hyperventilated
and had a low Paco2 of 2.0–3.5 kPa. In group 2 (A2, _n_ = 10; B2, _n_ = 10), the piglets were resuscitated in a normal ventilatory modus (Paco2: 4.5–6.0 kPa). The animals in group 3 (A3, _n_
= 10; B3, _n_ = 10) were normoventilated and had added CO2 to reflect a condition of hypercapnia (Paco2: 8.0–9.5 kPa). For hyperventilating the piglets (group 1), peak inspiratory pressure
and ventilatory rate were elevated and adjusted after evaluating end-tidal CO2 and blood gases. We resuscitated with ambient air or pure oxygen for 30 min in accordance with our previous
studies. After resuscitation, the piglets were reoxygenated for 150 min by 21% O2 and normal CO2 (4.5–6.0 kPa), which was the same for all groups. Hb with normal values was measured at
baseline and after surgery (7.7 ± 0.18 g/dL; CO-OXIMETER 270; Instrumentation Laboratory, Lexington, MA). Temperature-corrected acid/base status and blood sugar were measured regularly with
a Blood Gas Analyzer 860 (Ciba Corning Diagnostics, Midfield, MA), and the i.v. infusion was occasionally adjusted to keep s-glucose in the decided range (2–10 mM). The head was fixed in a
stereotactic frame (David Kopf Instruments, Tujunga, CA). The skull was exposed, and a 2.0-mm hole was drilled through the skull 5.5 mm laterally and 10 mm anteriorly to the bregma in the
right hemisphere. The dura was carefully pierced, and a microdialysis probe (CMA 10 20/04, outer diameter 500 μm, cut-off 20.000 D) was inserted stereotactically through the hole into the
corpus striatum area (19–21 mm from the surface of the skull, depending on body weight). All CMA equipment was purchased from CMA/Microdialysis AB (Stockholm, Sweden). Microdialysis probes
sampled extracellular glycerol throughout the experiment, and this was analyzed immediately (35). Because of contamination of the vials, microdialysis results were excluded in two animals
(one in A2 and one in B1 resulting in _n_ = 29 in both main groups). At the end of the experiment, the piglets were given an i.v. overdose of 150 mg/kg pentobarbital. The brains were removed
immediately; corpus striatum was snap-frozen in liquid nitrogen and stored at −70°C. PREPARATION OF CEREBRAL TISSUE EXTRACTS. For zymography and total MMP activity, tissue from corpus
striatum from the left hemisphere was pulverized under liquid nitrogen, and proteins were extracted using ice-cold lysis buffer [Tris-HCl (pH 7.5) containing protease inhibitor cocktail
without EDTA with 1% NP-40] at a ratio of 500 μL/50 mg wet weight tissue. Extracts were incubated on ice for 15 min and then centrifuged at 12,000 × _g_ for 15 min at 4°C. The supernatants
were retained, and protein concentration of the samples was measured by the BCA method (Pierce, Cheshire, UK). For the oxygen radical absorbance capacity (ORAC) assay, cerebral tissue was
pulverized under liquid nitrogen, and 50 mg of frozen material was added to 1.0 mL of ice-cold 75 mM of K2HPO4/NaH2PO4 phosphate buffer (pH 7.0). The tissue samples were homogenized using a
Polytron (PT 1200; Kinematica AG, Lucerne, Switzerland) at speed 1 for 30 s under nitrogen flush and centrifuged at 16,000 × _g_ for 5 min at 4°C (Biofuge A; Hereaeus-Christ, Hanau,
Germany). The pellet was resuspended in homogenization buffer and centrifuged as described above. The two supernatants were combined in a volumetric flask, filled up to 2.0 mL of total
volume, and used for the ORAC assay. TOTAL MMP ACTIVITY. Total MMP activity in tissue extracts was measured using a fluorogenic peptide substrate (cat. no. ES001; R&D Systems) according
to the protocol recommended by the manufacturer. The substrate can be cleaved by MMP-1, -2, -7, -8, -9, -12, and -13. The term “total MMP activity” refers to the total activity of these
enzymes and is measured by relative fluorogenic unit (RFU). Because of the limited amount of tissue, data from three animals were not obtained (two in group A and one in group B; A: _n_ =
28; B: _n_ = 29). GELATIN ZYMOGRAPHY. Equal amounts (10 μg) of cerebral tissue protein extracts were loaded onto the electrophoretic gel and assayed for gelatinase activity using 10%
SDS-polyacrylamide gel (containing 0.1% gelatin), with minor modifications according to the methods described elsewhere (39). Human MMP-2 and MMP-9 standards (CC073; Chemicon, Temecula, CA)
were used. The gels were stained with Coomassie blue R-250 and subsequently destained before being scanned in an imager (Kodak Image Station 440CF). The images were analyzed using software
from Total Lab v2.01. The results were calculated by densitometry, normalized to a sample used as an internal standard on every zymography run. When the gelatin gel was incubated with 200 μM
of EDTA, no lysis zones were detected, demonstrating that the metal-dependent lysis zones were most likely the result of gelatinase activity (40). Cerebral MMP-9 expression could not be
detected by gelatin zymography. Because of tissue limitations, three animals were excluded (one at baseline, one in A2, and one in B2). REAL-TIME QUANTITATIVE REVERSE TRANSCRIPTASE-PCR.
Total RNA was extracted from the tissue samples as described by the manufacturer, using Trizol (Invitrogen). The isolated RNA was treated with DNase (cat. No. M6101; Promega). Reverse
transcription was performed according to the manufacturer's protocol (cat. no. N808-0234; Applied Biosystems) with 125 ng of RNA per 50 μL of reverse transcriptase reaction.
Sequence-specific PCR primers and TaqMan probes for MMP-2 were designed using the Primer Express software version 1.5 (Applied Biosystems). Real-time quantitative reverse transcriptase-PCR
assays for pig MMP-2 (forward primer: 5′-GTG GTG CGT GTG AAG TAT GG-3′; reverse primer: 5′-GCC ATC CTT GTC GAA GTT GT-3′; TaqMan probe: FAM 5′-AGC TGT TAT ACT CCT TGC CGT T-TAMRA-3′) were
designed using the Primer Express software version 1.5 (Applied Biosystems). The housekeeping gene 18S (Applied Biosystems) was included as an endogenous normalization control to adjust for
unequal amounts of RNA. Quantification of mRNA was performed using the ABI Prism 7700 (Applied Biosystems). Each sample (each reaction: 2.5 μL of cDNA, total volume 25 μL) was run in
triplicate. Cycling parameters were 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Standard curves were run on the same plate, and the relative standard curve
method was used to calculate the relative gene expression (Livak KJ; ABI Prism 7700 Sequence Detection System, User Bulletin 2, PE Applied Systems). The predeveloped 18S assay from Applied
Biosystems is designed for rodent and human. The probe therefore was tested by running a standard curve to verify that the predeveloped 18S assay was suitable for pig. The product was also
run on a 1% agarose gel to make sure that there were no nonspecific products present. ORAC ASSAY. The ORAC assay was performed as described elsewhere (41) but with minor modifications.
Measurements were performed on a Wallac 1420 Victor2 96-well plate reader (Wallac Oy, Turku, Finland) with fluorescence filter. Excitation wavelength was 540 nm, and emission wavelength was
570 nm. The fluorescence probe was β-phycoerythrin (β-PE), and peroxyl radical generator was 2,2′-azobis (2-amidinopropane) dihydrochloride. 6-Hydroxy-2,5,7,8-tetramethyl-2-carboxylic acid
(Trolox), a water-soluble analogue of vitamin E, was used as control standard. All reagents and samples were diluted with phosphate buffer (75 mM, pH 7.0), and the reaction was conducted at
37°C. Each sample was analyzed in duplicate at four concentrations using a “forward-then-reverse” ordering in each plate row. Trolox and blanks were applied at both ends of each row in the
96-well plate to improve reproducibility of the fluorescence measurements. Total volume in each well was 0.2 mL. The plate was preheated to 37°C, and to each well, 10 μL of tissue extracts,
Trolox (20 μM, final concentration 1 μM) or blank (phosphate buffer), and 180 μL of β-PE (3.78 mg/L) were added. The plate was shaken for 30 s (speed 3.3 mm orbital), and initial
fluorescence was measured. The reaction was started by adding 10 μL of 2,2′-azobis (2-amidinopropane) dihydrochloride (80 mM) to each well and shaking the plate for 10 s. Measurements were
taken every 3 min until the fluorescence was <5% of the initial reading. All fluorescent measurements were expressed relative to the initial reading. Results were calculated by using the
area differences under the β-PE decay curves between the blank and a sample. The final results (ORAC value) were calculated by linear regression of areas _versus_ sample concentration and
expressed as micromol Trolox equivalents (TE) per gram of cerebral tissue (μmol TE/g). ORAC was measured in the normoventilated piglets A2 and B2 only. Because of loss of tissue, four
piglets were excluded (two at baseline, one in group A2, and one in group B2). STATISTICS. Statistical analysis was performed by SPSS11. For studying the relationship between glycerol, MMP-2
and total MMP-activity as dependent variables, and O2 and CO2 as independent variables, univariate ANOVA was used. For accommodating for multiple comparisons, the _p_ values were adjusted
according to Bonferroni. Comparing within two groups, independent _t_ test was used. All values are given as mean ± SEM. A level of _p_ < 0.05 was considered statistically significant.
RESULTS PIGLETS. There were no significant differences between the groups with respect to the number of animals, body weight, age, sex, rectal temperature, cardiovascular, or biochemical
variables at baseline. Furthermore, hypoxemia time, MABP, base excess, and pH were similar between groups at baseline, at the end of the insult, and at the end of the experiment (Tables 1
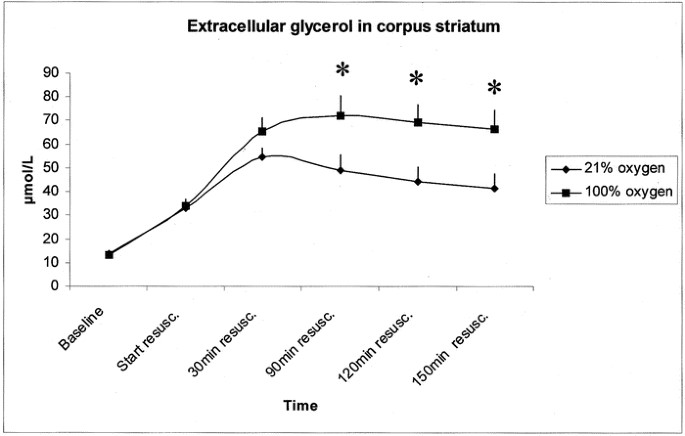
and 2). Extracellular glycerol and MMP-2 activity was not influenced by the Pco2 level; hence, the subgroups were combined. MICRODIALYSIS. Extracellular glycerol concentration in the piglets
that were resuscitated with ambient air (group A, _n_ = 29) and 100% O2 (group B, _n_ = 29) are shown in Figure 1. The values were 50% higher when resuscitation was done with 100% O2
compared with ambient air at 90, 120, and 150 min after resuscitation (_p_ < 0.05, independent sample _t_ test at each time point). TOTAL MMP ACTIVITY. Broad matrix degrading capacity was
examined to study whether any differences in total MMP activity could be seen between the normal controls at baseline (_n_ = 9) and the piglets at the end of the experiment [_n_ = 28 (A)
and _n_ = 29 (B)]. Total MMP activity was 2-fold higher in resuscitated animals compared with controls (10169 ± 815 _versus_ 5790 ± 1412 RFU; _p_ = 0.018). GELATINOLYTIC ACTIVITY. Gelatin
zymography was applied on cerebral extracts to determine the gelatinolytic activity of MMP-2 (Fig. 2). Gelatinolytic activity was detected at 68 kD, corresponding to MMP-2 proenzyme (42).
MMP-2 activity was significantly higher in resuscitated piglets (_n_ = 58) compared with baseline (_n_ = 8). MMP-2 activity was also significantly higher in piglets that were resuscitated
with 100% O2 (_n_ = 29) compared with ambient air (_n_ = 29, _p_ = 0.001). MRNA EXPRESSION OF MMP-2. MMP-2 activity was not influenced by the Pco2 level (_p_ = 0.4; Fig. 3); hence, the mRNA
and ORAC were analyzed in the normoventilated groups A2 and B2. To elucidate further the mechanism behind the augmented MMP activity, we measured the mRNA expression of MMP-2 by quantitative
real-time PCR. As shown in Figure 4, mRNA expression of MMP-2 was higher in the normoventilated group that was resuscitated with 100% O2 (_n_ = 9) than in the normoventilated group that was
resuscitated with ambient air (_n_ = 10, _p_ < 0.05). ORAC Total antioxidant capacity, denoted as ORAC values, was measured in cerebral tissue extracts from corpus striatum at baseline
and in the normoventilated (A2 and B2) piglets (Fig. 5). ORAC was considerably lowered in the piglets that were resuscitated with 100% O2 (_n_ = 9) compared with baseline (_p_ = 0.001),
indicating that total antioxidant capacity was lowered during the experiment. By contrast, ORAC response was not decreased in piglets that were resuscitated with ambient air (_n_ = 9)
compared with baseline (_n_ = 7). Piglets that were resuscitated with 100% O2 had mean ORAC values that were 50% lower than the ones that were resuscitated with 21% O2 (_p_ = 0.001).
DISCUSSION The findings of the current study indicate that resuscitation of piglets with 100% O2 after global hypoxia significantly increases cerebral extracellular glycerol and MMP-2
levels. These changes were concomitant with a marked reduction of total endogenous antioxidant capacity assessed by ORAC. Furthermore, cerebral leakage of glycerol reflecting brain damage
increased 50% more after resuscitation with 100% O2 compared with resuscitation with ambient air. The glycerol data obtained at 90, 120, and 150 min after onset of resuscitation indicate
that resuscitation with 100% O2 adds further cerebral damage after global hypoxia. To our knowledge, it has not been previously reported that MMP-2 activity and MMP-2 mRNA in newborn animals
are more up-regulated after resuscitation with 100% O2 than with ambient air. In adult animals, abnormal MMP activity has been implicated in cerebral ischemia (43). MMP-2 and MMP-9
expression levels were significantly increased soon after the ischemic onset (23,24,44). MMP expression after hypoxia might contribute to debris digestion, edema formation, blood-brain
barrier breakdown (22,23), and hemorrhagic transformation (25), although some beneficial contribution of MMPs in neuronal plasticity and tissue regeneration cannot be ruled out (45,46). The
applied ORAC assay provides significant information regarding the antioxidant capacity of various biologic tissue samples (41,47,48). We chose this assay because it directly measures a broad
spectrum of different types of antioxidant activities over a given time span, representing a relevant _in vivo_ situation (41). In normoventilated piglets that were resuscitated with
ambient air, we did not observe a significant change of the ORAC. In contrast, a marked reduction (50%) of ORAC was found in piglets that were resuscitated with 100% O2 compared with the
ones that were resuscitated with ambient air and baseline. This indicates that these piglets have less total antioxidant capacity left in cerebral tissue. Free radicals have previously been
reported to induce gene expression of several MMPs (49). That MMP-2 mRNA level was significantly higher in piglets that were resuscitated with 100% O2 compared with 21% O2 suggests that
higher levels of free radicals not only could be responsible for posttranslational modifications of MMPs but also could affect their transcriptional regulation. It is interesting that these
changes occur early after reoxygenation, placing MMPs upstream in the pathologic cascade of events that may lead to more conspicuous brain damage across time. In this context, further
studies should be undertaken to investigate the pathologic and histologic long-term consequences for the brain after resuscitation with 100% O2. In our experimental protocol, we did mRNA
expression profiling of MMP-2 3 h after hypoxia. Because of our experimental protocol, we unfortunately could not determine the exact time course of MMP-2 mRNA expression during the
experiment. However, our ORAC analysis after 3 h revealed that an oxidative stress had occurred during 3 h after hypoxia, causing a significant drop in endogenous antioxidant capacity. This
drop was more significant in the 100% group than in the ambient air group. The generation of reactive free radicals is an acute event (30–60 min after hypoxia) (50). Furthermore, we know
that activation of different genes, including MMP-2, can be driven by free radicals (51), and it is most likely that a maximum mRNA expression occurs later than 60 min as a result of a gene
transcription and processing of mRNA. It could be claimed that the peak MMP-2 transcript level might be reached between 2 and 3 h after hypoxia or even later (_e.g._ 6–12 h) and that an
underestimation of mRNA expression especially in the ambient air group had occurred 3 h after hypoxia. However, our data do not support this. First, our protein expression study of MMP-2 3 h
after hypoxia demonstrates a significantly increased MMP-2 level in the 100% oxygen group, compared with the ambient group. Second, significantly higher glycerol level in corpus striatum
was found in the 100% group, compared with the ambient air group. These data suggest that more brain damage has occurred in the piglets that were resuscitated with 100% O2, which at least
partly could be due to augmented MMP-2, and we suggest that the elevated generation of free radicals is the driving force in these adverse processes observed in the 100% oxygen group in
comparison with the ambient group. With our outcome variables, there were no significant differences comparing different Pco2 levels during resuscitation. This is in contrast to a study that
showed that different CO2 levels influence cerebral outcome after hypoxia-ischemia (52). In a different model, piglets that were younger than 4 d had a low CO2 reactivity and did not fully
autoregulate in response to MABP changes during the first days of life. At 4 d of age, adult responses have developed in the piglets (53). This may explain the lack of significant
differences between the CO2 groups in our study based on piglets <36 h of age. One limitation of the study is that the piglets were observed for only 2.5 h after resuscitation. A 24- or
48-h follow-up of the piglets for histologic examination may have been valuable. To investigate neuronal damage in the most vulnerable areas of the piglet brain—basal ganglia and thalamus—it
would be necessary to do histologic analysis at least 8 h after resuscitation. However, histologic analysis in other cerebral regions of hypoxic piglets have not demonstrated significant
differences between 21 and 100% oxygen even 4 d after hypoxia (54). Despite this, our study indicates the importance of adjusting down the oxygen level during resuscitation after global
hy-poxia. In most clinical situations, infants are coupled to pulse oximeter, and oxygen may be lowered when saturation reaches levels that are too high. Thus, the current accepted
recommendation to use 100% oxygen in the resuscitation of asphyxiated newborn infants should be discussed and investigated further. In conclusion, the current study documented a good
correspondence between the increased levels of MMP-2 and glycerol, a marker of tissue injury, in a piglet model of global hypoxemia and subsequent resuscitation with ambient air or 100% O2.
Cerebral glycerol values were 50% increased and MMP-2 levels were considerably up-regulated in the piglets that were resuscitated with 100% O2 compared with ambient air. ABBREVIATIONS * ECM:
extracellular matrix * MABP: mean arterial blood pressure * MMP: matrix metalloproteinase * ORAC: oxygen radical absorbance capacity * Paco2: arterial carbon dioxide tension * PE:
phycoerythrin * RFU: relative fluorogenic unit REFERENCES * Saugstad OD 2004 Physiology of resuscitation. In: Polin RA, Fox WF, Abman SH (eds) _Fetal and Neonatal Physiology_. Saunders,
Philadelphia, pp 765–772 Chapter Google Scholar * Ramji S, Ahuja S, Thirupuram S, Rootwelt T, Rooth G, Saugstad OD 1993 Resuscitation of asphyxic newborn infants with room air or 100%
oxygen. _Pediatr Res_ 34: 809–812 Article CAS Google Scholar * Saugstad OD, Rootwelt T, Aalen O 1998 Resuscitation of asphyxiated newborn infants with room air or oxygen: an international
controlled trial: the Resair 2 study. _Pediatrics_ 102: e1 Article CAS Google Scholar * Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J 2001 Resuscitation with room air
instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. _Pediatrics_ 107: 642–647 Article CAS Google Scholar * Ramji S, Rasaily R, Mishra PK, Narang A,
Jayam S, Kapoor AN, Kambo I, Mathur A, Saxena BN 2003 Resuscitation of asphyxiated newborns with room air or 100% oxygen at birth: a multicentric clinical trial. _Indian Pediatr_ 40: 510–517
CAS PubMed Google Scholar * Levine CR, Davis JM 2001 Resuscitation with 100% oxygen: should we change our ways?. _Pediatr Res_ 50: 432 Article CAS Google Scholar * Lefkowitz W 2002
Oxygen and resuscitation: beyond the myth. _Pediatrics_ 109: 517–519 Article Google Scholar * Saugstad OD 2003 Oxygen toxicity at birth: the pieces are put together. _Pediatr Res_ 54: 789
Article Google Scholar * Niermeyer S, Vento M 2004 Is 100% oxygen necessary for the resuscitation of newborn infants?. _J Matern Fetal Neonatal Med_ 15: 75–84 Article CAS Google Scholar
* Kondo M, Itoh S, Isobe K, Kondo M, Kunikata T, Imai T, Onishi S 2000 Chemiluminescence because of the production of reactive oxygen species in the lungs of newborn piglets during
resuscitation periods after asphyxiation load. _Pediatr Res_ 47: 524–527 Article CAS Google Scholar * Suzuki YJ, Forman HJ, Sevanian A 1997 Oxidants as stimulators of signal transduction.
_Free Radic Biol Med_ 22: 269–285 Article CAS Google Scholar * Jankov RP, Negus A, Tanswell AK 2001 Antioxidants as therapy in the newborn: some words of caution. _Pediatr Res_ 50:
681–687 Article CAS Google Scholar * Saliba E, Henrot A 2001 Inflammatory mediators and neonatal brain damage. _Biol Neonate_ 79: 224–227 Article CAS Google Scholar * Marklund N, Salci
K, Lewen A, Hillered L 1997 Glycerol as a marker for post-traumatic membrane phospholipid degradation in rat brain. _Neuroreport_ 8: 1457–1461 Article CAS Google Scholar * Hillered L,
Valtysson J, Enblad P, Persson L 1998 Interstitial glycerol as a marker for membrane phospholipid degradation in the acutely injured human brain. _J Neurol Neurosurg Psychiatry_ 64: 486–491
Article CAS Google Scholar * Frykholm P, Hillered L, Langstrom B, Persson L, Valtysson J, Watanabe Y, Enblad P 2001 Increase of interstitial glycerol reflects the degree of ischaemic
brain damage: a PET and microdialysis study in a middle cerebral artery occlusion-reperfusion primate model. _J Neurol Neurosurg Psychiatry_ 71: 455–461 Article CAS Google Scholar *
Sternlicht MD, Werb Z 2001 How matrix metalloproteinases regulate cell behavior. _Annu Rev Cell Dev Biol_ 17: 463–516 Article CAS Google Scholar * Vu TH, Werb Z 2000 Matrix
metalloproteinases: effectors of development and normal physiology. _Genes Dev_ 14: 2123–2133 Article CAS Google Scholar * Visse R, Nagase H 2003 Matrix metalloproteinases and tissue
inhibitors of metalloproteinases: structure, function, and biochemistry. _Circ Res_ 92: 827–839 Article CAS Google Scholar * Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH 2001
Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. _J Cereb Blood Flow Metab_ 21: 1393–1400
Article CAS Google Scholar * Jourquin J, Tremblay E, Decanis N, Charton G, Hanessian S, Chollet AM, Le Diguardher T, Khrestchatisky M, Rivera S 2003 Neuronal activity-dependent increase
of net matrix metalloproteinase activity is associated with MMP-9 neurotoxicity after kainate. _Eur J Neurosci_ 18: 1507–1517 Article Google Scholar * Romanic AM, White RF, Arleth AJ,
Ohlstein EH, Barone FC 1998 Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. _Stroke_ 29:
1020–1030 Article CAS Google Scholar * Rosenberg GA, Estrada EY, Dencoff JE 1998 Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in
rat brain. _Stroke_ 29: 2189–2195 Article CAS Google Scholar * Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH 1999 Early appearance of activated matrix
metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. _J Cereb Blood Flow Metab_ 19: 1020–1028 Article CAS Google Scholar * Heo
JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ 1999 Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. _J Cereb Blood Flow Metab_ 19:
624–633 Article CAS Google Scholar * Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH 1999 Early appearance of activated matrix metalloproteinase-9 and blood-brain
barrier disruption in mice after focal cerebral ischemia and reperfusion. _Brain Res_ 842: 92–100 Article CAS Google Scholar * Rivera S, Ogier C, Jourquin J, Timsit S, Szklarczyk AW,
Miller K, Gearing AJ, Kaczmarek L, Khrestchatisky M 2002 Gelatinase B and TIMP-1 are regulated in a cell- and time-dependent manner in association with neuronal death and glial reactivity
after global forebrain ischemia. _Eur J Neurosci_ 15: 19–32 Article Google Scholar * Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR 1997 Increased gelatinase A (MMP-2) and
gelatinase B (MMP-9) activities in human brain after focal ischemia. _Neurosci Lett_ 238: 53–56 Article CAS Google Scholar * Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L 2002
Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. _J Neurosci_ 22: 920–930 Article CAS Google Scholar * Ben Yosef Y, Lahat
N, Shapiro S, Bitterman H, Miller A 2002 Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation. _Circ Res_ 90: 784–791 Article CAS Google Scholar * Kim GW, Gasche
Y, Grzeschik S, Copin JC, Maier CM, Chan PH 2003 Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: role of matrix metalloproteinase-9 in early
blood-brain barrier disruption?. _J Neurosci_ 23: 8733–8742 Article CAS Google Scholar * Ferriero DM 2001 Oxidant mechanisms in neonatal hypoxia-ischemia. _Dev Neurosci_ 23: 198–202
Article CAS Google Scholar * Martin LJ, Brambrink AM, Price AC, Kaiser A, Agnew DM, Ichord RN, Traystman RJ 2000 Neuronal death in newborn striatum after hypoxia-ischemia is necrosis and
evolves with oxidative stress. _Neurobiol Dis_ 7: 169–191 Article CAS Google Scholar * Feet BA, Brun NC, Hellstrom-Westas L, Svenningsen NW, Greisen G, Saugstad OD 1998 Early cerebral
metabolic and electrophysiological recovery during controlled hypoxemic resuscitation in piglets. _J Appl Physiol_ 84: 1208–1216 Article CAS Google Scholar * Froen JF, Munkeby BH,
Stray-Pedersen B, Saugstad OD 2002 Interleukin-10 reverses acute detrimental effects of endotoxin-induced inflammation on perinatal cerebral hypoxia-ischemia. _Brain Res_ 942: 87–94 Article
CAS Google Scholar * Williams CE, Mallard C, Tan W, Gluckman PD 1993 Pathophysiology of perinatal asphyxia. _Clin Perinatol_ 20: 305–325 Article CAS Google Scholar * Greisen G 1997
Cerebral blood flow and energy metabolism in the newborn. _Clin Perinatol_ 24: 531–546 Article CAS Google Scholar * Volpe JJ 2001 Hypoxic-ischemic encephalopathy: biochemical and
physiological aspects. In: Fletcher J, Hund R (eds) _Neurology of the Newborn_. W.B. Saunders Company, Philadelphia, pp 217–276 Google Scholar * Kleiner DE, Stetler-Stevenson WG 1994
Quantitative zymography: detection of picogram quantities of gelatinases. _Anal Biochem_ 218: 325–329 Article CAS Google Scholar * Loy M, Burggraf D, Martens KH, Liebetrau M, Wunderlich
N, Bultemeier G, Nemori R, Hamann GF 2002 A gelatin in situ-overlay technique localizes brain matrix metalloproteinase activity in experimental focal cerebral ischemia. _J Neurosci Methods_
116: 125–133 Article CAS Google Scholar * Cao G, Prior RL 1999 Measurement of oxygen radical absorbance capacity in biological samples. _Methods Enzymol_ 299: 50–62 Article CAS Google
Scholar * Southgate KM, Fisher M, Banning AP, Thurston VJ, Baker AH, Fabunmi RP, Groves PH, Davies M, Newby AC 1996 Upregulation of basement membrane-degrading metalloproteinase secretion
after balloon injury of pig carotid arteries. _Circ Res_ 79: 1177–1187 Article CAS Google Scholar * Planas AM, Sole S, Justicia C, Farre ER 2000 Estimation of gelatinase content in rat
brain: effect of focal ischemia. _Biochem Biophys Res Commun_ 278: 803–807 Article CAS Google Scholar * Mun-Bryce S, Rosenberg GA 1998 Gelatinase B modulates selective opening of the
blood-brain barrier during inflammation. _Am J Physiol_ 274: 203–211 Google Scholar * Rivera S, Khrestchatisky M 1999 Matrix metalloproteinases and tissue inhibitors of metalloproteinases
in neuronal plasticity and pathology. In: Baudry M, Davis JL, Thomsen C (eds) _Advances in Synaptic Plasticity_. The MIT Press, Cambridge, pp 53–86 Google Scholar * Yong VW, Power C,
Forsyth P, Edwards DR 2001 Metalloproteinases in biology and pathology of the nervous system. _Nat Rev Neurosci_ 2: 502–511 Article CAS Google Scholar * Cao G, Verdon CP, Wu AH, Wang H,
Prior RL 1995 Automated assay of oxygen radical absorbance capacity with the COBAS FARA II. _Clin Chem_ 41: 1738–1744 CAS PubMed Google Scholar * Cao G, Giovanoni M, Prior RL 1996
Antioxidant capacity in different tissues of young and old rats. _Proc Soc Exp Biol Med_ 211: 359–365 Article CAS Google Scholar * Kameda K, Matsunaga T, Abe N, Hanada H, Ishizaka H, Ono
H, Saitoh M, Fukui K, Fukuda I, Osanai T, Okumura K 2003 Correlation of oxidative stress with activity of matrix metalloproteinase in patients with coronary artery disease. Possible role for
left ventricular remodelling. _Eur Heart J_ 24: 2180–2185 Article CAS Google Scholar * Zweier JL, Kuppusamy P, Lutty GA 1988 Measurement of endothelial cell free radical generation:
evidence for a central mechanism of free radical injury in postischemic tissues. _Proc Natl Acad Sci USA_ 85: 4046–4050 Article CAS Google Scholar * Zhang HJ, Zhao W, Venkataraman S,
Robbins ME, Buettner GR, Kregel KC, Oberley LW 2002 Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves
reactive oxygen species. _J Biol Chem_ 277: 20919–20926 Article CAS Google Scholar * Vannucci RC, Towfighi J, Heitjan DF, Brucklacher RM 1995 Carbon dioxide protects the perinatal brain
from hypoxic-ischemic damage: an experimental study in the immature rat. _Pediatrics_ 95: 868–874 CAS PubMed Google Scholar * Haaland K, Karlsson B, Skovlund E, Lagercrantz H, Thoresen M
1995 Postnatal development of the cerebral blood flow velocity response to changes in CO2 and mean arterial blood pressure in the piglet. _Acta Paediatr_ 84: 1414–1420 Article CAS Google
Scholar * Rootwelt T, Loberg EM, Moen A, Oyasaeter S, Saugstad OD 1992 Hypoxemia and reoxygenation with 21% or 100% oxygen in newborn pigs: changes in blood pressure, base deficit, and
hypoxanthine and brain morphology. _Pediatr Res_ 32: 107–113 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank H. Nilsen, J. Lindstad, S. Pettersen, G. Dyrhaug, and
E.L. Sagen for excellent technical assistance. We are also grateful for valuable advice from G. Aamodt and Prof. T. Egeland in Section of Biostatistics, Rikshospitalet University Hospital,
Norway. We also thank S. Rivera, Université de la Méditerranée Neurobiologie des Interactions Cellulaires, Marseille, France. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Pediatric Research, Rikshospitalet University Hospital, Oslo, N-0027, Norway Berit H Munkeby, Wenche B Børke & Ola D Saugstad * Institute for Surgical Research and Surgical Department,
Rikshospitalet University Hospital, N-0027 Oslo, Norway Kristin Bjørnland * Centre for Occupational and Environmental Medicine, Rikshospitalet University Hospital, Oslo, N-0027, Norway Liv I
B Sikkeland * Matforsk AS, Norwegian Food Research Institute, Osloveien, N-1430 Ås 1, Norway Grethe I A Borge * Research Institute for Internal Medicine, Rikshospitalet University Hospital,
Oslo, 0027, Norway Bente Halvorsen Authors * Berit H Munkeby View author publications You can also search for this author inPubMed Google Scholar * Wenche B Børke View author publications
You can also search for this author inPubMed Google Scholar * Kristin Bjørnland View author publications You can also search for this author inPubMed Google Scholar * Liv I B Sikkeland View
author publications You can also search for this author inPubMed Google Scholar * Grethe I A Borge View author publications You can also search for this author inPubMed Google Scholar *
Bente Halvorsen View author publications You can also search for this author inPubMed Google Scholar * Ola D Saugstad View author publications You can also search for this author inPubMed
Google Scholar CORRESPONDING AUTHOR Correspondence to Berit H Munkeby. ADDITIONAL INFORMATION This work was granted by The Norwegian SIDS Society, University of Oslo, AGA AB Medical Research
Fund, The Laerdal Foundation for Acute Medicine, The Norwegian Society of Anesthesiology, The Norwegian Women's Public Health and Medinnova SF. B.H.M. is a research fellow at the
Department Group for Clinical Medicine, University of Oslo. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Munkeby, B., Børke, W., Bjørnland, K. _et
al._ Resuscitation with 100% O2 Increases Cerebral Injury in Hypoxemic Piglets. _Pediatr Res_ 56, 783–790 (2004). https://doi.org/10.1203/01.PDR.0000141988.89820.E3 Download citation *
Received: 04 February 2004 * Accepted: 28 May 2004 * Issue Date: 01 November 2004 * DOI: https://doi.org/10.1203/01.PDR.0000141988.89820.E3 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative